Abstract
Mucopolysaccharidosis IVA (MPS IVA; Morquio A syndrome) is an autosomal recessive lysosomal storage disorder resulting from a deficiency of N-acetylgalactosamine-6-sulfate sulfatase (GALNS) activity. Diagnosis can be challenging and requires agreement of clinical, radiographic, and laboratory findings. A group of biochemical genetics laboratory directors and clinicians involved in the diagnosis of MPS IVA, convened by BioMarin Pharmaceutical Inc., met to develop recommendations for diagnosis. The following conclusions were reached. Due to the wide variation and subtleties of radiographic findings, imaging of multiple body regions is recommended. Urinary glycosaminoglycan analysis is particularly problematic for MPS IVA and it is strongly recommended to proceed to enzyme activity testing even if urine appears normal when there is clinical suspicion of MPS IVA. Enzyme activity testing of GALNS is essential in diagnosing MPS IVA. Additional analyses to confirm sample integrity and rule out MPS IVB, multiple sulfatase deficiency, and mucolipidoses types II/III are critical as part of enzyme activity testing. Leukocytes or cultured dermal fibroblasts are strongly recommended for enzyme activity testing to confirm screening results. Molecular testing may also be used to confirm the diagnosis in many patients. However, two known or probable causative mutations may not be identified in all cases of MPS IVA. A diagnostic testing algorithm is presented which attempts to streamline this complex testing process.
Introduction
Mucopolysaccharidosis IVA (MPS IVA; OMIM #253000), also known as Morquio A syndrome, is an autosomal recessive lysosomal storage disorder resulting from mutations in the gene encoding N-acetylgalactosamine-6-sulfate sulfatase (GALNS; EC 3.1.6.4) (Tomatsu et al 2005a). GALNS is required for the degradation of two glycosaminoglycans (GAGs), chondroitin-6-sulfate (C6S) (Matalon et al 1974; Singh et al 1976) and keratan sulfate (KS) (Glossl and Kresse 1982; Yutaka et al 1982). Defects in GALNS can lead to disturbance of lysosomal breakdown of KS and C6S with subsequent storage within the lysosomes and disruption of cell function and metabolism causing tissue and organ dysfunction. Some of the excess GAGs may also be excreted in urine with abnormal KS excretion often occurring in MPS IVA and B. MPS IVB (OMIM #253010), although phenotypically similar to MPS IVA, is a separate disorder and is caused by mutations in a different gene, the GLB1 gene which encodes β-galactosidase (EC 3.2.1.23).
As in all mucopolysaccharidosis (MPS) disorders, the tissue distribution pattern of the specific GAGs accumulated determines the clinical manifestations of the disorder. In MPS IVA these manifestations can include short stature, skeletal abnormalities, cervical instability, limited endurance, visual and auditory impairment, oral health challenges, cardiovascular abnormalities, and significant respiratory system compromise (Montano et al 2007; Hendriksz et al 2012). The accumulation of KS in cartilage, as opposed to bone, is responsible for the skeletal manifestations characteristic of MPS IVA (and B) (Hollister et al 1975). Developmental delay, seizures, and other serious CNS involvement are not typically a component of MPS IVA (McKusick and Neufeld 1983). There is a wide spectrum of phenotypic presentations (Beck et al 1986). Severely affected patients typically do not survive beyond the second or third decade of life while patients with the attenuated form of the disease may survive for over 70 years (Tomatsu et al 2011). An international MPS IVA registry found that 64 % of patients registered were below 18 years of age (Montano et al 2007). The heterogeneity of the mutations in the GALNS gene (Tomatsu et al 2005a) is likely responsible for the variable phenotypic presentation; however, additional genetic, environmental, and metabolic factors may also play a role.
This variability of phenotypic presentation can delay the diagnosis by years or even decades (Tylki-Szymanska et al 1998; Gosele et al 2000). Several caveats in MPS IVA diagnostic testing can further complicate and delay the diagnostic process. The combination of clinical and laboratory diagnostic challenges make MPS IVA particularly prone to both delayed diagnoses and misdiagnoses. Unfortunately, the time between onset of initial symptoms and diagnosis is typically on the order of years (Holzgreve et al 1981; Montano et al 2007). However, due to the progressive and often life-threatening nature of the disease (Montano et al 2007), early and accurate diagnosis is critical for optimal patient management. Clinicians and laboratories should both be aware of the possible complications of diagnostic testing for MPS IVA and work together to ensure that an accurate diagnosis is reached in a timely manner.
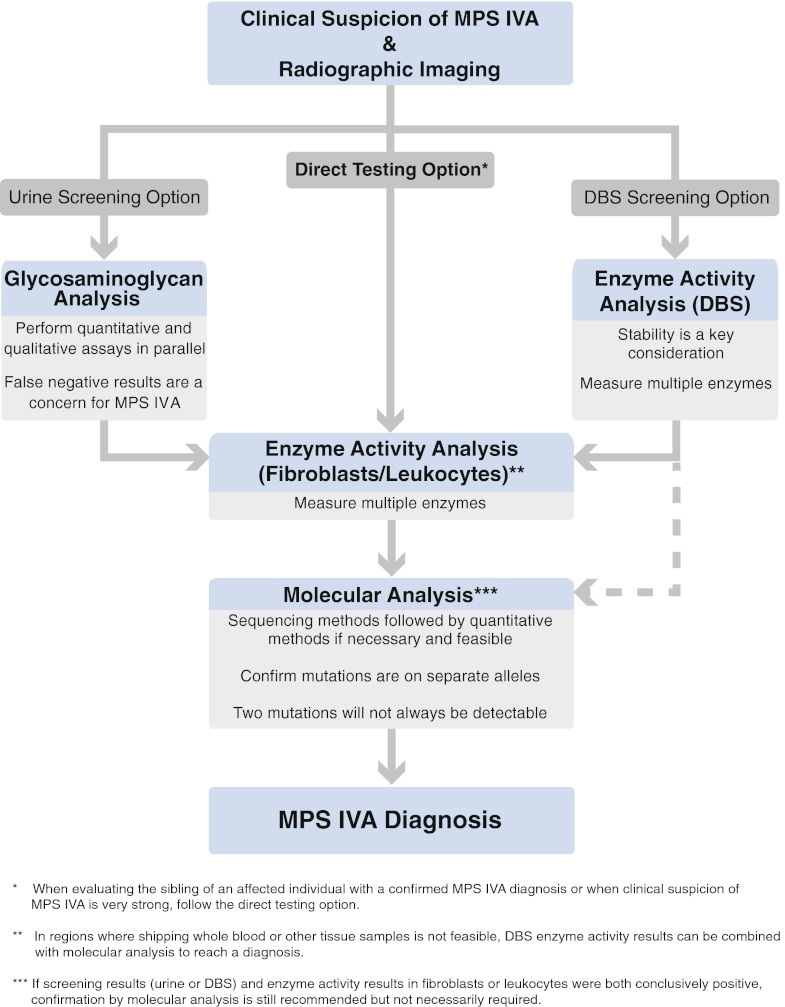
With the goal of illuminating the MPS IVA diagnostic process, a group of biochemical genetics laboratory directors and clinicians involved in the diagnosis of MPS IVA participated in surveys and met in working groups to develop recommendations for an MPS IVA diagnostic algorithm. The working groups met in Prague on June 16–18, 2011 and in San Diego on February 6–7, 2012. BioMarin Pharmaceutical Inc. administered the surveys and organized the meetings. A diagnostic algorithm was developed (Fig. 1). Additional background information was compiled and recommendations regarding key diagnostic elements were established in support of the algorithm.
Fig. 1.
Algorithm for the diagnosis of MPS IVA. MPS mucopolysaccharidosis; DBS dried blood spot
Establishing clinical suspicion
The path to diagnosis begins with the development of clinical suspicion of MPS IVA (Table 1). Clinical suspicion can originate from clinical findings, radiographic findings, or a combination of both. Patients typically appear normal at birth then begin to develop symptoms at varying rates depending upon the severity of their disease (Montano et al 2007). Severely affected patients will usually present with signs and symptoms within the first year of life while patients with attenuated disease may not develop initial symptoms until later in childhood or adolescence (Montano et al 2007). Initial signs and symptoms vary from patient to patient and may be present alone or in combination (Montano et al 2007). The presence or absence of any particular sign or symptom does not rule out or confirm MPS IVA. Many patients are initially seen by specialists such as orthopedic surgeons, ophthalmologists, or rheumatologists who may not be familiar with MPS IVA (Aldenhoven et al 2009; Lehman et al 2011; Summers and Ashworth 2011). Increased awareness of MPS disorder signs and symptoms, especially those which do not fit the classic phenotype, among these specialists is needed to facilitate prompt referral to a physician familiar with the diagnosis of inherited metabolic disorders such as a clinical geneticist or a metabolic specialist.
Table 1.
Signs and symptoms that should generate clinical suspicion of MPS IVA
| Skeletal abnormalities | Non-skeletal abnormalities |
|---|---|
| Short stature | Respiratory compromisea |
| Abnormal gait | Endurance limitations |
| Genu valgum | Recurrent respiratory infections |
| Spinal abnormalities | Sleep apnea |
| Odontoid hypoplasia | Snoring |
| Cervical instability | Cardiac valve abnormalities |
| Kyphosis/Gibbus | Muscular weakness |
| Scoliosis | Visual impairment |
| Chest abnormalities | Corneal clouding |
| Pectus carinatum | Astigmatism |
| Pectus excavatum | Hearing lossa |
| Joint abnormalities | Conductive |
| Joint hypermobility | Sensorineural |
| Joint pain | Dental abnormalities and oral health challengesa |
aThese abnormalities may be manifestations of underlying skeletal abnormalities
Common patient-reported initial skeletal symptoms include short stature, abnormal gait, genu valgum, pectus carinatum (or, more rarely, pectus excavatum), and spinal abnormalities (Montano et al 2007). Spinal abnormalities include odontoid hypoplasia, cervical instability, kyphosis/gibbus, and scoliosis (Tomatsu et al 2011). Gibbus in particular is often the first sign noticed in MPS IVA (Northover et al 1996). However, in patients with attenuated disease, hip stiffness and pain are more likely to be the initial signs reported (Wraith 1995). Diagnosis tends to be very challenging in these cases because they do not exhibit the classical signs of the disease. Patients with attenuated MPS IVA may undergo extensive medical treatment, such as corrective orthopedic surgeries, to address their symptoms prior to being identified as MPS IVA cases (Prat et al 2008). This can pose a significant risk for the patient as MPS IVA is often attended with anesthetic and perioperative complications (Theroux et al 2012) which may not be anticipated in the absence of an MPS IVA diagnosis.
Regardless of severity, as the disease progresses, more signs and symptoms appear. Joint hypermobility (of the wrist in particular) may develop and can be especially helpful in establishing clinical suspicion as it is unique to MPS IVA (Tomatsu et al 2011) and MPS IVB (Gucev et al 2008) among the MPS disorders. In fact, joint hypermobility is quite rare among all of the lysosomal storage diseases which generally tend to cause joint stiffness (Aldenhoven et al 2009). Aspartylglucosaminuria (OMIM #208400) is an exception to this generalization (Isenberg and Sharp 1975; Beaudet 1983). The absence of intellectual disability is also helpful in differentiating MPS IVA from several other lysosomal storage diseases.
Although MPS IVA has historically been considered a skeletal disease, the significance of the non-skeletal features has recently been described (Hendriksz et al 2012). Non-skeletal abnormalities may also provide key insight into the clinical diagnosis of MPS IVA. Signs of respiratory compromise, such as limited endurance, frequent respiratory tract infections, sleep apnea, and snoring, are common in MPS IVA (Hendriksz et al 2012). Other non-skeletal findings include mitral and/or aortic value regurgitation and thickening, conductive and sensorineural hearing loss, and muscle weakness (Hendriksz et al 2012). Visual impairment in MPS IVA differs slightly from other MPS disorders. Corneal clouding, although common, is milder, astigmatism can occur, and reported cases of glaucoma have been open-angle as opposed to closed-angle as reported for other MPS disorders (Hendriksz et al 2012). Dental abnormalities, including spaced dentition, pointed cusps, spade-shaped incisors, and enamel hypoplasia are also characteristic of MPS IVA (Levin et al 1975; Nelson et al 1988; Hendriksz et al 2012). Due to both dental and upper extremity abnormalities, maintaining oral health can be particularly challenging (James et al 2012).
A patient’s surgical history can be another element used to establish clinical suspicion. Adenoidectomy, tonsillectomy, hernia repair, and ear ventilation tubes can all be suggestive of an MPS disorder while cervical decompression and/or fusion and limb alignment surgeries at a young age are common in MPS IVA in particular (Tomatsu et al 2011).
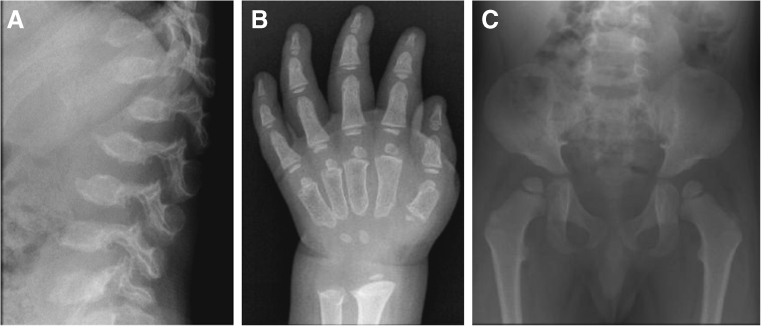
When MPS IVA is suspected, radiographic imaging should be performed as a component of the diagnostic process. Due to the wide variation and subtleties of radiographic findings in MPS IVA, it is recommended to obtain a skeletal survey to allow evaluation of multiple parts of the body, including skull, complete spine (including lateral views), hips, and limbs (particularly hands and wrists). Radiographic findings in MPS IVA (Fig. 2) can include odontoid hypoplasia, atlantoaxial subluxation, thickened skull, J- or omega-shaped sella turcica, flared ribs, short thorax with wide anterior posterior diameter, constricted iliac wings, underdeveloped acetabula, flattened capital femoral epiphyses, coxa valga, universal platyspondyly, anterior beaking of the vertebrae, short ulna, and delayed bone age or dysplastic carpal/tarsal and metacarpal/metatarsal bones.
Fig. 2.
Example radiographic findings specific for MPS IVA. a anterior beaking of the vertebrae; b short ulna and delayed bone age or dysplastic metacarpals; c constricted iliac wings, underdeveloped acetabula, and flattened capital femoral epiphyses
Although classified by the International Working Group on Constitutional Diseases of Bone as part of the dysostosis multiplex group (Spranger 1992), MPS IVA can appear similar to several other skeletal dysplasias not classified as part of that group (Table 2). While a comprehensive review of these disorders is beyond the scope of this article, a few key differential diagnoses are described in detail to highlight specific diagnostic challenges. Since MPS IVA can be conclusively confirmed or ruled out using laboratory testing and many other skeletal disorders are diagnosed based on clinical evidence alone, performing laboratory tests for MPS IVA whenever it is considered a possible differential diagnosis is recommended.
Table 2.
Select non-dysostosis multiplex skeletal disorders which may be confused with MPS IVA
| Category | Differential diagnosis | Distinguishing clinical/radiographic features | References |
|---|---|---|---|
| Spondyloepiphyseal dysplasia (SED) | SED congenita | Symptoms present from birth | (Fraser et al 1969) |
| Dyggve-Melchior-Clausen syndrome (pseudo-Morquio syndrome type 1) | Intellectual disability and lace-like appearance of the iliac crests in radiographs | (Nakamura et al 1997; Nishimura et al 2010) | |
| Smith-McCort syndrome | Lace-like appearance of the iliac crests in radiographs | (Nakamura et al 1997; Cohn et al 2003; Nishimura et al 2010) | |
| SED, Maroteaux type (pseudo-Morquio syndrome type 2) | Smooth and uniform platyspondylysis, no anterior beaking of the vertebrae, upper femoral metaphyses are enlarged and short | (Doman et al 1990; Megarbane et al 2004; Nishimura et al 2010) | |
| X-linked SED tarda | Coxa vara, disproportionately long arms relative to height, superior and inferior humping of vertebral bodies | (Whyte et al 1999) | |
| Autosomal recessive SED tarda | Intellectual disability | (Kohn et al 1987b) | |
| Spondylometaphyseal dysplasia (SMD) | SMD, Kozlowski type | Overfaced vertebral pedicles, irregular proximal femoral growth plates | (Krakow et al 2009) |
| Brachyolmia | Types 1, 2, and 3 | Lack of long bone involvement | (Shohat et al 1989) |
| Other | Legg-Calve-Perthes disease | Involvement limited to hips | (Miyamoto et al 2007) |
Radiographically, skeletal changes in MPS IVA can appear very similar to those seen in the osteochondrodysplasias classified by the International Working Group on Constitutional Diseases of Bone (Spranger 1992) as spondyloepiphyseal dysplasias (SEDs). Congenital SEDs can usually be differentiated from MPS IVA because symptoms are present at birth. However, there are also several forms of SED in which affected individuals may appear normal at birth, making differentiation from MPS IVA more difficult. Two SEDs, Dyggve-Melchior-Clausen syndrome (DMC; OMIM #223800) and SED, Maroteaux type (OMIM #184095), were previously known as pseudo-Morquio syndrome types 1 and 2, respectively, due to their skeletal resemblance to MPS IVA (Nishimura et al 2010).
DMC, originally reported as Morquio-Ullrich’s disease (Dyggve et al 1962), is caused by mutations in the DYM gene (El Ghouzzi et al 2003) and can be clinically differentiated from MPS IVA because patients are intellectually disabled. However, mutations in the same gene can also cause Smith-McCort syndrome (SMC; OMIM #607326), a condition radiographically identical to DMC in which intelligence and psychomotor development are normal (Cohn et al 2003). In radiographs, both DMC and SMC appear similar to MPS IVA, including the presence of atlantoaxial instability caused by odontoid hypoplasia, but can be differentiated by a characteristic lace-like appearance of the iliac crests (Nakamura et al 1997) which is absent in MPS IVA.
SED, Maroteaux type is part of a family of skeletal dysplasias caused by dominant mutations in the TRPV4 gene (Nishimura et al 2010). SED, Maroteaux type can be differentiated from MPS IVA clinically by the absence of the previously discussed non-skeletal features; however, radiographically, the conditions are very similar (Doman et al 1990). Radiographic differences are seen mainly in the spine and the hip (Doman et al 1990; Megarbane et al 2004). In MPS IVA, platyspondyly is irregular, anterior beaking often develops on the vertebrae, and there is coxa valga with flattened epiphyses as opposed to SED, Maroteaux type in which the platyspondyly is smooth and uniform, anterior beaking of the vertebrae is not present, and the upper femoral metaphyses are enlarged and short (Doman et al 1990; Megarbane et al 2004). SED, Maroteaux type may also be referred to as spondyloepimetaphyseal dysplasia (SEMD) of Maroteaux (Megarbane et al 2004).
A third SED, X-linked SED tarda (X-linked SED; OMIM #313400), although distinct from classical MPS IVA, can appear very similar to mild cases of MPS IVA, but can be differentiated by coxa vara, disproportionately long arms relative to height, and characteristic superior and inferior humping of vertebral bodies (Whyte et al 1999) which are not seen in MPS IVA. An autosomal recessive variant of SED has also been identified, however, unlike MPS IVA, intellectual disability is a feature (Kohn et al 1987a). Additional differential diagnoses include spondylometaphyseal dysplasia, Kozlowski type (SMDK; OMIM #184252) and brachyolmia (OMIM #271530, 271630, 613678, 113500). Individuals with SMDK have platyspondyly, overfaced vertebral pedicles, irregular proximal femoral growth plates, and carpal ossification delay (Krakow et al 2009). Brachyolmias are a heterogeneous group of skeletal dysplasias characterized radiographically by generalized platyspondyly without significant long bone abnormalities (Shohat et al 1989).
If a mild case of MPS IVA presents with hip pain alone and additional areas of the skeleton are not imaged, it is also possible to misdiagnose the patient as having Legg-Calve-Perthes disease (OMIM #120140), a form of avascular necrosis of the femoral head (Miyamoto et al 2007). At least one such case in which an MPS IVA patient was initially diagnosed with bilateral Legg-Calve-Perthes disease has occurred recently (manuscript under development).
Although clinical findings and radiographs can provide substantial insight, laboratory testing is required to reach a diagnosis of MPS IVA. It is also important to note that when an alternative skeletal dysplasia is suspected but MPS IVA is considered enough of a possibility that the clinician chooses to perform a laboratory test to rule out the MPS disorders, urinary screening is not an acceptable method to use alone due to high false negative rates for MPS IVA. This issue is discussed in detail in the urinary screening section.
Laboratory testing
Once clinical suspicion of MPS IVA has been established, laboratory testing can be used to reach a diagnosis. A variety of laboratory testing methods are currently available. Screening can be performed either by testing for abnormal elevation of urinary GAG levels and presence of excess KS or by measuring enzyme activity from a dried blood spot (DBS). Each screening method has advantages and disadvantages which will be discussed in detail. The screening method selected often depends upon the availability of testing and of samples. Alternatively, if suspicion of MPS IVA is strong, clinicians may elect to bypass screening assays. A comprehensive list of all testing laboratories is beyond the scope of this article, however, readers are directed to websites such as GeneTests (www.GeneTests.org) or the genetic test registry (GTR) (www.ncbi.nlm.nih.gov/gtr/) for a list of laboratories offering diagnostic testing.
Throughout the diagnostic process, maintaining open communication between the clinician and laboratory personnel facilitates efficiency and helps reduce errors. Information regarding the clinical suspicion of MPS IVA allows the laboratory to ensure that the most appropriate tests are performed and aids in the interpretation of results. Clear communication of the results to the clinician ensures that the results are acted on appropriately.
As diagnostic testing for this disorder is difficult, the laboratory process can be enhanced by participation in external proficiency testing programs. These programs can improve consistency by allowing laboratories to monitor their competency for a particular test over time and relative to other laboratories. Proficiency testing is currently offered for MPS urinary analysis and/or enzyme activity testing by the College of American Pathologists (CAP) and the European Research Network for Evaluation and Improvement of Screening, Diagnosis and Treatment of Inherited Disorders of Metabolism (ERNDIM).
A significant challenge for these programs, however, is the lack of sufficient amounts of positive patient samples. Clinicians, laboratories and proficiency testing programs are encouraged to work together to overcome this challenge.
Urinary screening
Adequate sample quality is essential for urinary screening. Dilute urine samples can be particularly problematic and may not be accepted by the laboratory. A first morning void or 24 h collection is preferred and a minimum of 15 mL of sample is usually recommended; however, patient age must be taken into consideration. As shipping, handling, and logistical considerations for urine samples vary, clinicians should contact the laboratory for specific details.
Urinary screening is based on the excretion of excess GAGs that occurs in the MPS disorders. The urinary excretion of GAGs, when normalized to creatinine, is high in infants and young children, decreases with age, plateaus in the second decade of life, and remains constant through adulthood (de Jong et al 1989; Whitley et al 1989a; Piraud et al 1993; Gray et al 2007; Wood et al 2012). Evaluations can be done for both the total amount of GAG excreted (quantitative analysis) and the specific types of GAG excreted (qualitative analysis). In the case of MPS IVA, evaluating both simultaneously is critical to avoid false negative results as KS can be present without elevating the total amount of GAG above the upper limits for unaffected individuals (Whitley et al 1989a; Piraud et al 1993; Tomatsu et al 2004; Gray et al 2007). Determining both total urinary GAG quantity and GAG identity is strongly recommended, especially if MPS IVA is considered a possibility.
Some of the methods for analyzing total urinary GAG used in the past, such as spot tests and turbidity tests, are no longer recommended. Spot tests for MPS have very poor specificity (12–29 % false positives) and sensitivity (19–35 % false negatives) and do not allow for interpretation of results by patient age or sample concentration (Pennock 1976; de Jong et al 1991). The cetylpyridinium chloride (CPC) assay, a turbidity test based on GAG precipitation, also has a high false negative rate reported to range from 11 to 30 % depending upon the pH, with MPS IVA being the most commonly missed MPS (de Jong et al 1989). Another test for total urinary GAG, the uronic acid-carbazole test is particularly unsuited for use when MPS IVA or B is suspected because it is based on the measurement of uronic acid in GAGs; however, KS, the main GAG excreted in MPS IVA and B, does not contain uronic acid residues. Therefore, MPS IVA and B cannot be reliably detected by the uronic acid-carbazole test (de Jong et al 1989; Frazier et al 2008).
Currently, the analysis of total urinary GAG is usually performed in a quantitative manner by spectrophotometric analysis of urine combined with a cationic blue dye such as dimethylmethylene blue (DMB) (Whitley et al 1989b). Total GAG is reported relative to creatinine to normalize for urinary concentration and age-based reference ranges are used to interpret results (de Jong et al 1989). As previously discussed, although most MPS IVA patients do excrete KS, many do not excrete enough to elevate their total urinary GAG level above the reference range for unaffected individuals. This results in an unacceptably high false negative rate when this quantitative analysis is performed alone, 15 % for MPS in general and higher for MPS IVA in particular (Whitley et al 1989a; Tomatsu et al 2004; Gray et al 2007). To reduce false negative rates, running a qualitative analysis simultaneously is strongly recommended (Piraud et al 1993; Gray et al 2007).
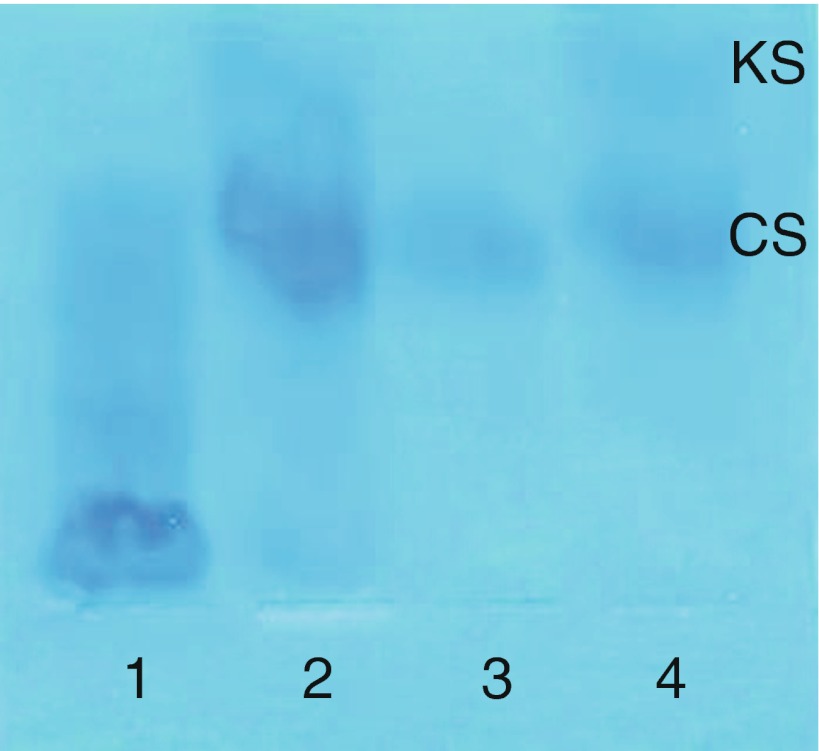
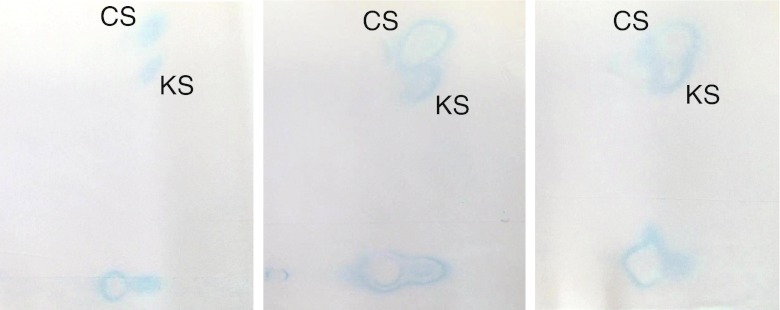
Qualitative analyses isolate GAGs from urine then separate them using either thin layer chromatography or electrophoresis (Wessler 1968; Humbel and Chamoles 1972). GAG identity is determined by relative position on the plate or gel. While the results are not quantitative, they can provide substantial insight into which MPS disorder is present and may reveal the presence of KS for some patients whose total urinary GAG levels are not elevated (Piraud et al 1993). Unfortunately, KS can be challenging to separate and visualize. Separation can be improved by using either multiple-step 1D electrophoresis (Hopwood and Harrison 1982) (Fig. 3) or 2D electrophoresis (Hata and Nagai 1972) (Fig. 4). However, despite the improved separation, interpretation of results is still subjective and can be challenging (Fig. 5).
Fig. 3.
Multiple-step 1D electrophoresis qualitative urinary GAG analysis (Hopwood and Harrison 1982). KS keratan sulfate, CS chondroitin sulfate, lane 1: MPS I/II control, lane 2: MPS IVA patient, lane 3: unaffected control, lane 4: MPS IVA positive control
Fig. 4.
Positive 2D electrophoresis qualitative urinary GAG analysis results for MPS IVA. KS keratan sulfate, CS chondroitin sulfate
Fig. 5.
Challenging to interpret urinary GAG 2D electrophoresis results from MPS IVA patients illustrating the difficulty in discerning the presence of keratan sulfate. All patients were subsequently confirmed to have MPS IVA by enzyme activity analysis
Tandem mass spectrometry detection (MS-MS) and quantification of several GAG species or KS alone is an attractive alternative to the methods previously described (Oguma et al 2007; Ohashi et al 2009; Tomatsu et al 2010; Hintze et al 2011). A new method for quantifying urinary KS alone, as opposed to total urinary GAG, has recently been described (Martell et al 2011) and is based on the method that has been previously published (Oguma et al 2007). Keratanase II is used to digest KS and the resulting disaccharides are analyzed by LC-MS/MS. While currently used (non MS-MS) methods of urinary GAG analysis are either qualitatively specific for KS but non-quantitative or non-specific for KS and only quantitative for total GAG, this new method has the advantage of being both KS-specific and quantitative. Additionally, the LC-MS/MS method is likely to provide higher sensitivity than other methods. Reported assay validation results show separation between MPS IVA patient (n = 160) and unaffected control (n = 186) KS ranges in urine and provide compelling evidence for the clinical utility of this method. However, the degree of separation is small, particularly for older patients (0.2–1.3 (unaffected) vs. 1.7–37.9 (affected) μg/mg creatinine for patients >15 years of age) (Martell et al 2011). The increased sensitivity and specificity of this technology has provided renewed interest in the analysis of KS in blood. While some research suggests that blood KS may be an additional biomarker of interest in MPSIVA patients, further research is needed to understand its use and determine the best methods for analysis (Oguma et al 2007; Martell et al 2011; Hintze et al 2011). Finally, another new method has been described for analysis of non-reducing ends of urinary GAGs by LC-MS. Currently this method does not provide a measure of non-reducing ends for MPS IVA (Lawrence et al 2012). MS/MS methodology for the analysis of KS is not widely available in diagnostic, clinical settings which limit its current usefulness to physicians. However, with increased interest in MS-MS assays and the wider availability of the required reagents, MS-MS methodologies are likely to have a large impact in the diagnosis of and monitoring of MPS IVA in the future.
Ultimately, while both quantitative and qualitative urinary GAG analyses can provide insight into the diagnosis, they are still considered screening methods and can produce false positive and false negative results (Piraud et al 1993). The relatively high rate of false negatives is particularly problematic in mild cases where a clinician may not suspect MPS IVA and is interested in using urinary analysis to rule out the diagnosis. It is this testing strategy in patients with borderline or normal total urinary GAGs and/or KS that often leads to the diagnosis being missed or delayed. While most MPS IVA patients excrete KS (Tomatsu et al 2004; Martell et al 2011), the absence of KS excretion in MPS IVA has been reported in the past (Fujimoto and Horwitz 1983). This may have been due to the limited sensitivity of the technology available at the time. However, regardless of the technology used, urine analysis is likely to be more challenging in older patients especially those with the mild form of the disease who do not present with symptoms until adolescence. One of the main sources of KS is cartilage, so as bone growth centers close in patients reaching puberty, the rate of KS accumulation slows and urinary excretion is substantially reduced and may even disappear (Longdon and Pennock 1979). Therefore, false negative results become more likely as patients age. Increased disease awareness may also lead to increased false negative rates as milder cases with lower KS levels are screened more often. Whether or not the new LC-MS/MS method will be able to overcome these challenges remains to be seen. Regarding the specificity of urinary analysis for MPS IVA, patients with MPS IVB and multiple sulfatase deficiency (OMIM # 272200) also excrete KS (Arbisser et al 1977; Groebe et al 1980) and, although unexpected, elevated KS has been reported in other MPS disorders and mucolipidoses (Tomatsu et al 2005b). KS can also be elevated in other lysosomal disorders such as Fucosidosis (OMIM # 230000) and GM1 gangliosidosis (OMIM #230500, 230600, 230650) which is caused by the same enzyme deficiency as MPS IVB (Ullrich and Kresse 1996). Hence the absence of KS does not conclusively rule out MPS IVA and the presence of KS does not conclusively diagnose MPS IVA. Proceeding to enzyme activity analysis even if urinary analysis results are negative is strongly recommended if there is clinical suspicion of MPS IVA.
In addition to being used to screen for MPS, urinary analysis is also commonly used in other MPS disorders to track a patient’s response to enzyme replacement therapy (ERT) (Wraith et al 2004; Harmatz et al 2006; Muenzer et al 2006). Should ERT become available for MPS IVA, using urinary analysis to track MPS IVA patient responses may present a significant challenge. New methodology, such as the LC-MS/MS assay, or possibly even new biomarkers, may be required to successfully quantify biochemical patient outcomes.
Enzyme activity analysis
Diagnosis of MPS IVA requires the demonstration of a deficiency in GALNS activity. Fibroblasts and leukocytes are the recommended sample types for this analysis. Prenatal samples, such as dissected chorionic villi, cultured chorionic villus cells, and amniocytes, can also be used, facilitating prenatal diagnosis (Zhao et al 1990; Kleijer et al 2000). Additionally, protocols for evaluating GALNS activity in a DBS have recently been proposed as screening methods (Duffey et al 2010; Camelier et al 2011).
Fibroblast samples are recommended for enzyme activity analysis because the impact of environmental and logistical factors during shipment can be minimized and corrected through culturing of the cells in the receiving laboratory. The culturing process also allows for the generation of a large number of cells for analysis and more cells can be grown at a later time if further analyses are needed. On the other hand, skin punch biopsies are invasive and a significant amount of time is required prior to analysis to culture the cells (ranging from 2 to 10 weeks) which can occasionally fail to grow or become contaminated. The total time from sample collection to the reporting of results to the clinician can be relatively lengthy, particularly if problems are encountered during cell culture and the culturing process has to be repeated. Some laboratories also require that the fibroblasts from the skin punch biopsy are cultured prior to being sent to the laboratory which requires considerable time, effort, and expertise on the part of the establishment sending the sample.
Leukocytes isolated from whole blood allow for more rapid analysis as cell culture is not required. Typically, the time from sample collection to the reporting of results to the clinician is less than 2 weeks. Whole blood samples normally provide a sufficient number of cells for analysis; however, additional cells cannot be generated by cell culture, so another sample must be obtained if the initial sample is insufficient or if the results are inconclusive. Furthermore, leukocyte samples are susceptible to environmental extremes during shipping in countries with a hot climate (Burin et al 2000). Sample deterioration may also become an issue when shipping long distances results in a prolonged amount of time spent in transit. This has lead many laboratories to require arrival of the sample within 24 to 48 h post-draw. Ultimately, the measurement of lysosomal enzymes in blood requires careful attention to the quality of the incoming sample including measurement of controls to verify sample integrity.
DBS samples are a convenient sample type both in regions where it is difficult to ship whole blood or tissue samples and for newborn screening programs. However, as described for leukocytes, proper sample collection and shipping are critical to the success of the analysis (Camelier et al 2011). A video on DBS collection technique is available online (Fundación para el Estudio de las Enfermedades Neurometabólicas 2011). The date of collection should always be noted on the card to aid in interpretation of results. The card should be dried at room temperature for at least 4 h. Due to the temperature sensitive nature of GALNS in a DBS (Camelier et al 2011), cards should be stored at 4°C after drying and shipped promptly; the longer the period of time between collection and analysis, the higher the risk of a false positive result. If high temperatures are possible during shipment, the card should be shipped in an insulated container with ice packs (Camelier et al 2011).
While measurement of GALNS activity in DBS samples is very useful for screening, this method is not as robust as it is in fibroblasts or leukocytes due to the lower number of cells present in the sample. Furthermore, more data are needed to evaluate GALNS stability in a DBS, especially since DBS samples are more likely to be exposed to environmental extremes during shipping than leukocytes. For these reasons, DBS results should not be used alone to reach a diagnosis. Confirming positive DBS results by enzyme activity analysis in fibroblasts or leukocytes is strongly recommended. Alternatives are discussed in the section entitled “Reaching a diagnosis”.
It is important to note that if a patient has recently received a blood transfusion or a bone marrow/stem cell transplant, enzyme activity analysis in both leukocyte and DBS samples could give inaccurate results. If there is any concern for this type of interference, consultation with the laboratory is recommended or, at minimum, this information should be included on the sample requisition. Fibroblast samples will not be impacted by these treatments and are recommended in these instances.
GALNS acts on two substrates, N-acetylgalactosamine-6-sulfate (GalNAc-6S) (Matalon et al 1974) and galactose-6-sulfate (Gal-6S) (Glossl and Kresse 1982; Yutaka et al 1982). GalNAc-6S is a component of C6S and Gal-6S is a component of KS. Both of these substrates are used currently to demonstrate deficient GALNS activity. The GalNAc-6S based assay uses radio-labeled natural substrate (Glossl and Kresse 1978) and the Gal-6S based assay uses fluorogenic artificial substrate (van Diggelen et al 1990). The radio-labeled substrate, a tritiated disulfated trisaccharide prepared from C6S, was developed first (Glossl and Kresse 1978). Using this substrate, GALNS activity is determined based on the amount of radioactivity released from the substrate with a lack of GALNS activity resulting in low generation of signal. Although this method is still in use, many laboratories have opted to switch to an assay based on a fluorogenic substrate (van Diggelen et al 1990). The fluorogenic assay uses 4-methylumbelliferyl-β-D-galactopyranoside-6-sulfate (4MU-Gal6S) as the substrate and is accomplished in two steps. First, GALNS present in the sample removes the 6-sulfate, and then exogenous β-galactosidase removes the galactoside, freeing the 4-methylumbelliferone adduct, which fluoresces under high pH. The addition of exogenous β-galactosidase to the reaction mixture is critical because conditions in which β-galactosidase is deficient (MPS IVB, I-cell disease (OMIM #252500), and GM1 gangliosidosis) would result in significant GALNS activity underestimation and possibly misdiagnoses (van Diggelen et al 1990). Removal of endogenous phosphates and sulfates is also important as they are lysosomal sulfatase inhibitors (van Diggelen et al 1990). Modification of the original published protocol (van Diggelen et al 1990) by increasing the substrate concentration ten-fold is recommended to increase assay sensitivity.
In addition to these two currently used methods, a tandem mass spectrometry based method using a novel substrate has recently been developed (Duffey et al 2010). Incorporation of this new assay into a multiplexed lysosomal storage disease panel for use in newborn screening programs is being considered (Khaliq et al 2011).
Regardless of the methods used, performing enzyme activity analysis for MPS IVA should also involve the evaluation of additional enzymes to control for sample integrity and to rule out differential diagnoses.
To confirm that low GALNS activity is not due to sample degradation, the activity of a reference enzyme with similar stability in the same sample should be measured. If a DBS is used, measuring a reference enzyme in the same sample to confirm integrity is still recommended, but may not be sufficient to rule out an effect of handling on GALNS activity because the stability of GALNS as compared to other enzymes in a DBS is not known. Simultaneously collecting and shipping a negative control DBS sample (from an unaffected healthy individual) to confirm sample handling has not compromised GALNS activity is recommended (Camelier et al 2011). However, obtaining control samples may be difficult logistically for some laboratories, particularly for routine testing of a large number of patients. Parental or familial samples are not recommended for this purpose as their GALNS activity level may be below normal if they are carriers.
It is also important to confirm that the low GALNS activity is not being caused by a different disease. Multiple sulfatase deficiency (MSD) and mucolipidosis II or III (ML II/III) also impact GALNS activity. MSD is a disease in which the activities of several sulfatases, including GALNS, are deficient (Dierks et al 2003) and ML II/III impair mannose-6-phosphate guided enzyme targeting causing lysosomal enzyme activities to be low in fibroblasts, elevated in plasma, and relatively unaltered in leukocytes (Neufeld and McKusick 1983). Both diseases can be alternative causes of low GALNS activity depending on the sample type used. The possibility of MSD should be evaluated by measuring the activity of a second sulfatase such as arylsulfatase B (EC 3.1.6.12) or iduronate-2-sulfatase (EC 3.1.6.13). If a leukocyte sample or a DBS was used for GALNS analysis, ML II/III is not a concern as GALNS activity is not decreased by ML II/III in this specific sample type. However, if fibroblasts were used, ML II/III must be ruled out. This can be accomplished by measuring a second mannose-6-phosphate targeted enzyme such as β-galactosidase, arylsulfatase B, iduronate-2-sulfatase, β-hexosaminidase (EC 3.2.1.52), or α-iduronidase (EC 3.2.1.76) in fibroblasts.
Lastly, ruling out MPS IVB during enzyme analysis is also highly recommended. MPS IVA and B can present with very similar symptoms and can both cause elevated urinary KS (McKusick and Neufeld 1983). Therefore, β-galactosidase, the enzyme deficient in MPS IVB, is commonly tested in conjunction with GALNS. As mentioned previously, β-galactosidase activity in fibroblasts or plasma can also be used to rule out ML II/III (not necessary if leukocytes were used), allowing for the exclusion of two diseases with one measurement. Additional examples of enzymes that can be used to exclude more than one condition, including some additional alternative MPS disorders, when testing for MPS IVA are shown in Table 3.
Table 3.
Enzymes that can be measured in addition to GALNS to exclude more than one alternative condition
| MSD | ML II/IIIa | MPS | |
|---|---|---|---|
| β-galactosidase | ● | MPS IVB | |
| Arylsulfatase B (ASB) | ● | ● | MPS VI |
| Iduronate-2-sulfatase | ● | ● | MPS II |
| α-iduronidase | ● | MPS I |
aEnzyme levels in leukocytes will not be affected by ML II/III
Care should be taken when interpreting enzyme activity results. Reference ranges vary significantly depending on the units, sample type, and methodology used, as well as among laboratories (Table 4). Laboratories should clearly indicate reference ranges along with patient results and provide an interpretation in this context.
Table 4.
Laboratory-specific reference ranges from five example laboratories. Reference ranges vary based on methods, sample type, and units used for reporting, they also vary among laboratories even using the same method
| Substrate | Sample type | Laboratory | Units | Normal range | Affected range |
|---|---|---|---|---|---|
| 4MU-Gal-6S | fibroblasts | Lab #1 | nmol/h/mg protein | 3.9–45.9 | 0–0.12 |
| Lab #2 | nmol/17 h/mg protein | 12–26 | 0.5–1.2 | ||
| Lab #3 | 76–255 | 0–10 | |||
| Lab #4 | 40–170 | <16 | |||
| leukocytes | Lab #1 | nmol/h/mg protein | 11.0–44.6 | 0.08–3.2 | |
| Lab #2 | nmol/17 h/mg protein | 87–180 | 2–11 | ||
| Lab #3 | 34–347 | 0–10 | |||
| DBS | Lab #4 | μmol/L/20 h | >8 | <4.5 | |
| GalNAc6S-glcA-[1-3H]galitolNAc6S | fibroblasts | Lab #5 | pmol/min/mg protein | 18–72 | <6 |
| leukocytes | Lab #5 | 39–166 | <2.7 |
Molecular analysis
Molecular analysis, also known as mutation analysis, can be used to confirm enzyme activity results and facilitate genetic counseling of the family. The goal is to identify genetic mutations that result in decreased or absent GALNS enzyme activity. Because MPS IVA is a recessive disease, both GALNS alleles must contain a pathogenic mutation for a patient to be affected. In addition to pathogenic mutations, polymorphisms have also been reported in the GALNS gene (Tomatsu et al 2005a). When a novel mutation is identified, further investigation may be needed to determine whether or not it is pathogenic in nature.
The GALNS gene is located on chromosome 16q24.3 (Baker et al 1993; Masuno et al 1993), contains 14 exons (Nakashima et al 1994), and generates a 1566 nucleotide mRNA (Tomatsu et al 1991). A review of 148 unique mutations was published in 2005 (Tomatsu et al 2005a) and the identification of additional mutations continues at a steady pace to date. Mutation types reported in the Human Gene Mutation Database (HGMD) include missense, nonsense, splicing, small insertions and deletions, gross insertions and deletions, and complex rearrangements (Table 5). There is limited published data regarding mutation analysis of the GALNS gene, however the most common mutations published to date are reported to be missense mutations c.1156C>T (p.Arg386Cys), c.901G>T (p.Gly301Cys), and c.337A>T (p.Ile113Phe) (Tomatsu et al 2005a). However, their allelic frequencies are only 8.9, 6.8, and 5.7 %, respectively, demonstrating the allelic heterogeneity of MPS IVA (Tomatsu et al 2005a). Allelic frequencies are also very population-dependent. For example, the c.337A>T (p.Ile113Phe) mutation was actually found to be much more common (identified in 32 % of patients) in a cohort of United Kingdom/Eire patients (n = 89) evaluated at the Central Manchester University Hospitals in the United Kingdom (unpublished data).
Table 5.
GALNS mutations as reported by the HGMD database (http://www.hgmd.org/)
| Mutation type | # of unique mutations |
|---|---|
| Missense | 120 |
| Nonsense | 11 |
| Splicing | 14 |
| Small deletions | 18 |
| Small insertions | 3 |
| Small indels | 1 |
| Gross deletions | 4 |
| Gross insertions/duplications | 2 |
| Complex rearrangements | 2 |
Accessed June 11, 2012
Molecular analysis is typically performed using a blood sample. DNA in dried blood samples is very stable (Chaisomchit et al 2005) and, therefore, can be shipped easily. Specific collection cards are available for samples intended for DNA analysis; collection card selection should be as per receiving laboratory recommendations. It is important to note that blood samples from patients who have undergone a recent blood transfusion or a bone marrow/stem cell transplant at any time may give inaccurate results as these samples will likely be contaminated with donor DNA. These samples are not recommended for genotype analysis. If the initial biochemical diagnosis has been performed on fibroblasts, then the same cells could also be used as a source of patient DNA. Saliva samples and buccal swabs are additional acceptable sources of DNA.
In standard DNA sequencing-based methods, coding regions of the GALNS gene and small segments of immediately adjacent intronic regions are evaluated. Numerous MPS IVA mutations are believed to be “private” and found in only one individual or family (Tomatsu et al 2005a). Although many mutations have been reported in the literature, novel, unreported mutations are still commonly detected (Table 6). Unfortunately, conclusively determining whether a novel mutation is pathogenic may not always be possible. It is notable that some of the known pathogenic mutations in MPS IVA result in conservative amino acid changes, such as the common pathogenic mutation p.Ile113Phe, highlighting the difficulty in predicting the pathogenic nature of a novel mutation without associating enzyme activity testing results. Development of a global database of mutations with associated degree of compromised enzyme activity and phenotypic correlations could potentially facilitate improved dissemination of information and help reduce the number of mutations with unknown significance identified by GALNS sequencing.
Table 6.
Current rate of diagnostic challenges arising from molecular analysis for MPS IVA
| Laboratorya | # of patients (alleles) analyzed | Frequency of patients (alleles) with novel mutations | Frequency of enzymatically confirmed MPS IVA patients (alleles) lacking one or more pathogenic mutations |
|---|---|---|---|
| Adelaide laboratory | 17 (34) | 35 % (26 %) | 0 (0) |
| Willink biochemical genetics laboratory | 89 (178) | 45 % (34 %) | 10 % (5 %) |
| Greenwood Genetic Center | 6 (12) | 50 % (42 %) | 16 % (8 %) |
aLocal population genetics affect the variety of mutations seen and detection rates
While DNA sequencing-based methods identify missense mutations, nonsense mutations, and small insertions and deletions in the coding region of the gene, mutations that cause splicing alterations (Pajares et al 2012) or changes in copy number (Fukuda et al 1996) may be missed. If a pathogenic mutation is not found in each allele, evaluation of cDNA may be helpful in identifying intronic mutations causing splicing errors (Pajares et al 2012) and quantitative methods of molecular analysis can be used to identify mutations affecting copy number, such as large insertions and deletions (Fukuda et al 1996). A variety of methods are available including real-time PCR, comparative genomic hybridization (CGH), and multiplex ligation-dependent probe amplification (MLPA). Unfortunately, even when both sequencing and quantitative methods are used, some mutations, such as those in promoter regions, will still not be detectable. Undetectable mutations have been reported in the literature to occur in approximately 14 % of alleles (Tomatsu et al 2005a).
When two pathogenic mutations are successfully identified, it is important to confirm that they are on separate alleles (in trans) because more than one mutation may be present on the same allele (in cis). It is also important to identify any in cis mutations because their presence may impact future prenatal diagnoses. To confirm that the mutations are in trans, analyzing the parental DNA is recommended. If each parent has one of the mutations, they are likely present in trans in the patient. If both of the mutations are identified in one parent, the two identified mutations are likely in cis in the patient. In this case, the patient is either a carrier (not affected) or the mutation in the second allele has not yet been identified.
One notable exception to the mutations coming from both parents is a recently reported case of maternal uniparental isodisomy (Catarzi et al 2012). This case provides evidence for the need to perform parental testing in all cases, even when the patient is found to be homozygous for a known mutation. It is also possible that a change could occur de novo in the germ cell. These considerations highlight the importance of parental testing in being able to provide families with accurate genetic counseling regarding recurrence risk.
Ultimately, although molecular analysis results can confirm a diagnosis and allow for genetic counseling of the family, results can also be inconclusive and complicate the diagnostic process. Discussing the limitations of the analysis with the patient and their family prior to testing is recommended.
Reaching a diagnosis
There are multiple paths to a diagnosis (Fig. 1). To reach an accurate diagnosis, it is critical that laboratory results from different assays and sample types corroborate the diagnosis. Any discrepancies should be investigated and resolved. The full diagnostic process involves screening followed by enzyme activity analysis in fibroblasts or leukocytes and confirmation by molecular analysis. However, all three steps are not always required and may not provide clinically relevant information in all cases. When clinical suspicion of MPS IVA is especially strong or when evaluating the sibling of an affected individual, screening may be unnecessary. In other cases, if screening results (urine or DBS) and enzyme activity results in fibroblasts or leukocytes are both conclusively positive, confirmation by molecular analysis is still recommended but not necessarily required. In special situations, such as in regions where it is not feasible to transport whole blood or other tissue samples, diagnostic evaluation of enzyme activity in fibroblasts or leukocytes might not be possible. In these situations DBS enzyme activity results from two independently collected DBSs can be combined with molecular analysis to reach a diagnosis. On the other hand, the combination of an isolated DBS enzyme activity result with the detection of urinary KS, while being strongly suggestive of MPS IVA, is not considered adequate for a definitive diagnosis as KS excretion is not specific to MPS IVA alone; in addition to being elevated in MPS IVB there is some evidence that KS can be elevated in other MPS disorders as well as mucolipidoses (Tomatsu et al 2005b).
The accurate diagnosis of MPS IVA rests on a thorough clinical evaluation and multiple laboratory measures. Alone, neither clinical features, nor laboratory results are sufficient given the clinical and laboratory challenges and complexities involved in the diagnosis of MPS IVA.
Conclusions
Variable clinical presentation and laboratory testing caveats make MPS IVA particularly challenging to diagnose. Both skeletal and non-skeletal symptoms should contribute to clinical suspicion. Radiographic imaging of multiple areas of the body is particularly important in MPS IVA. If urinary screening is carried out, quantitative analysis should not be performed without qualitative analysis because total urinary GAGs are not elevated in a significant proportion of MPS IVA patients and KS may be detectable by qualitative analysis even when total urinary GAGs are not elevated. However, KS is not always excreted in detectable quantities by all MPS IVA patients regardless of the method used. False negatives can occur even if quantitative and qualitative analyses are both performed. Proceeding to enzyme activity analysis even if urine appears normal is recommended when there is suspicion of MPS IVA. Enzyme activity testing of GALNS is essential in diagnosing MPS IVA; fibroblasts and leukocytes are the recommended sample types. Sample integrity should be confirmed as part of enzyme activity analysis and MPS IVB should be ruled out, particularly following positive urine results, because MPS IVA and B both cause urinary KS excretion. If GALNS activity is low, additional conditions causing low GALNS activity, MSD and ML II/III (if fibroblasts were used), should also be ruled out. If a DBS is used, collection and shipping conditions are of critical importance and cannot be understated. DBS results should be confirmed by enzyme activity testing in fibroblasts or leukocytes, or by molecular testing if it is not feasible to ship whole blood or other tissue samples. Using molecular analysis to confirm an MPS IVA diagnosis and provide genetic counseling to the family is recommended. When feasible, quantification-based methods of molecular analysis should be used to find mutations that could not be identified by sequencing-based methods. Even with currently available technology, two pathogenic mutations may not always be found in every case of MPS IVA. Clinicians and laboratories should both be aware of the possible complexities of diagnostic testing for MPS IVA and work together to ensure that an accurate diagnosis is reached in a timely manner.
Acknowledgements
Both Global MPS IVA Laboratory Diagnostics Scientific Summits were arranged and sponsored by BioMarin Pharmaceutical Inc (BioMarin). BioMarin also provided editorial and administrative assistance during the drafting of this manuscript.
Competing interests
Authors Bainbridge, Burin, Church, D’Almeida, van Diggelen, Fietz, Harmatz, Hendriksz, Lukacs, Pasquali, and Wood received consultant fees and limited travel reimbursement from BioMarin Pharmaceutical Inc. (BioMarin) for participating in the Global MPS IVA Laboratory Diagnostics Scientific Summit in Prague on June 16th–18th, 2011. Authors Bainbridge, Burin, Chien, Church, van Diggelen, Giugliani, Harmatz, Hendriksz, Hwu, Lukacs, Pasquali, Thompson, Tylee, Wood, and Yu received consultant fees and limited travel reimbursement from BioMarin for participating in the Global MPS IVA Laboratory Diagnostics Scientific Summit in San Diego on February 6th–7th, 2012. Authors Bainbridge, Beck, Burin, Chien, D’Almeida, van Diggelen, Fietz, Giugliani, Harmatz, Hendriksz, Hwu, Ketteridge, Lukacs, Thompson, Tylee, and Wood were compensated by BioMarin for completing a survey on the diagnosis of MPS IVA. Authors Hawley and Miller are employees of BioMarin and have direct financial interest and investments in BioMarin. Authors Giugliani, Harmatz, Hendriksz, Lukacs, and Tylee have served or are serving on advisory boards for BioMarin. Authors Giugliani, Harmatz, Hendriksz, and Ketteridge are current or recent participants in BioMarin sponsored clinical trials. Authors Harmatz and Hendriksz have assisted in the design of clinical trials evaluating BioMarin products. Authors Beck, Harmatz, Hendriksz, and Lukacs have received research support from BioMarin. Authors Chien, D’Almeida, van Diggelen, Fietz, Giugliani, Harmatz, Hendriksz, Ketteridge, Thompson, Wood, and Yu have received consulting fees or other remuneration from BioMarin. Authors Church, D’Almeida, and Tylee have received travel grants from BioMarin. Authors Burin and Schenone have acted as expert witnesses on the subject of this manuscript. Through their laboratory, authors Church and Tylee provide a diagnostic service for MPS for samples being sent from Turkey which is funded by BioMarin, a dried blood spot diagnostic service for Fabry and Pompe diseases which is funded by Genzyme Corporation, and baseline lysosomal acid lipase measurement service for patients recruited into ongoing clinical trials for Wolman/CESD which is funded by Synageva BioPharma Corporation.
References
- Aldenhoven M, Sakkers RJ, Boelens J, de Koning TJ, Wulffraat NM. Musculoskeletal manifestations of lysosomal storage disorders. Ann Rheum Dis. 2009;68:1659–1665. doi: 10.1136/ard.2008.095315. [DOI] [PubMed] [Google Scholar]
- Arbisser AI, Donnelly KA, Scott CI, Jr, et al. Morquio-like syndrome with beta galactosidase deficiency and normal hexosamine sulfatase activity: mucopolysacchariodosis IVB. Am J Med Genet. 1977;1:195–205. doi: 10.1002/ajmg.1320010205. [DOI] [PubMed] [Google Scholar]
- Baker E, Guo XH, Orsborn AM, et al. The morquio A syndrome (mucopolysaccharidosis IVA) gene maps to 16q24.3. Am J Hum Genet. 1993;52:96–98. [PMC free article] [PubMed] [Google Scholar]
- Beaudet AL. Disorders of glycoprotein degradation: mannosidosis, fucosidosis, sialidosis, and aspartylglycosaminuria. In: Stanbury JB, Wyngaarden JB, Fredrickson DS, Goldstein JL, Brown MS, editors. The metabolic basis of inheritied disease. New York: McGraw-Hill; 1983. pp. 788–802. [Google Scholar]
- Beck M, Glossl J, Grubisic A, Spranger J. Heterogeneity of Morquio disease. Clin Genet. 1986;29:325–331. doi: 10.1111/j.1399-0004.1986.tb01262.x. [DOI] [PubMed] [Google Scholar]
- Burin M, Dutra-Filho C, Brum J, Mauricio T, Amorim M, Giugliani R. Effect of collection, transport, processing and storage of blood specimens on the activity of lysosomal enzymes in plasma and leukocytes. Braz J Med Biol Res. 2000;33:1003–1013. doi: 10.1590/S0100-879X2000000900003. [DOI] [PubMed] [Google Scholar]
- Camelier MV, Burin MG, De MJ, Vieira TA, Marasca G, Giugliani R. Practical and reliable enzyme test for the detection of mucopolysaccharidosis IVA (Morquio syndrome type A) in dried blood samples. Clin Chim Acta. 2011;412:1805–1808. doi: 10.1016/j.cca.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Catarzi S, Giunti L, Papadia F, et al. Morquio A syndrome due to maternal uniparental isodisomy of the telomeric end of chromosome 16. Mol Genet Metab. 2012;105:438–442. doi: 10.1016/j.ymgme.2011.11.196. [DOI] [PubMed] [Google Scholar]
- Chaisomchit S, Wichajarn R, Janejai N, Chareonsiriwatana W. Stability of genomic DNA in dried blood spots stored on filter paper. Southeast Asian J Trop Med Public Health. 2005;36:270–273. [PubMed] [Google Scholar]
- Cohn DH, Ehtesham N, Krakow D, et al. Mental retardation and abnormal skeletal development (Dyggve-Melchior-Clausen dysplasia) due to mutations in a novel, evolutionarily conserved gene. Am J Hum Genet. 2003;72:419–428. doi: 10.1086/346176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JG, Hasselman JJ, van Landeghem AA, Vader HL, Wevers RA. The spot test is not a reliable screening procedure for mucopolysaccharidoses. Clin Chem. 1991;37:572–575. [PubMed] [Google Scholar]
- de Jong JG, Wevers RA, Laarakkers C, Poorthuis BJ. Dimethylmethylene blue-based spectrophotometry of glycosaminoglycans in untreated urine: a rapid screening procedure for mucopolysaccharidoses. Clin Chem. 1989;35:1472–1477. [PubMed] [Google Scholar]
- Dierks T, Schmidt B, Borissenko LV, et al. Multiple sulfatase deficiency is caused by mutations in the gene encoding the human C(alpha)-formylglycine generating enzyme. Cell. 2003;113:435–444. doi: 10.1016/S0092-8674(03)00347-7. [DOI] [PubMed] [Google Scholar]
- Doman AN, Maroteaux P, Lyne ED. Spondyloepiphyseal dysplasia of Maroteaux. J Bone Joint Surg Am. 1990;72:1364–1369. [PubMed] [Google Scholar]
- Duffey TA, Khaliq T, Scott CR, Turecek F, Gelb MH. Design and synthesis of substrates for newborn screening of Maroteaux-Lamy and Morquio A syndromes. Bioorg Med Chem Lett. 2010;20:5994–5996. doi: 10.1016/j.bmcl.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyggve HV, Melchior JC, Clausen J. Morquio-Ullrich’s disease: an inborn error of metabolism? Arch Dis Child. 1962;37:525–534. doi: 10.1136/adc.37.195.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ghouzzi V, Dagoneau N, Kinning E, et al. Mutations in a novel gene Dymeclin (FLJ20071) are responsible for Dyggve-Melchior-Clausen syndrome. Hum Mol Genet. 2003;12:357–364. doi: 10.1093/hmg/ddg029. [DOI] [PubMed] [Google Scholar]
- Fraser GR, Friedmann AI, Maroteaux P, Glen-Bott AM, Mittwoch U. Dysplasia spondyloepiphysaria congenita and related generalized skeletal dysplasias among children with severe visual handicaps. Arch Dis Child. 1969;44:490–498. doi: 10.1136/adc.44.236.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier SB, Roodhouse KA, Hourcade DE, Zhang L. The quantification of glycosaminoglycans: a comparison of HPLC, carbazole, and alcian blue methods. Open Glycosci. 2008;1:31–39. doi: 10.2174/1875398100801010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto A, Horwitz AL. Biochemical defect of non-keratan-sulfate-excreting Morquio syndrome. Am J Med Genet. 1983;15:265–273. doi: 10.1002/ajmg.1320150210. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Tomatsu S, Masuno M, et al. Mucopolysaccharidosis IVA: submicroscopic deletion of 16q24.3 and a novel R386C mutation of N-acetylgalactosamine-6-sulfate sulfatase gene in a classical Morquio disease. Hum Mutat. 1996;7:123–134. doi: 10.1002/(SICI)1098-1004(1996)7:2<123::AID-HUMU6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Fundación para el Estudio de las Enfermedades Neurometabólicas (2011) Collection of DBS Samples for Screening for Lysosomal Diseases. Video retreived from http://www.youtube.com/watch?v=dvrWgmiFrBA Accessed 5-24-2012
- Glossl J, Kresse H. A sensitive procedure for the diagnosis of N-acetyl-galactosamine-6-sulfate sulfatase deficiency in classical Morquio’s disease. Clin Chim Acta. 1978;88:111–119. doi: 10.1016/0009-8981(78)90157-2. [DOI] [PubMed] [Google Scholar]
- Glossl J, Kresse H. Impaired degradation of keratan sulphate by Morquio A fibroblasts. Biochem J. 1982;203:335–338. doi: 10.1042/bj2030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosele S, Dithmar S, Holz FG, Volcker HE. Late diagnosis of Morquio syndrome. Clinical histopathological findings in a rare mucopolysaccharidosis. Klin Monbl Augenheilkd. 2000;217:114–117. doi: 10.1055/s-2000-10394. [DOI] [PubMed] [Google Scholar]
- Gray G, Claridge P, Jenkinson L, Green A. Quantitation of urinary glycosaminoglycans using dimethylene blue as a screening technique for the diagnosis of mucopolysaccharidoses: an evaluation. Ann Clin Biochem. 2007;44:360–363. doi: 10.1258/000456307780945688. [DOI] [PubMed] [Google Scholar]
- Groebe H, Krins M, Schmidberger H, et al. Morquio syndrome (mucopolysaccharidosis IV B) associated with beta-galactosidase deficiency. Report of two cases. Am J Hum Genet. 1980;32:258–272. [PMC free article] [PubMed] [Google Scholar]
- Gucev ZS, Tasic V, Jancevska A, et al. Novel beta-galactosidase gene mutation p.W273R in a woman with mucopolysaccharidosis type IVB (Morquio B) and lack of response to in vitro chaperone treatment of her skin fibroblasts. Am J Med Genet A. 2008;146A:1736–1740. doi: 10.1002/ajmg.a.32318. [DOI] [PubMed] [Google Scholar]
- Harmatz P, Giugliani R, Schwartz I, et al. Enzyme replacement therapy for mucopolysaccharidosis VI: a phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. J Pediatr. 2006;148:533–539. doi: 10.1016/j.jpeds.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Hata R, Nagai Y. A rapid and micro method for separation of acidic glycosaminoglycans by two-dimensional electrophoresis. Anal Biochem. 1972;45:462–468. doi: 10.1016/0003-2697(72)90208-4. [DOI] [PubMed] [Google Scholar]
- Hendriksz CJ, Al-Jawad M, Berger KI et al (2012) Clinical overview and treatment options for non-skeletal manifestations of mucopolysaccharidosis type IVA. J Inherit Metab Dis. doi:10.1007/s10545-012-9459-0 [DOI] [PMC free article] [PubMed]
- Hintze JP, Tomatsu S, Fujii T, et al. Comparison of liquid chromatography-tandem mass spectrometry and sandwich ELISA for determination of keratan sulfate in plasma and urine. Biomark Insights. 2011;6:69–78. doi: 10.4137/BMI.S7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister DW, Cohen AH, Rimoin DL, Silberberg R. The Morquio syndrome (mucopolysaccharidosis IV): morphologic and biochemical studies. Johns Hopkins Med J. 1975;137:176–183. [PubMed] [Google Scholar]
- Holzgreve W, Grobe H, von Figura K, Kresse H, Beck H, Mattei JF. Morquio syndrome: clinical findings in 11 patients with MPS IVA and 2 patients with MPS IVB. Hum Genet. 1981;57:360–365. doi: 10.1007/BF00281685. [DOI] [PubMed] [Google Scholar]
- Hopwood JJ, Harrison JR. High-resolution electrophoresis of urinary glycosaminoglycans: an improved screening test for the mucopolysaccharidoses. Anal Biochem. 1982;119:120–127. doi: 10.1016/0003-2697(82)90674-1. [DOI] [PubMed] [Google Scholar]
- Humbel R, Chamoles NA. Sequential thin layer chromatography of urinary acidic glycosaminglycans. Clin Chim Acta. 1972;40:290–293. doi: 10.1016/0009-8981(72)90287-2. [DOI] [PubMed] [Google Scholar]
- Isenberg JN, Sharp HL. Aspartylglucosaminuria: psychomotor retardation masquerading as a mucopolysaccharidosis. J Pediatr. 1975;86:713–717. doi: 10.1016/S0022-3476(75)80355-6. [DOI] [PubMed] [Google Scholar]
- James A, Hendriksz CJ, Addison O. The oral health needs of children, adolescents and young adults affected by a mucopolysaccharide disorder. JIMD Reports. 2012;2:51–58. doi: 10.1007/8904_2011_46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq T, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis IVA. Clin Chem. 2011;57:128–131. doi: 10.1373/clinchem.2010.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijer WJ, Geilen GC, Garritsen V, et al. First-trimester diagnosis of Morquio disease type A. Prenat Diagn. 2000;20:183–185. doi: 10.1002/(SICI)1097-0223(200003)20:3<183::AID-PD774>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kohn G, Elrayyes ER, Makadmah I, Rosler A, Grunebaum M. Spondyloepiphyseal dysplasia tarda: a new autosomal recessive variant with mental retardation. J Med Genet. 1987;24:366–369. doi: 10.1136/jmg.24.6.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow D, Vriens J, Camacho N, et al. Mutations in the gene encoding the calcium-permeable ion channel TRPV4 produce spondylometaphyseal dysplasia, Kozlowski type and metatropic dysplasia. Am J Hum Genet. 2009;84:307–315. doi: 10.1016/j.ajhg.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R, Brown JR, Al-Mafraji K, Lamanna WC, Beitel JR, Boons GJ, Esko JD, Crawford BE. Disease-specific non-reducing end carbohydrate biomarkers for mucopolysaccharidoses. Nat Chem Biol. 2012;8:197–204. doi: 10.1038/nchembio.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman TJ, Miller N, Norquist B, Underhill L, Keutzer J. Diagnosis of the mucopolysaccharidoses. Rheumatology (Oxford) 2011;50(Suppl 5):v41–v48. doi: 10.1093/rheumatology/ker390. [DOI] [PubMed] [Google Scholar]
- Levin LS, Jorgenson RJ, Salinas CF. Oral findings in the Morquio syndrome (mucopolysaccharidosis IV) Oral Surg Oral Med Oral Pathol. 1975;39:390–395. doi: 10.1016/0030-4220(75)90082-1. [DOI] [PubMed] [Google Scholar]
- Longdon K, Pennock CA. Abnormal keratan sulphate excretion. Ann Clin Biochem. 1979;16:152–154. doi: 10.1177/000456327901600132. [DOI] [PubMed] [Google Scholar]
- Martell LA, Cunico RL, Ohh J, Fulkerson W, Furneaux R, Foehr ED. Validation of an LC-MS/MS assay for detecting relevant disaccharides from keratan sulfate as a biomarker for Morquio A syndrome. Bioanalysis. 2011;3:1855–1866. doi: 10.4155/bio.11.172. [DOI] [PubMed] [Google Scholar]
- Masuno M, Tomatsu S, Nakashima Y, et al. Mucopolysaccharidosis IV A: assignment of the human N-acetylgalactosamine-6-sulfate sulfatase (GALNS) gene to chromosome 16q24. Genomics. 1993;16:777–778. doi: 10.1006/geno.1993.1266. [DOI] [PubMed] [Google Scholar]
- Matalon R, Arbogast B, Justice P, Brandt IK, Dorfman A. Morquio’s syndrome: deficiency of a chondroitin sulfate N-acetylhexosamine sulfate sulfatase. Biochem Biophys Res Commun. 1974;61:759–765. doi: 10.1016/0006-291X(74)91022-5. [DOI] [PubMed] [Google Scholar]
- McKusick VA, Neufeld EF. The mucopolysaccharide storage diseases. In: Stanbury JB, Wyngaarden JB, Fredrickson DS, Goldstein JL, Brown MS, editors. The metabolic basis of inherited disease. New York: McGraw-Hill; 1983. pp. 751–777. [Google Scholar]
- Megarbane A, Maroteaux P, Caillaud C, Le MM. Spondyloepimetaphyseal dysplasia of Maroteaux (pseudo-Morquio type II syndrome): report of a new patient and review of the literature. Am J Med Genet A. 2004;125A:61–66. doi: 10.1002/ajmg.a.20442. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Matsuda T, Kitoh H, et al. A recurrent mutation in type II collagen gene causes Legg-Calve-Perthes disease in a Japanese family. Hum Genet. 2007;121:625–629. doi: 10.1007/s00439-007-0354-y. [DOI] [PubMed] [Google Scholar]
- Montano AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007;30:165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- Muenzer J, Wraith JE, Beck M, et al. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kurokawa T, Nagano A, Nakamura S, Taniguchi K, Hamazaki M. Dyggve-Melchior-Clausen syndrome without mental retardation (Smith-McCort dysplasia): morphological findings in the growth plate of the iliac crest. Am J Med Genet. 1997;72:11–17. doi: 10.1002/(SICI)1096-8628(19971003)72:1<11::AID-AJMG3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Tomatsu S, Hori T, et al. Mucopolysaccharidosis IV A: molecular cloning of the human N-acetylgalactosamine-6-sulfatase gene (GALNS) and analysis of the 5′-flanking region. Genomics. 1994;20:99–104. doi: 10.1006/geno.1994.1132. [DOI] [PubMed] [Google Scholar]
- Nelson J, Broadhead D, Mossman J. Clinical findings in 12 patients with MPS IV A (Morquio’s disease). Further evidence for heterogeneity. Part I: Clinical and biochemical findings. Clin Genet. 1988;33:111–120. doi: 10.1111/j.1399-0004.1988.tb03421.x. [DOI] [PubMed] [Google Scholar]
- Neufeld EF, McKusick VA. Disorders of lysosomal enzyme synthesis and localization: I-cell disease and psuedo-hurler polydystrophy. In: Stanbury JB, Wyngaarden JB, Fredrickson DS, Goldstein JL, Brown MS, editors. The metabolic basis of inheritied disease. New York: McGraw-Hill; 1983. pp. 778–787. [Google Scholar]
- Nishimura G, Dai J, Lausch E, et al. Spondylo-epiphyseal dysplasia, Maroteaux type (pseudo-Morquio syndrome type 2), and parastremmatic dysplasia are caused by TRPV4 mutations. Am J Med Genet A. 2010;152A:1443–1449. doi: 10.1002/ajmg.a.33414. [DOI] [PubMed] [Google Scholar]
- Northover H, Cowie RA, Wraith JE. Mucopolysaccharidosis type IVA (Morquio syndrome): a clinical review. J Inherit Metab Dis. 1996;19:357–365. doi: 10.1007/BF01799267. [DOI] [PubMed] [Google Scholar]
- Oguma T, Tomatsu S, Montaño AM, Okazaki O. Analytical method for determination of disaccharides derived from keratan, heparan and dermatan sulfates in human serum and plasma by high-performance liquid chromatography/turbo-ionspray ionization tandem mass spectrometry. Anal Biochem. 2007;368:79–86. doi: 10.1016/j.ab.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Ohashi A, Montaño AM, Colon JE, Oguma T, Luisiri A, Tomatsu S. Sacral dimple: incidental findings from newborn evaluation. Mucopolysaccharidosis IVA disease. Acta Paediatr. 2009;98:768–772. doi: 10.1111/j.1651-2227.2009.01134.x. [DOI] [PubMed] [Google Scholar]
- Pajares S, Alcalde C, Couce ML, et al. Molecular analysis of mucopolysaccharidosis IVA (Morquio A) in Spain. Mol Genet Metab. 2012;106:196–201. doi: 10.1016/j.ymgme.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Pennock CA. A review and selection of simple laboratory methods used for the study of glycosaminoglycan excretion and the diagnosis of the mucopolysaccharidoses. J Clin Pathol. 1976;29:111–123. doi: 10.1136/jcp.29.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piraud M, Boyer S, Mathieu M, Maire I. Diagnosis of mucopolysaccharidoses in a clinically selected population by urinary glycosaminoglycan analysis: a study of 2,000 urine samples. Clin Chim Acta. 1993;221:171–181. doi: 10.1016/0009-8981(93)90031-X. [DOI] [PubMed] [Google Scholar]
- Prat C, Lemaire O, Bret J, Zabraniecki L, Fournie B. Morquio syndrome: diagnosis in an adult. Joint Bone Spine. 2008;75:495–498. doi: 10.1016/j.jbspin.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Shohat M, Lachman R, Gruber HE, Rimoin DL. Brachyolmia: radiographic and genetic evidence of heterogeneity. Am J Med Genet. 1989;33:209–219. doi: 10.1002/ajmg.1320330214. [DOI] [PubMed] [Google Scholar]
- Singh J, Di FN, Niebes P, Tavella D. N-acetylgalactosamine-6-sulfate sulfatase in man. Absence of the enzyme in Morquio disease. J Clin Invest. 1976;57:1036–1040. doi: 10.1172/JCI108345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger J. International classification of osteochondrodysplasias. The International Working Group on Constitutional Diseases of Bone. Eur J Pediatr. 1992;151:407–415. doi: 10.1007/BF02072219. [DOI] [PubMed] [Google Scholar]
- Summers CG, Ashworth JL. Ocular manifestations as key features for diagnosing mucopolysaccharidoses. Rheumatology (Oxford) 2011;50(Suppl 5):v34–v40. doi: 10.1093/rheumatology/ker392. [DOI] [PubMed] [Google Scholar]
- Theroux MC, Nerker T, Ditro C, Mackenzie WG (2012) Anesthetic care and perioperative complications of children with Morquio syndrome. Paediatr Anaesth. doi:10.1111/j.1460-9592.2012.03904.x [DOI] [PubMed]
- Tomatsu S, Fukuda S, Masue M, et al. Morquio disease: isolation, characterization and expression of full-length cDNA for human N-acetylgalactosamine-6-sulfate sulfatase. Biochem Biophys Res Commun. 1991;181:677–683. doi: 10.1016/0006-291X(91)91244-7. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montano AM, Nishioka T, et al. Mutation and polymorphism spectrum of the GALNS gene in mucopolysaccharidosis IVA (Morquio A) Hum Mutat. 2005;26:500–512. doi: 10.1002/humu.20257. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Oguma T et al (2010) Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry. J Inherit Metab Dis. doi:10.1007/s10545-009-9013-x [DOI] [PubMed]
- Tomatsu S, Montano AM, Oikawa H, et al. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Curr Pharm Biotechnol. 2011;12:931–945. doi: 10.2174/138920111795542615. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Okamura K, Maeda H, et al. Keratan sulphate levels in mucopolysaccharidoses and mucolipidoses. J Inherit Metab Dis. 2005;28:187–202. doi: 10.1007/s10545-005-5673-3. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Okamura K, Taketani T, et al. Development and testing of new screening method for keratan sulfate in mucopolysaccharidosis IVA. Pediatr Res. 2004;55:592–597. doi: 10.1203/01.PDR.0000113767.60140.E9. [DOI] [PubMed] [Google Scholar]
- Tylki-Szymanska A, Czartoryska B, Bunge S, et al. Clinical, biochemical and molecular findings in a two-generation Morquio A family. Clin Genet. 1998;53:369–374. doi: 10.1111/j.1399-0004.1998.tb02747.x. [DOI] [PubMed] [Google Scholar]
- Ullrich K, Kresse H. Mucopolysaccharidoses. In: Blau N, Duran M, Blaskovics ME, editors. Physician’s guide to the laboratory diagnosis of metabolic diseases. London: Hodder Arnold Publishers; 1996. pp. 303–317. [Google Scholar]
- van Diggelen OP, Zhao H, Kleijer WJ, et al. A fluorimetric enzyme assay for the diagnosis of Morquio disease type A (MPS IV A) Clin Chim Acta. 1990;187:131–139. doi: 10.1016/0009-8981(90)90339-T. [DOI] [PubMed] [Google Scholar]
- Wessler E. Analytical and preparative separation of acidic glycosaminoglycans by electrophoresis in barium acetate. Anal Biochem. 1968;26:439–444. doi: 10.1016/0003-2697(68)90205-4. [DOI] [PubMed] [Google Scholar]
- Whitley CB, Draper KA, Dutton CM, Brown PA, Severson SL, France LA. Diagnostic test for mucopolysaccharidosis. II. Rapid quantification of glycosaminoglycan in urine samples collected on a paper matrix. Clin Chem. 1989;35:2074–2081. [PubMed] [Google Scholar]
- Whitley CB, Ridnour MD, Draper KA, Dutton CM, Neglia JP. Diagnostic test for mucopolysaccharidosis. I. Direct method for quantifying excessive urinary glycosaminoglycan excretion. Clin Chem. 1989;35:374–379. [PubMed] [Google Scholar]
- Whyte MP, Gottesman GS, Eddy MC, McAlister WH. X-linked recessive spondyloepiphyseal dysplasia tarda. Clinical and radiographic evolution in a 6-generation kindred and review of the literature. Medicine (Baltimore) 1999;78:9–25. doi: 10.1097/00005792-199901000-00002. [DOI] [PubMed] [Google Scholar]
- Wood T, Bodamer OA, Burin MG, et al. Expert recommendations for the laboratory diagnosis of MPS VI. Mol Genet Metab. 2012;106:73–82. doi: 10.1016/j.ymgme.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Wraith JE. The mucopolysaccharidoses: a clinical review and guide to management. Arch Dis Child. 1995;72:263–267. doi: 10.1136/adc.72.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraith JE, Clarke LA, Beck M, et al. Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human alpha-L-iduronidase (laronidase) J Pediatr. 2004;144:581–588. doi: 10.1016/j.jpeds.2004.01.046. [DOI] [PubMed] [Google Scholar]
- Yutaka T, Okada S, Kato T, Inui K, Yabuuhi H. Galactose 6-sulfate sulfatase activity in Morquio syndrome. Clin Chim Acta. 1982;122:169–180. doi: 10.1016/0009-8981(82)90276-5. [DOI] [PubMed] [Google Scholar]
- Zhao H, van Diggelen OP, Thoomes R, et al. Prenatal diagnosis of Morquio disease type A using a simple fluorometric enzyme assay. Prenat Diagn. 1990;10:85–91. doi: 10.1002/pd.1970100204. [DOI] [PubMed] [Google Scholar]