Abstract
Tripura is a north-eastern state of India with a total geographical area of 10,491 sq.km. Labeobata, L. calbasu, L. gonius of the genus Labeo Cuvier and Cirrhinusreba of genus Cirrhinus Oken are the most popular and commercially important native minor carps of the state. During a detailed survey on the protozoan parasites of the minor carps of Tripura, two new species of myxozoan (Myxozoa: Bivalvulida) parasites Myxobolus tripurensis sp.n. and Myxoboluspotularis sp.n. were encountered on the gill filaments of the host fishes. Distinctive characteristics of the plasmodia and the spores have been described.
Keywords: Minor carps, Myxobolus tripurensis sp.n., Myxobolus potularis sp.n., Parasite
Introduction
Tripura by virtue of its location in the sub-Himalayan belt has a rich and diversified fish fauna. The Myxosporeans are the most destructive pathogens within the protozoan phyla, causing large scale mortalities in natural and culture conditions (Shulman 1966). During the course of present investigations on the protozoan parasites of minor carps of the natural and cultured water bodies of the four different districts of Tripura (latitude 22°51′–24°32′N and longitude 90°10′–92°21′E), two new myxosporidian species Myxobolus tripurensis sp.n. and Myxobolus potularis sp.n. were encountered on the gill filaments and gill rakers of the host fishes. The description of the new species was done based on the guidelines of Lom and Dykova (1992).
Materials and methods
During the survey, fish samples were collected from the natural habitat and the aquaculture ponds of all the four districts of Tripura. The host fishes were identified as per Jhingran (1975), Talwar and Jhingran (1991) and Jayaram and Dhas (2000). The sporogonic plasmodia encountered were dissected out carefully with sterile forceps and smeared on grease-free glass slides with 0.5 % NaCl solution drops and covered with cover slips and examined under the transmission light microscope (TLM). Fresh spores were treated with Lugol’s iodine solution for detection of iodinophilous vacuole. The Indian ink method of Lom and Vavra (1963) was used for the detection of any external mucous coating around the spores.
For making the permanent preparations air-dried smears were Giemsa stained after fixation in acetone-free absolute methanol. The measurements were done with help of a calibrated ocular micrometre on basis of 20 fresh spores treated with Lugol’s iodine. The measurements of all the parameters presented are in μm as mean ± SD followed in parenthesis by the range. Drawings were made on fresh or stained material with the help of a mirror type camera lucida and the computer programme Corel Draw Version 14. The abbreviations used in this paper are as follows: length of the spore (LS), width of spore (WS), length of large polar capsule (LLPC), width of large polar capsule (WLPC), length of small polar capsule (LSPC), width of small polar capsule (WSPC), length of polar capsule (LPC), width of polar capsule (WPC), diameter of iodinophilous vacuole (DIV).
Results and discussion
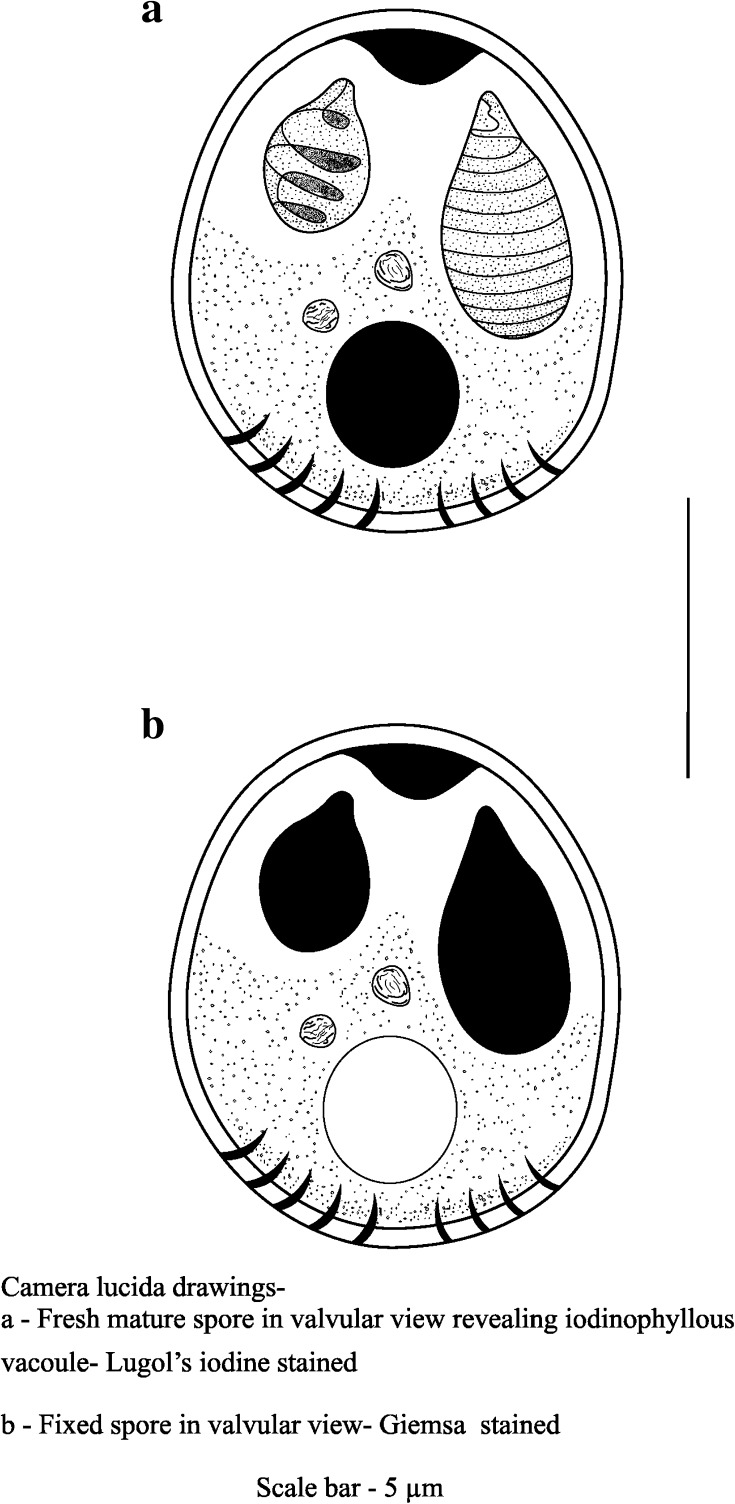
Myxobolus tripurensis sp.n. (Fig. 1; Plate 1)
Fig. 1.
Myxobolus tripurensis sp.n.
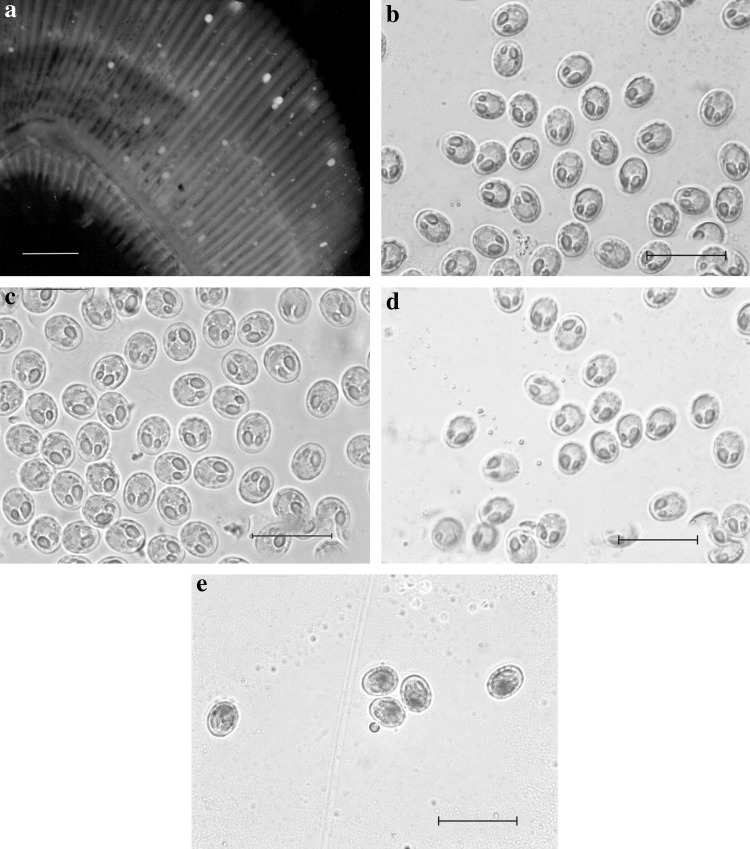
Plate 1.
Myxobolus tripurensis sp.n. a Photomicrograph of plasmodia or cyst attached on the gill filament of Labeo calbasu. b–d Photomicrographs of spores showing variations in appearences. e Spores stained with Lugol's iodine solution. Scale bars: a 700 μm, b–e 20 μm
Plasmodia
Creamy yellow oval shaped cysts found attached on the gill filaments and on the gill rakers of the host fishes.
Spores
The spores are small sized and round to oval in valvular view and is lenticular in sutural view. The two shell valves are thick-walled and symmetrical to each other. At the posterior portion of the spore there are suture markings. Mature spores are of size 8.1–9.4 (8.9 ± 0.41) μm in length and 6.5–7.0 (6.7 ± 0.19) μm in breadth.
The two polar capsules are distinctly different sized. The larger polar capsule measuring 4.0–4.5 (4.2 ± 0.18) μm in length and 2.0–2.6 (2.3 ± 0.21) μm in breadth is elongated and oval with eight to nine closely packed polar filaments. The smaller capsule measuring 2.5–2.8 (2.6 ± 0.10) μm in length and 1.6–1.9 (1.7 ± 0.12) μm in breadth is oval in shape with three to four loosely coiled polar filaments. An intercapsular projection is present between the two polar capsules.
The crescent shaped granular protoplasm extends into the intercapsular space medially. An iodinophilous vacuole, 2.2–2.6 (2.5 ± 0.15) μm in diameter was observed on treatment with Lugol’s iodine. No mucous envelope was seen around the spores on processing with Indian ink preparation.
The species under description shows very close affinity to Myxobolus lalithae Gupta and Khera (1988) described from the gills of Labeo calbasu; M. undasuturae, Sarkar (1994) reported from the urinary bladder of Amblypharyngodon mola; M. labeosus, Sarkar (1995) from the mesentery of spleen of Labeo fimbriatus and M. mrigalhitae, Basu and Haldar (2003) from gills of mrigal-rohu hybrid.
Myxobolus lalithae shows resemblance to the present species by the presence of unequal capsules and the presence of an intercapsular ridge. However, the detailed morphometric studies of the present species revealed that the spores and the polar capsules were much smaller compared to the spores of M. lalithae (LS: 10.0; WS: 8.4; LLPC: 5.8; WLPC: 3.0; LSPC: 4.8; WSPC: 2.8). M. undasuturae (LS: 10.6; WS: 5.7; LLPC: 6.1; WLPC: 2.3; LSPC: 4.8; WSPC: 2.8) shows apparent resemblance to the present species; however, the absence of inter capsular ridge and the elongated pyriform shape of the polar capsules differs from the present species.
The present species resembles M. mrigalhitae (LS: 10.8; WS: 7.9; LLPC: 4.8; WLPC: 2.9; LSPC: 3.0; WSPC: 2.1) by the presence of two unequal capsules and iodinophyllous vacuole as well as the parietal folds in the posterior extremities of the spore. However, the pyriform shape and the larger dimensions of the spore differ from the present species. M. labeosus (LS: 9.2; WS: 7.6; LLPC: 6.1; WLPC: 2.7; LSPC: 4.0; WSPC: 2.3) differs from the present species in the absence of iodinophyllous vacuole, morphometry and the orientation of the polar capsules.
In view of all the cited differences with the closely related species the present species is designated as a new species and named hereby as Myxobolus tripurensis sp.n. (Table 1).
Table 1.
Statistical measurements of morphometric parameters of Myxobolus tripurensis sp.n.
| Morphometric parameters | Range | X | SD | SE | CV (%) |
|---|---|---|---|---|---|
| LS | 8.1–9.4 | 8.9 | 0.41 | 0.09 | 4.61 |
| WS | 6.5–7.0 | 6.7 | 0.19 | 0.04 | 2.84 |
| LLPC | 4.0–4.5 | 4.2 | 0.18 | 0.04 | 4.28 |
| WLPC | 2.0–2.6 | 2.3 | 0.21 | 0.05 | 9.13 |
| LSPC | 2.5–2.8 | 2.6 | 0.10 | 0.02 | 3.85 |
| WSPC | 1.6–1.9 | 1.7 | 0.12 | 0.03 | 7.06 |
| DIV | 2.2–2.6 | 2.5 | 0.15 | 0.03 | 6.00 |
Taxonomic summary
Type host: Labeo calbasu (Hamilton 1822), Labeo bata (Hamilton 1822) and Cirrhinus reba (Hamilton 1822).
Type locality: Sonamura, West Tripura.
Type specimen: Holotype on slide MX/MB/003 and paratype on slide MX/MD/003/PR, collection of Parasitology Laboratory, Department of Zoology, University of Kalyani, Kalyani, West Bengal, India.
Prevalence: 1.7
Mean intensity: 1.05 %.
Infection loci: Gills.
Etymology: The specific name tripurensis represents the State of Tripura from where the host fishes were collected.
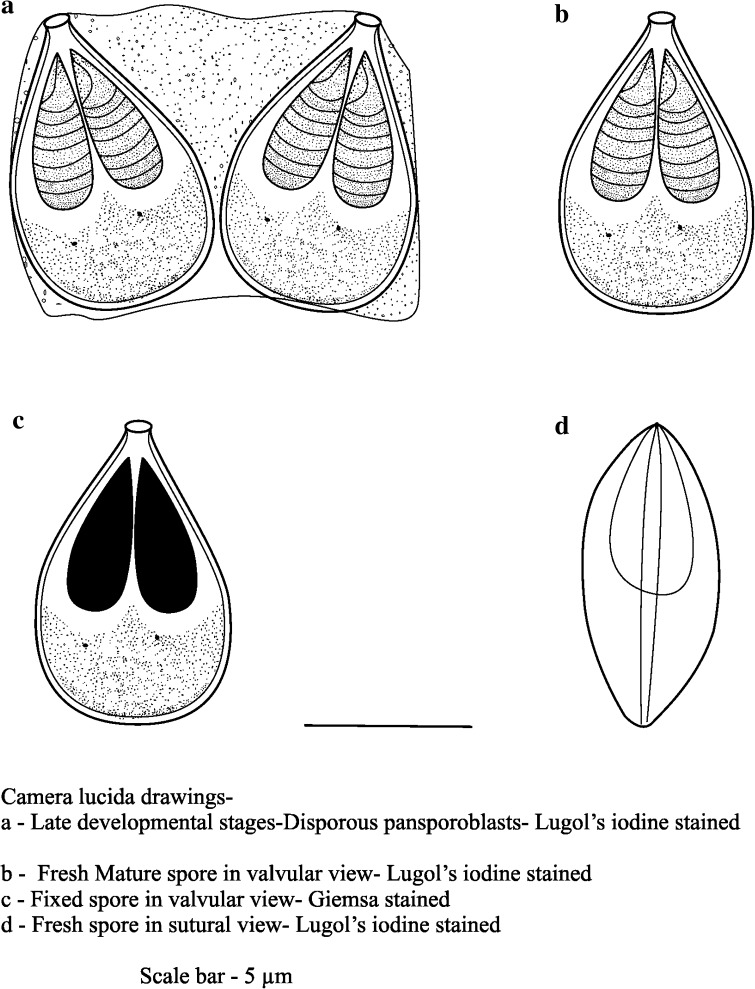
Myxobolus potularis sp.n. (Fig. 2, Plate 2)
Fig. 2.
Myxoboluspotularis sp.n.
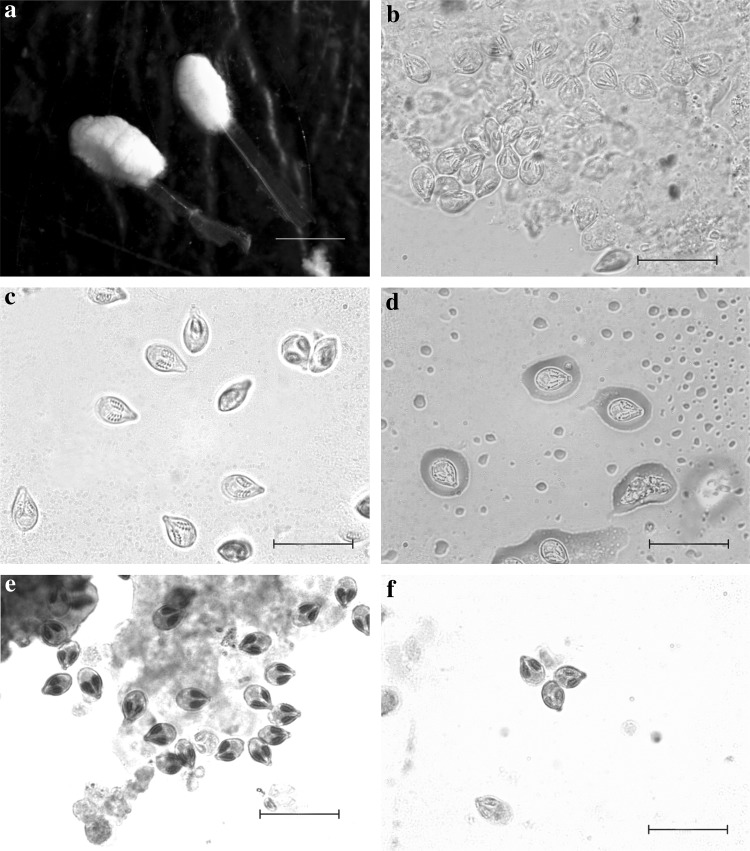
Plate 2.
Myxoboluspotularis sp.n. a Photomicrograph of plasmodia or cyst attached on the tip of a gill filament of Cirrhinus reba. b–d Photomicrographs of spores showing variations. e, f Giemsa stained spores. Scale bars: a 120 μm, b–f 20 μm
Plasmodia
Fully developed plasmodia are elongated and oval in shape and are conspicuous on lifting the gill operculum of the host fishes. The cysts are comparatively large-sized enveloping the gill filament tips. They are creamy yellow in colour and observed mostly as one or two cysts parasitising a single gill.
Spores
In front view, the mature spores are tear-shaped to oval with the anterior end bluntly pointed and posterior end greatly rounded and pot-like. Towards the anterior portion the spores take a nearly bottle-neck shape with a small knob which is clearly evident in fresh as well as stained preparations. The shell valves are completely devoid of any shell markings, suture ridge is present but the suture line is not clear. Mature spores measure 8.3–9.3 (9.0 ± 0.29) μm in length and 5.4–6.3 (6.0 ± 0.21) μm in breadth.
The two polar capsules are equal in size and is pyriform with greatly rounded or curved posterior end and is pointed towards the anterior proximity. The polar filaments are evidently visible in fresh as well as in stained condition. There are 7–8 tightly packed coils inside each capsule. The polar capsules measure 5.0–5.6 (5.4 ± 0.19) μm in length and 1.6–2.5 (2.2 ± 0.29) μm in breadth.
Granular and homogenous mass of cytoplasm fills the extra capsular region of the spore cavity and there are two very minute cytoplasmic nuclei. Spores lack mucous envelope surrounding the cyst wall and no iodinophyllous vacuole was detected on treatment with Lugols iodine solution.
The present species resembles Myxobolus nodularis Southwell and Prasad 1918 observed from the muscles of Rasbora daniconius (Southwell and Prasad 1918); M. mrigalae, Chakravarty (1939) reported from the scales of Cirrhinus cirrhosus; M. bengalensis, Chakravarty and Basu (1948) from the branchiae of Catla catla; M. potaili, Lalithakumari (1969) described from the branchiae of Labeo potail; M. dujardini, Lom and Noble 1984 reported from the fins, gills and the visceral organs of Rutilus spp., Leuciscus cephalus, Abramis spp., etc. M. anili, Sarkar (1989) found in the mesentry of Rhinomugil corsula; M. jiroveci, Lom et al. (1989) from the brain of Cottus gobio and M. molae, Sarkar 1993 reported from the kidney of Amblypharyngodon mola.
Though the spores of M. bengalensis (LS: 8.5–9.3; WS: 6.4–6.8; LPC: 4.2–5.4; WPC: 2.5–3.2) show remarkable similarity with the spores of the present species in the general appearance and the presence of the anterior protuberance, the presence of a spherical iodinophile vacuole which is lacking in the present species and the wider polar capsule of M. bengalensis diverts from the present species which is much narrower; thus the species under discussion distinguishes itself from M. bengalensis.
The present species perhaps shows the maximum morphological affinity with the spores of M. dujardini described by Lom and Noble (1984). Any how, on correlating the morphometry of the spores it was observed that M. dujardini (LS: 11.0–13.0; WS: 5.0–8.0) has much longer and wider spores than the present species, thus making it distinct. In spite of the presence of an anterior knob in the spores of M. potaili (LS: 7.2; WS: 5.4; LPC: 3.3; WPC: 2.0), it differs from the species under consideration by having striations in the shell valves which is absent in the present case. Though the spores of M. mrigalae (LS: 7.2; WS: 8.2; LLPC: 5.1; WLPC: 3.0; LSPC: 3.0; WSPC: 2.0) exhibit some superficial similarity in shape, they differ from the present case distinctly by the presence of two unequal polar capsules.
Spores of M. jiroveci (LS: 13.9; WS: 9.7; LPC: 6.2; WPC: 2.9) though has striking resemblance in the spore morphology with the present case, it differs in two significant aspects that is the presence of a distinct inter capsular appendix and very prominent markings along the sutural edge of the spore except in its anterior third, these two features lacking in the present case thus making the latter a different species. The spores of M. anili (LS: 10.7; WS: 8.6; LPC: 4.4; WPC: 2.0) are much longer and wider than the spores of the present species making it different from the latter whereas the polar capsules of M. nodularis (LS: 9.0; WS: 7.2; LPC: 3.0–4.0) are much smaller in length than the species under consideration. Further though superficially relating to M. molae (LS: 9.0; WS: 7.4; LPC: 6.0; WPC: 4.0), the detailed morphological comparison revealing a small round iodinophillous vacuole and the oval-shaped spore leads to the conclusion that the tear-shaped spores of the present species devoid of iodinophillous vacuole is distinct from M. molae.
All the above cited disparities with the closely related species justify the designation of this myxozoan as a new species and is hereby christened as Myxobolus potularis sp.n. (Table 2).
Table 2.
Statistical measurements of morphometric parameters of Myxobolus potularis sp.n.
| Morphometric parameters | Range | X | SD | SE | CV (%) |
|---|---|---|---|---|---|
| LS | 8.3–9.3 | 9.0 | 0.29 | 0.06 | 3.22 |
| WS | 5.4–6.3 | 6.0 | 0.21 | 0.05 | 3.50 |
| LPC | 5.0–5.6 | 5.4 | 0.19 | 0.04 | 3.52 |
| WPC | 1.6–2.5 | 2.2 | 0.29 | 0.06 | 13.18 |
Taxonomic summary
Type host: Labeo calbasu (Hamilton 1822), Labeo bata (Hamilton 1822), Labeo gonius (Hamilton 1822) and Cirrhinus reba (Hamilton 1822).
Type locality: Udaipur, South Tripura.
Type specimen: Holotype on slide MX/MC/005 and paratype on slide MX/MC/008, collection of Parasitology Laboratory, Department of Zoology, University of Kalyani, Kalyani, West Bengal, India.
Prevalence: 1.7
Mean Intensity: 1.05 %.
Infection loci: Gill filaments.
Etymology: The specific epithet potularis symbolises the peculiar pot shaped structure of the spore of the parasite.
Acknowledgments
The Head of the Department of Zoology, University of Kalyani and the authorities of ICAR, Tripura Centre are humbly acknowledged for providing the necessary laboratory facilities.
References
- Basu S, Haldar DP. Three new species of Myxobolus Butschli, 1882 from different food fishes of West Bengal. Acta Protozool. 2003;42:245–251. [Google Scholar]
- Chakravarty MM. Studies on myxosporidia from the fishes of Bengal, with a note on the myxosporidian infection in aquaria fishes. Archiv fur Protistenkunde. 1939;92:169–178. [Google Scholar]
- Chakravarty MM, Basu SP. Observations on some myxosporidians parasitic in fishes. Proc Zool Soc (Bengal) 1948;1:23–33. [Google Scholar]
- Gupta S, Khera S. On one new and one already known species of the genus Myxobolus from freshwater fishes of India. Res Bull Sci Punjab Univ. 1988;39:173–179. [Google Scholar]
- Jayaram KC, Dhas JJ (2000) Revision of the genus Labeo Cuvier from the Indian region with a discussion on its phylogeny and zoogeography (Pisces: Cypriniformes, Cyprinidae, Cyprininiae). Rec Zool Survey India. Occ. Paper No. 183
- Jhingran AG. Fish and fisheries of India. Delhi: Hindustan Publishing Corporation; 1975. [Google Scholar]
- Lalithakumari PS. Studies on parasitic protozoa (Myxosporidia) of freshwater fishes of Andhra Pradesh, India. Rivista Parasitologica. 1969;30:153–226. [PubMed] [Google Scholar]
- Lom J, Dykova I. Protozoan parasites of fishes. Development of aquaculture and fisheries science. Amsterdam: Elsevier; 1992. [Google Scholar]
- Lom J, Noble ER. Revised classification of class Myxosporea Butschli, 1881. Folia Parasitol (Prague) 1984;31:193–205. [Google Scholar]
- Lom J, Vavra J. Mucous envelopes of spores of the subphylum Cnidospora (Doflein) Vestnik Ceskoslovenske zoologiske spolecnosti. 1963;27:4–6. [Google Scholar]
- Lom J, Feist SW, Dykova I, Kepr T. Brain myxoboliasis of bullhead, Cottus gobio L., due to Myxobolus jiroveci sp. nov. light and electron microscope observations. J Fish Dis. 1989;12:15–27. doi: 10.1111/j.1365-2761.1989.tb01235.x. [DOI] [Google Scholar]
- Sarkar NK. Myxobolus anili sp. nov. (Myxozoa: Myxosporea) from a marine teleost fish Rhinomugil corsula (Hamilton) Proc Zool Soc Calcutta. 1989;42:71–74. [Google Scholar]
- Sarkar NK. On two new species of Myxobolus Butshli, 1882 (Myxozoa: Mysosporea) from some freshwater fish of West Bengal, India. Proc Zool Soc Calcutta. 1993;46:61–66. [Google Scholar]
- Sarkar NK. Three new species of Myxosporidia (Myxozoa: Bivalvulida) infecting some freshwater fishes of West Bengal, India. Proc Zool Soc Calcutta. 1994;47:63–69. [Google Scholar]
- Sarkar NK. Observations on two new Myxosporidia (Myxozoa: Myxosporea) from fishes of bhery-fishery of West Bengal, India. Acta Protozool. 1995;34:67–70. [Google Scholar]
- Shulman SS. Myxosporidia of the U.S.S.R. New Delhi: Amerind Publishing Company; 1966. [Google Scholar]
- Southwell T, Prasad B. Notes from the Bengal fisheries laboratory No.5. Parasites of Indian fishes with note on carcinoma in the climbing perch. Rec Indian Museum. 1918;15:341–355. [Google Scholar]
- Talwar DK, Jhingran AG. Inland fishes of India and adjacent countries. New Delhi: Oxford and IBH; 1991. [Google Scholar]