Abstract
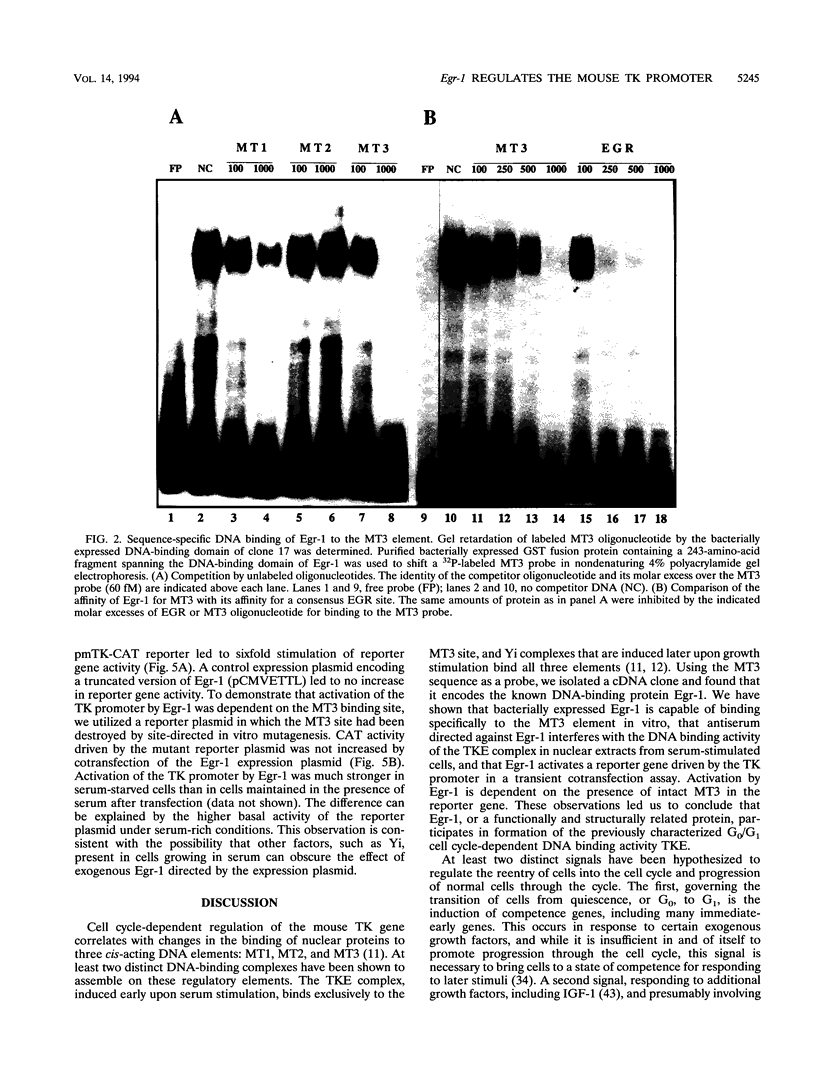
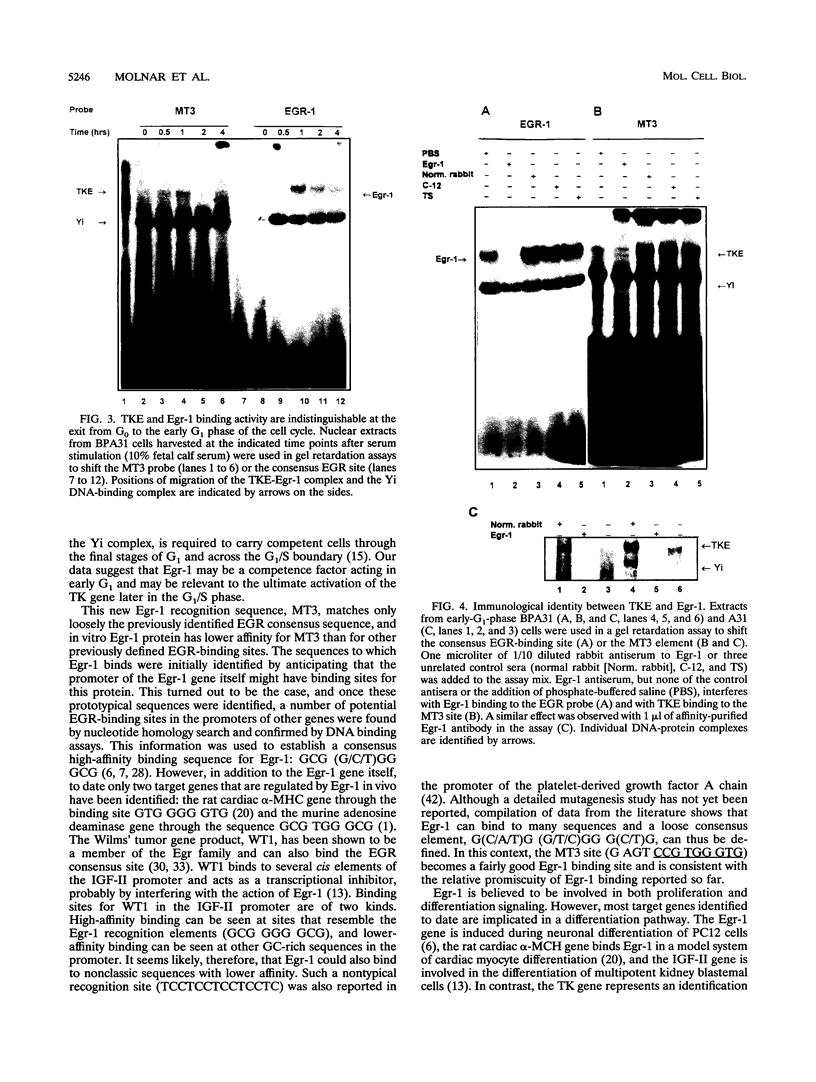
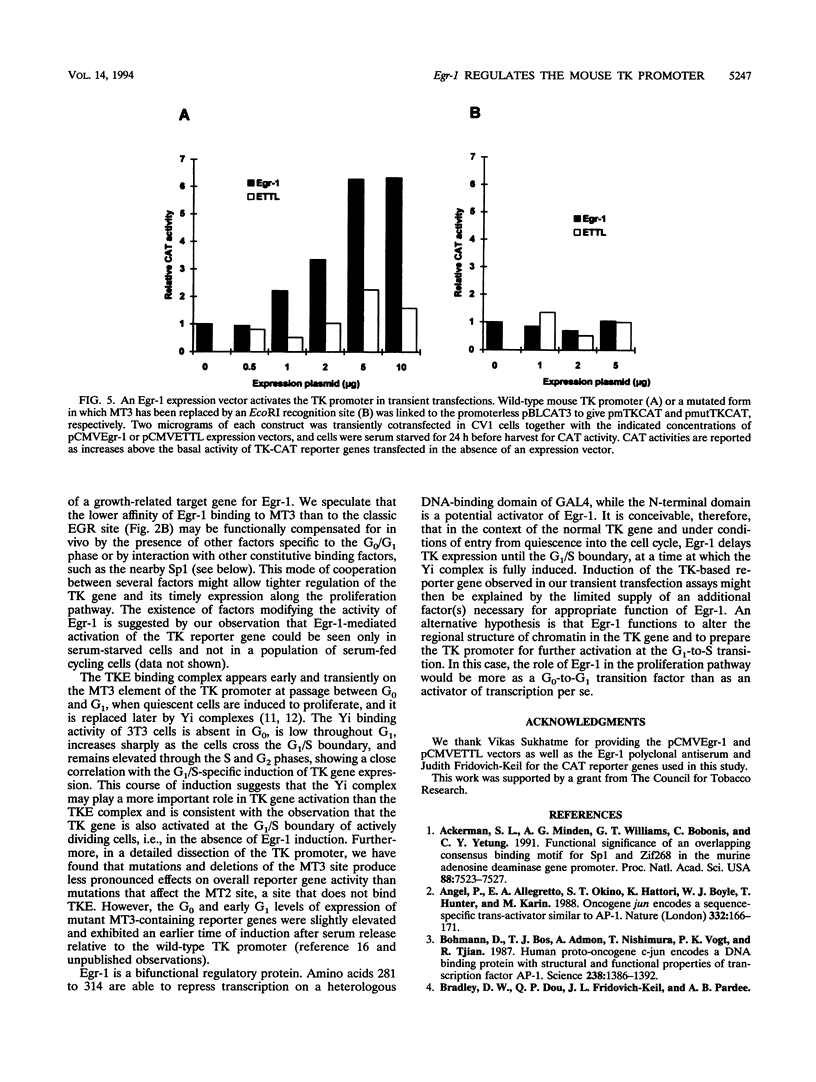
Production of thymidine kinase (TK) protein parallels the onset of DNA synthesis during the cell cycle. This process is regulated at transcriptional, posttranscriptional, and translational levels to cause a 40- to 50-fold increase in cytosolic enzymatic activity as cells progress from G1 to S phase. Transcriptional activation of the mouse TK gene through the cell cycle is dependent upon previously characterized cis elements of the proximal promoter, called MT1, MT2, and MT3, that bind at least two different complexes: TKE during the transition of cells from quiescence (G0) to G1, and Yi later at the G1/S boundary. To identify the transcription factors involved in this regulation, we screened a mouse fibroblast cDNA expression library with a labeled MT3 oligonucleotide probe and isolated a clone that encodes Egr-1, an immediate-early transcription factor, whose expression is regulated by serum or growth factors during the G0-to-G1 transition when cells reenter the cell cycle. Electrophoretic mobility shift assays demonstrate that Egr-1 is involved in the TKE complex that binds to the MT3 element and that expression of Egr-1 induces transcription of a mouse TK-chloramphenicol acetyltransferase reporter in transient transfections. These results suggest a role for Egr-1 in regulating expression of the TK gene at the G0-to-G1 transition.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. L., Minden A. G., Williams G. T., Bobonis C., Yeung C. Y. Functional significance of an overlapping consensus binding motif for Sp1 and Zif268 in the murine adenosine deaminase gene promoter. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7523–7527. doi: 10.1073/pnas.88.17.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Allegretto E. A., Okino S. T., Hattori K., Boyle W. J., Hunter T., Karin M. Oncogene jun encodes a sequence-specific trans-activator similar to AP-1. Nature. 1988 Mar 10;332(6160):166–171. doi: 10.1038/332166a0. [DOI] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Cao X. M., Koski R. A., Gashler A., McKiernan M., Morris C. F., Gaffney R., Hay R. V., Sukhatme V. P. Identification and characterization of the Egr-1 gene product, a DNA-binding zinc finger protein induced by differentiation and growth signals. Mol Cell Biol. 1990 May;10(5):1931–1939. doi: 10.1128/mcb.10.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B. A., Lau L. F., Nathans D. A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with "zinc finger" sequences. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7857–7861. doi: 10.1073/pnas.85.21.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B., Nathans D. DNA binding site of the growth factor-inducible protein Zif268. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8737–8741. doi: 10.1073/pnas.86.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E., Koeller D. M., Casey J. L., Drake J. C., Chabner B. A., Elwood P. C., Zinn S., Allegra C. J. Autoregulation of human thymidylate synthase messenger RNA translation by thymidylate synthase. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8977–8981. doi: 10.1073/pnas.88.20.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppock D. L., Pardee A. B. Control of thymidine kinase mRNA during the cell cycle. Mol Cell Biol. 1987 Aug;7(8):2925–2932. doi: 10.1128/mcb.7.8.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Q. P., Fridovich-Keil J. L., Pardee A. B. Inducible proteins binding to the murine thymidine kinase promoter in late G1/S phase. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1157–1161. doi: 10.1073/pnas.88.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Q. P., Markell P. J., Pardee A. B. Thymidine kinase transcription is regulated at G1/S phase by a complex that contains retinoblastoma-like protein and a cdc2 kinase. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3256–3260. doi: 10.1073/pnas.89.8.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond I. A., Madden S. L., Rohwer-Nutter P., Bell G. I., Sukhatme V. P., Rauscher F. J., 3rd Repression of the insulin-like growth factor II gene by the Wilms tumor suppressor WT1. Science. 1992 Jul 31;257(5070):674–678. doi: 10.1126/science.1323141. [DOI] [PubMed] [Google Scholar]
- Fridovich-Keil J. L., Gudas J. M., Bryan I. B., Pardee A. B. Improved expression vectors for eukaryotic promoter/enhancer studies. Biotechniques. 1991 Nov;11(5):572–579. [PubMed] [Google Scholar]
- Fridovich-Keil J. L., Gudas J. M., Dou Q. P., Bouvard I., Pardee A. B. Growth-responsive expression from the murine thymidine kinase promoter: genetic analysis of DNA sequences. Cell Growth Differ. 1991 Feb;2(2):67–76. [PubMed] [Google Scholar]
- Fridovich-Keil J. L., Markell P. J., Gudas J. M., Pardee A. B. DNA sequences required for serum-responsive regulation of expression from the mouse thymidine kinase promoter. Cell Growth Differ. 1993 Aug;4(8):679–687. [PubMed] [Google Scholar]
- Gashler A. L., Swaminathan S., Sukhatme V. P. A novel repression module, an extensive activation domain, and a bipartite nuclear localization signal defined in the immediate-early transcription factor Egr-1. Mol Cell Biol. 1993 Aug;13(8):4556–4571. doi: 10.1128/mcb.13.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M. K., Merrill G. F. Thymidine kinase synthesis is repressed in nonreplicating muscle cells by a translational mechanism that does not affect the polysomal distribution of thymidine kinase mRNA. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4987–4991. doi: 10.1073/pnas.86.13.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas J. M., Knight G. B., Pardee A. B. Nuclear posttranscriptional processing of thymidine kinase mRNA at the onset of DNA synthesis. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4705–4709. doi: 10.1073/pnas.85.13.4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M. P., Gupta M., Zak R., Sukhatme V. P. Egr-1, a serum-inducible zinc finger protein, regulates transcription of the rat cardiac alpha-myosin heavy chain gene. J Biol Chem. 1991 Jul 15;266(20):12813–12816. [PubMed] [Google Scholar]
- Ito M., Conrad S. E. Independent regulation of thymidine kinase mRNA and enzyme levels in serum-stimulated cells. J Biol Chem. 1990 Apr 25;265(12):6954–6960. [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Kauffman M. G., Kelly T. J. Cell cycle regulation of thymidine kinase: residues near the carboxyl terminus are essential for the specific degradation of the enzyme at mitosis. Mol Cell Biol. 1991 May;11(5):2538–2546. doi: 10.1128/mcb.11.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- Lemaire P., Revelant O., Bravo R., Charnay P. Two mouse genes encoding potential transcription factors with identical DNA-binding domains are activated by growth factors in cultured cells. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4691–4695. doi: 10.1073/pnas.85.13.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire P., Vesque C., Schmitt J., Stunnenberg H., Frank R., Charnay P. The serum-inducible mouse gene Krox-24 encodes a sequence-specific transcriptional activator. Mol Cell Biol. 1990 Jul;10(7):3456–3467. doi: 10.1128/mcb.10.7.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden S. L., Cook D. M., Morris J. F., Gashler A., Sukhatme V. P., Rauscher F. J., 3rd Transcriptional repression mediated by the WT1 Wilms tumor gene product. Science. 1991 Sep 27;253(5027):1550–1553. doi: 10.1126/science.1654597. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Morris J. F., Tournay O. E., Cook D. M., Curran T. Binding of the Wilms' tumor locus zinc finger protein to the EGR-1 consensus sequence. Science. 1990 Nov 30;250(4985):1259–1262. doi: 10.1126/science.2244209. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Stiles C. D. Serum-inducible genes. Adv Cancer Res. 1989;53:1–32. doi: 10.1016/s0065-230x(08)60277-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stuart P., Ito M., Stewart C., Conrad S. E. Induction of cellular thymidine kinase occurs at the mRNA level. Mol Cell Biol. 1985 Jun;5(6):1490–1497. doi: 10.1128/mcb.5.6.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhatme V. P., Cao X. M., Chang L. C., Tsai-Morris C. H., Stamenkovich D., Ferreira P. C., Cohen D. R., Edwards S. A., Shows T. B., Curran T. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988 Apr 8;53(1):37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Sukhatme V. P. Early transcriptional events in cell growth: the Egr family. J Am Soc Nephrol. 1990 Dec;1(6):859–866. doi: 10.1681/ASN.V16859. [DOI] [PubMed] [Google Scholar]
- Wang Z. Y., Deuel T. F. An S1 nuclease-sensitive homopurine/homopyrimidine domain in the PDGF A-chain promoter contains a novel binding site for the growth factor-inducible protein EGR-1. Biochem Biophys Res Commun. 1992 Oct 15;188(1):433–439. doi: 10.1016/0006-291x(92)92403-k. [DOI] [PubMed] [Google Scholar]
- Yang H. C., Pardee A. B. Insulin-like growth factor I regulation of transcription and replicating enzyme induction necessary for DNA synthesis. J Cell Physiol. 1986 Jun;127(3):410–416. doi: 10.1002/jcp.1041270309. [DOI] [PubMed] [Google Scholar]