Summary
Background
Membranous nephropathy leads to end-stage renal disease in more than 20% of patients. Although immunosuppressive therapy benefits some patients, trial evidence for the subset of patients with declining renal function is not available. We aimed to assess whether immunosuppression preserves renal function in patients with idiopathic membranous nephropathy with declining renal function.
Methods
This randomised controlled trial was undertaken in 37 renal units across the UK. We recruited patients (18–75 years) with biopsy-proven idiopathic membranous nephropathy, a plasma creatinine concentration of less than 300 μmol/L, and at least a 20% decline in excretory renal function measured in the 2 years before study entry, based on at least three measurements over a period of 3 months or longer. Patients were randomly assigned (1:1:1) by a random number table to receive supportive treatment only, supportive treatment plus 6 months of alternating cycles of prednisolone and chlorambucil, or supportive treatment plus 12 months of ciclosporin. The primary outcome was a further 20% decline in renal function from baseline, analysed by intention to treat. The trial is registered as an International Standard Randomised Controlled Trial, number 99959692.
Findings
We randomly assigned 108 patients, 33 of whom received prednisolone and chlorambucil, 37 ciclosporin, and 38 supportive therapy alone. Two patients (one who received ciclosporin and one who received supportive therapy) were ineligible, so were not included in the intention-to-treat analysis, and 45 patients deviated from protocol before study end, mostly as a result of minor dose adjustments. Follow up was until primary endpoint or for minimum of 3 years if primary endpoint was not reached. Risk of further 20% decline in renal function was significantly lower in the prednisolone and chlorambucil group than in the supportive care group (19 [58%] of 33 patients reached endpoint vs 31 [84%] of 37, hazard ratio [HR] 0·44 [95% CI 0·24–0·78]; p=0·0042); risk did not differ between the ciclosporin (29 [81%] of 36) and supportive treatment only groups (HR 1·17 [0·70–1·95]; p=0·54), but did differ significantly across all three groups (p=0·003). Serious adverse events were frequent in all three groups but were higher in the prednisolone and chlorambucil group than in the supportive care only group (56 events vs 24 events; p=0·048).
Interpretation
For the subset of patients with idiopathic membranous nephropathy and deteriorating excretory renal function, 6 months' therapy with prednisolone and chlorambucil is the treatment approach best supported by our evidence. Ciclosporin should be avoided in this subset.
Funding
Medical Research Council, Novartis, Renal Association, Kidney Research UK.
Introduction
Membranous nephropathy is the most common cause of primary nephrotic syndrome in adults, and according to figures from the Netherlands,1 30–50 people per million population develop the disorder every 5 years (6–10 per million population per year). Membranous nephropathy results in substantial morbidity and is an important cause of end-stage renal disease, which accounted for expenditure of US$40 billion in the USA in 2008.2 Optimum treatment for membranous nephropathy is controversial despite several controlled trials having assessed available treatments, not least because the disorder has a variable natural history and many severely affected individuals can undergo spontaneous remission. Only a subset of patients (25–30% in most series) develops progressive loss of kidney function, and since available therapies have substantial adverse effects, some believe that aggressive therapy should be reserved for this subgroup.3,4 However, no large prospective randomised controlled trials (RCTs) in this worst-affected subset exist.
Studies of the natural history of membranous nephropathy show that once excretory renal function starts to decline, continued deterioration can be expected,5 which suggests that the really important clinical question in membranous nephropathy is whether treatments that are effective in less severely affected patients are also beneficial in patients showing definite signs of decline in renal function.6,7 Thus, findings from prospective RCTs are essential to inform decisions about treatment of this disorder.
Idiopathic membranous nephropathy is often managed with immunosuppressive drugs. Until recently, evidence that this nephropathy is autoimmune in origin was circumstantial.8 However, autoantibodies to the phospholipase A2 receptor (PLA2R1) have now been noted in most affected individuals,9 and a predisposition to the disorder has been very strongly linked to two genetic regions (one in the MHC and the other in the PLA2R1 gene itself10)—findings that support an immunological pathogenesis and provide a rationale for immunosuppressive therapy, especially treatment targeted at B lymphocytes.
When our study was designed, combined treatment with prednisolone and chlorambucil11 and single-agent therapy with ciclosporin were supported by RCT evidence.12 We did a questionnaire survey as part of our preliminary research and noted that nephrologists were uncertain about the relative risks of intervention with immunosuppressive therapy compared with supportive therapy alone. Therefore, we aimed to test the hypothesis that immunosuppressive therapy, either with prednisolone and chlorambucil or with ciclosporin, preserves renal function in patients with idiopathic membranous nephropathy with declining renal function compared with supportive therapy alone.
Methods
Trial design and participants
In this randomised controlled trial, patients were recruited from 37 of 45 renal units in acute hospitals throughout the UK that obtained local ethical approval. Inclusion criteria were: age 18–75 years; biopsy-proven diagnosis of membranous nephropathy (we did not impose a limit on the time since biopsy), regarded as idiopathic with no evidence of an underlying cause (such as drugs, infections, or tumours); and serum or plasma creatinine concentration of less than 300 μmol/L together with a 20% or greater decline in excretory renal function (measured by creatinine clearance or estimated with the Cockcroft-Gault calculation, and later by the Modification of Diet in Renal Disease [MDRD] formula13) that was based on at least three measurements over a period of 3 months or longer within the 2 years before study entry.
We excluded patients whose membranous nephropathy was a result of secondary causes (defined according to usual clinical practice). Other exclusion criteria were: known infection with hepatitis B or C virus or HIV; known malignant disease; positive antibodies to double-stranded DNA; current treatment with gold, penicillamine, non-steroidal anti-inflammatory drugs, cytotoxic drugs, or ciclosporin; more than 3 months' treatment with corticosteroids in the preceding 2 years; pregnancy or unreliable contraception; or a previous adverse reaction to prednisolone, methylprednisolone, chlorambucil or ciclosporin.
Ethics approval was obtained from the South West Multicentre Research Ethics Committee (reference MREC/97/6/12). Each participating centre also obtained local ethical approval. All patients gave written informed consent.
Randomisation
Eligible patients were randomly assigned by a member of staff in the clinical trials office at the Glasgow Royal Infirmary, Glasgow, UK, who was not otherwise involved in the trial. A random numbers table had been prepared to allocate patients to one of three groups: supportive therapy alone, supportive therapy plus 6 months of prednisolone and chlorambucil, or supportive therapy plus 12 months of ciclosporin. Treatment allocation was communicated by fax to the clinician entering the patient into the trial. We did not attempt to mask patients or investigators.
Procedures
We recorded baseline data for the supportive treatment alone group at randomisation, because these patients were effectively continuing their existing management. In the two groups receiving immunosuppressive treatment in addition to supportive therapy, baseline data were recorded when the new treatment began. We could not always start immunosuppressive treatment immediately after randomisation because the new treatments had to be prescribed and delivered.
The treatment schedules were based on best available evidence at the time. All patients received supportive therapy, including renin-angiotensin blockade, statins, and anticoagulants as indicated. Those assigned to supportive therapy plus 6 months' prednisolone and chorambucil11 received intravenous methyl prednisolone 1 g per day for 3 consecutive days then oral prednisolone 0·5 mg/kg per day for 28 days during months 1, 3, and 5. Intravenous prednisolone was administered in hospital. During months 2, 4, and 6, patients received oral chlorambucil at a starting dose of 0·15 mg/kg per day. We gave this reduced dose because the parent drug and its metabolites are renally excreted and our preliminary work14 had shown that a dose of 0·2 mg/kg per day was poorly tolerated in patients with impaired excretory renal function. We reduced the dose further if the patient developed leucopenia (weekly full blood counts were advised) and interrupted it if leucopenia was severe.
Those assigned to supportive therapy plus 12 months' of ciclosporin received a starting dose of 5 mg/kg per day,12 adjusted according to trough blood concentrations of the drug to achieve a concentration of 100–200 μg/L. We reduced the dose if toxicity was evident.
We followed up patients until they met the primary endpoint, or for a minimum of 3 years if they did not do so. The trial was not formally analysed until 3 years after all patients had begun treatment. All surviving trial patients remain under routine follow-up at their renal units.
The primary endpoint was a further 20% decline in excretory renal function from baseline readings, calculated in all patients with the Cockcroft-Gault equation (standard methodology at the start of the trial).
Secondary endpoints were proteinuria (measured with 24-h urinary collections or estimated from protein–creatinine ratios by multiplying the ratio [in mg/mmol] by 10) and severe adverse events. The primary investigator (PWM) identified which adverse events were serious and categorised them according to the most affected body system. We report all serious adverse events as defined by the Medicines and Healthcare products Regulatory Agency (MHRA) guidance15—(ie, any adverse event, adverse reaction, or unexpected adverse reaction that results in death, is life-threatening, results in admission to hospital or extends the length of an existing hospital stay, results in persistent or serious disability or incapacity, or consists of a congenital anomaly or birth defect). We also regarded as serious other important medical events that might have jeopardised the patient or needed intervention to prevent one of these outcomes. We recorded information about deaths and development of end-stage renal disease.
In accordance with the Medical Research Council's guidelines for good clinical practice, a trial steering committee and a data monitoring committee were established to receive yearly reports for primary endpoints, adverse events, and deaths.
Statistical analysis
To have 90% power to detect a reduction in frequency of the primary endpoint from 80% in the supportive treatment group to 40% in the immunosuppression groups with p<0·05, we calculated that 35 patients would be needed in each group (105 in total). After allowing for an estimated dropout rate of 10%, we concluded that we needed to recruit 116 patients.
Primary analysis followed the principles of intention to treat, and secondary analysis assessed all patients who received at least one dose of treatment. Unless otherwise stated, p values and estimates of treatment effects are based on two-way comparisons. We did not make adjustments for multiple comparisons. We analysed time to further 20% decline in renal function by the log-rank test and calculated hazard ratios [HRs] with Cox proportional hazards regression. We used the log-rank test for other survival endpoints, and repeated measures analysis of variance fowr continuous longitudinal data (eg, proteinuria). We analysed serious adverse event data with the log-rank test on the basis of time to first serious adverse event. We did statistical analyses using SAS software (version 9.2).
In 2003, the trial was shown to comply with the requirements of the EU clinical trials directive, and in 2004, a clinical trial authorisation (CTA) was obtained from the Medicines and Healthcare products Regulatory Authority (CTA number 18524/0001/001). In 2008, the trial was adopted onto the National Institute for Health Research portfolio and assigned the UK Clinical Research Network identification number 2579. The trial is registered as an International Standard Randomised Controlled Trial, number 99959692.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility to submit for publication.
Results
We randomly assigned 108 patients between April 1, 1998, and March 31, 2008, at a steady rate of about one patient per month (figure 1). We discovered that two patients were ineligible after randomisation, so no follow-up data are available for them, and they weren't included in the primary analysis. Of the 106 patients included in the intention-to-treat analysis, 37 were assigned to receive supportive therapy alone, 33 to prednisolone and chlorambucil, and 36 to ciclosporin. 37 of the 45 centres that obtained ethics approval entered patients into the trial.
Figure 1.
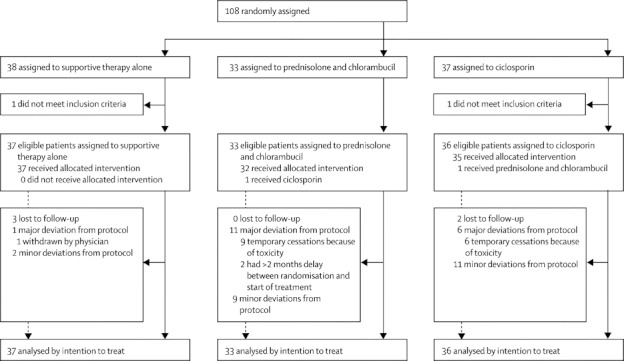
Trial profile
The groups had similar baseline measurements (table 1). Times between randomisation and baseline readings are longer in both immunosuppressive groups than in the supportive therapy alone group because of delays relating to prescription, delivery, and administration of treatment.
Table 1.
Baseline values
| Prednisolone and chlorambucil (n=33) | Ciclosporin (n=36) | Supportive therapy only (n=37) | ||
|---|---|---|---|---|
| Creatinine clearance (mL/min) | 50 (16) | 49 (18) | 50 (20) | |
| Systolic blood pressure (mm Hg) | 141 (16) | 143 (21) | 138 (19) | |
| Proteinuria (g per 24 h) | 10·1 (5·3) | 6·8 (4·7) | 9·1 (5·3) | |
| Age (years) | 58 (12) | 58 (11) | 56 (16) | |
| Time of baseline reading* | ||||
| <2 weeks before randomisation | 0 | 0 | 3 (8%) | |
| >2 weeks after randomisation | 11 (33%) | 11 (31%) | 1 (3%) | |
Data are mean (SD) or number (%).
Baseline readings were taken at start of study treatment in the immunosuppressive treatment groups.
We classed deviations from the defined protocol, including starting dose of intervention drugs, as either minor (eg, dose reductions because of toxicity) or major (eg, cessation of treatment, including temporary interruptions, or administration of the wrong treatment). We did not classify a delay between randomisation and start of treatment as a default protocol deviation. However, two patients had excessive delays (2 and 3 months, respectively), and we classified both as major deviations.
Angiotensin-converting enzyme inhibitor was given to 27 (82%) of 33 in the prednisolone and chlorambucil group, 36 (100%) of 36 patients in the ciclosporin group, and 34 (92%) of 37 of those who received supportive treatment only. We did not obtain information about angiotensin-receptor antagonist use.
The rate of occurrence of a further 20% decline in excretory renal function from baseline was fastest in the ciclosporin group and slowest in the prednisolone and chlorambucil group (figure 2). Risk of a further 20% decline in renal function was significantly lower in the prednisolone and chlorambucil group than in the supportive therapy group (19 [58%] of 33 patients vs 31 [84%] of 37 patients, HR 0·44 [95% CI 0·24–0·78]; p=0·0042), with no significant difference noted between the ciclosporin group (29 [81%] of 36 patients) and supportive care group (HR 1·17 [0·70–1·95]; p=0·54). The difference in the proportion of patients who reached the primary endpoint across all three groups was significant (p=0·003 for the three-way comparison; figure 2).
Figure 2.
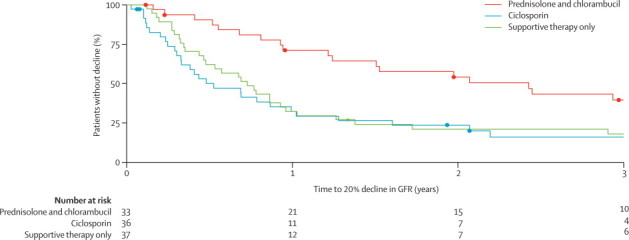
Kaplan-Meier analysis of further 20% decline in renal function
Deaths were censored. GFR=glomerular filtration rate.
Survival analysis showed no significant differences between groups: two (6·1%) of 33 patients in the prednisolone plus chlorambucil group died during the trial follow-up compared with two (5·6%) of 36 patients in the ciclosporin group and one (2·7%) of 37 in the supportive therapy group. We did not classify any deaths as likely to be related to trial treatments. Of the five deaths, two were due to myocardial infarction (one each in the prednisolone plus chlorambucil and ciclosporin groups), one to septicaemia (in the supportive treatment only group), and two to unknown causes.
Malignant disease was reported in two (6·1%) of 33 patients in the prednisolone plus chlorambucil group during follow-up: one had squamous-cell carcinoma of the skin and one had adenocarcinoma of the sigmoid colon. 11 patients reached end-stage renal disease: one (3·0%) of 33 in the prednisolone plus chlorambucil group compared with six (16·7%) of 36 in the ciclosporin group and four (10·8%) of 37 in the supportive therapy group.
The fall in proteinuria with time was greatest in the prednisolone plus chlorambucil group (figure 3). The difference in the mean reduction of protein in the urine for prednisolone and chlorambucil versus supportive therapy alone was −2·2 g in 24 h (p=0·014). The difference in the mean reduction for ciclosporin versus supportive care alone was −0·7 g in 24 h (p=0·46).
Figure 3.
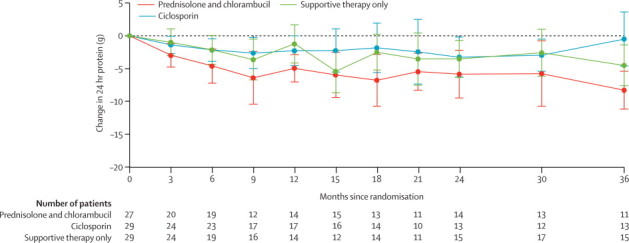
Change in measured or calculated 24-h urinary protein
Datapoints show mean change in 24-h urinary protein from baseline; error bars show 95% CI. The numbers of patients from whom readings were taken at each timepoint are presented; variation in numbers was due to dropout and missing readings at those timepoints.
We recorded 390 adverse events, of which 117 were deemed serious by PWM. These 117 events occurred in 54 patients (table 2). The number of patients with a serious adverse event by 1 year did not differ significantly between the ciclosporin and supportive care groups (17 [46%] of 37 patients in the ciclosporin group vs 11 [29%] of 38 in the supportive therapy only group; p=0·20), but the number of patients in the prednisolone and chlorambucil group with a serious adverse event by 1 year (17 [52%] of 33 patients) was significantly higher than in the supportive care group (p=0·048).
Table 2.
Serious adverse events by treatment and body system affected
| Prednisolone and chlorambucil | Ciclosporin | Supportive therapy only | ||
|---|---|---|---|---|
| Patients with at least one SAE* | 20/33 (61%) | 18/37 (49%) | 16/38 (42%) | |
| Number of SAEs† | 56/117 (48%) | 37/117 (32%) | 24/117 (21%) | |
| Likelihood to be related to treatmentठ| ||||
| None/unlikely | 20/56 (36%) | 18/37 (49%) | 24/24 (100%) | |
| Possible | 10/56 (18%) | 6/37 (16%) | 0 | |
| Likely | 26/56 (46%) | 13/37 (35%) | 0 | |
| Body system affected | ||||
| Haematological | 28 | 5 | 3 | |
| Dermatological | 4 | 2 | 0 | |
| Renal | 1 | 5 | 2 | |
| Neurological | 3 | 6 | 4 | |
| Cardiovascular | 4 | 3 | 3 | |
| Metabolic | 8 | 1 | 5 | |
| Gastroenterological | 3 | 3 | 2 | |
| Infection | 3 | 8 | 2 | |
| Other/not specified | 2 | 4 | 3 | |
Data are number, or number (%). SAE=serious adverse event.
Out of number of patients assigned to each treatment group; includes patients removed from the intention-to-treat analysis because they were deemed ineligible after randomisation.
Out of number of SAEs overall.
Out of number of SAEs in each treatment group.
Likelihood was assessed by PWM.
Haematological events were leucopenia, anaemia, and thrombocytopenia, although we did not note any lymphomas or leukaemias. Dermatological effects included rash and shingles. Renal effects were mainly deterioration of excretory renal function or hyperkalaemia. Neurological effects were tremor and headache. Cardiovascular effects were hypertension, myocardial infarction, chest pain, and pulmonary embolus. Metabolic effects included impairment of glucose tolerance. Gastroenterological effects included nausea, vomiting, diarrhoea, bleeding, and pancreatitis. Infection included septicaemia, pneumonia, and cellulitis. Other effects included cataract, fractured humerus, hernia repairs, and pulmonary sarcoid. We acknowledge that some potential adverse effects of prednisolone plus chlorambucil and ciclosporin are more longlasting than the effects reported with supportive care alone, especially the risk of lymphoma or other malignant diseases.
Discussion
These results suggest that supportive therapy plus prednisolone and chlorambucil is better at prevention of decline in excretory renal function than is supportive therapy plus ciclosporin or supportive therapy alone in patients with deteriorating function due to idiopathic membranous nephropathy. Ideally the results should be confirmed in a larger study, but because this trial took 10 years to recruit due to the difficulty of running a multicentre trial in slowly progressive glomerular disease, a similar larger study is unlikely to be done. 92% of all patients in this trial received angiotensin-converting enzyme inhibitors, showing that this aspect of supportive therapy was virtually universal in all three groups. The benefit to renal function was matched by a reduction in proteinuria with prednisolone and chlorambucil. In this severely affected subset of patients, we thought it would be unlikely that even responders would achieve complete remission of proteinuria, hence our decision to make excretory renal function our primary endpoint.
Adverse events were frequent, including in the supportive therapy group, suggesting that this group of patients is susceptible to major medical problems. Masking was impossible because of the nature of the treatments involved, but we accept that the fact that the study was unblinded might have affected the reporting of adverse effects. In the active intervention groups, the serious adverse effects were predictable from the side-effect profiles of the drugs—particularly headache, tremor, hypertension, deterioration in renal function, or infection in the ciclosporin group and predominantly haematological (especially leucopenia and anaemia) or metabolic (especially glucose intolerance) effects in the prednisolone and chlorambucil group. Dose adjustment of chlorambucil arose frequently and treatment interruptions were common, both accounting for most of the protocol departures in the prednisolone and chlorambucil group. Similarly, in the ciclosporin group, nephrotoxicity was a substantial problem despite the fact that we used target blood concentrations advocated in a previous RCT;12 dose reductions, treatment interruptions, and reaching of primary endpoint were frequent in this group.
Slow trial recruitment was undoubtedly determined by the eligibility criteria: deteriorating renal function in idiopathic membranous nephropathy has become less common with angiotensin-cascade blockade and other aspects of supportive management,16 and our detailed communications with renal units throughout the UK during the trial showed that the rarity of eligible patients was the main barrier to recruitment. Of the 37 units that entered patients, 31 recruited four patients or fewer. Renal units that declined to participate and apply for ethical approval were roughly equally divided into those concerned about the lack of active therapy in the supportive care only group or the potential toxicity of active therapy—clear evidence that considerable uncertainty surrounds appropriate management of this disorder, and that a definitive RCT is needed.
We contend that for patients with idiopathic membranous nephropathy, 6 months' therapy with alternating monthly cycles of prednisolone and chlorambucil is the treatment approach best supported by evidence, and our study extends this evidence to the important subset of patients with membranous nephropathy and deteriorating excretory renal function. Our findings do not support the use of ciclosporin in this group—the adverse effects on renal function make it unsuitable once renal function has started to decline. The ciclosporin starting dose in our study was based on an RCT reported by Cattran and colleagues.12 A later trial led by this group17 used a lower starting dose (3·5 mg/kg per day) but aimed for similar plasma concentrations and reported beneficial effects on proteinuria. Other investigators have used a lower dose of ciclosporin in small uncontrolled studies and also reported beneficial effects for proteinuria.18,19
Shortly after our study started, Ponticelli and colleagues20 reported that the choice of alkylating agent (between chlorambucil and the more familiar cyclophosphamide) for idiopathic membranous nephropathy might be immaterial, although cyclophosphamide might be less toxic. We decided not to change our study design, but we agree that cyclophosphamide could probably be substituted for chlorambucil. Uncontrolled studies using a combination of prednisolone and cyclophosphamide in severely affected patients have led to similar conclusions,1,21 and one RCT supports this approach in patients with well preserved renal function at entry.22 Our study shows that this form of therapy can still be effective in patients whose renal function has started to deteriorate. The use of alkylating agents (with their potent effects on B lymphocytes) for idiopathic membranous nephropathy has a rationale now that autoantibodies to PLA2R1 have been discovered in most patients.8,9 Immunosuppressive therapy is also logical in view of our previously published analysis of the genetic basis of idiopathic membranous nephropathy,10 showing that two genes predispose white people to this disorder, an immune-response gene in the HLA-DQA1 region and the PLA2R1 gene itself.
Rituximab for idiopathic membranous nephropathy has shown promise,23,24 but so far evidence for its effectiveness is not based on RCT data and the beneficial effects are mostly in reduction of proteinuria rather than preservation of excretory renal function. Furthermore, rituximab is expensive and is associated with important long-term safety concerns such as progressive multifocal leukoencephalopathy.25 However, progressive multifocal leukoencephalopathy has not been reported in patients with idiopathic membranous nephropathy given rituximab and might be associated with intensity of immunosuppression, since patients who got the disease received rituximab together with other agents. Future RCTs should assess the cost-effectiveness, efficacy, and safety of rituximab for patients with idiopathic membranous nephropathy, perhaps compared with prednisolone plus an alkylating agent (either cyclophosphamide or chlorambucil). Future trials should ideally include patient-reported outcomes and an analysis of the balance between treatment costs (including those associated with adverse effects) and costs of renal replacement therapy in untreated patients, so that the cost–benefit of delaying the need for renal replacement therapy and quality-of-life issues can be assessed.
Adverse effects, particularly haematological outcomes, were common in patients given prednisolone and chlorambucil in our study and often necessitated dose reduction or interruption of therapy. Clearly, the preservation of renal function that can be achieved with prednisolone and chlorambucil comes at a price, and careful monitoring of the therapy is needed.
Benefits of prednisolone and chlorambucil were maintained for at least 3 years of follow-up. Delaying end-stage renal disease, with its associated cardiovascular risk and increased morbidity and mortality, is of undoubted value to patients with idiopathic membranous nephropathy. However, even in patients given prednisolone and chlorambucil, only 40% had not had a further 20% decline in excretory renal function at 3 years. Clearly, more effective and safer forms of therapy are still needed for idiopathic membranous nephropathy. Until new treatments are available and have been properly tested, the evidence favours use of prednisolone and an alkylating agent in the most severely affected patients. This conclusion, and the lack of good RCTs in nephrology to address this and similar questions, are supported by a Cochrane review of the subject26 and an authoritative review of the international scientific literature on the treatment of glomerulonephritis (panel).27
Panel. Research in context.
Systematic review
We searched Medline and PubMed for articles published in any language between Jan 1, 1960, and April 12, 2012, with the keywords “membranous nephropathy” or “membranous glomerulonephritis” and “treatment”, “trial”, and “randomised”. The older scientific literature is best summarised in a 2004 Cochrane review26 and the current literature is well reviewed in a 2012 authoritative guideline on the treatment of glomerulonephritis.27
Interpretation
This study is the first to focus on the important subset of patients with membranous nephropathy whose excretory renal function is deteriorating. The results suggest that therapy with prednisolone and chlorambucil is better than with ciclosporin or supportive therapy alone. This patient population has serious morbidity, and the benefits of treatment need to be weighed against the adverse events. The protection of renal function offered by prednisolone and chlorambucil comes at a price, so this therapy needs to be closely monitored.
The supportive therapy only group effectively continued current treatment whereas the two intervention groups received new treatment, which led to some minor differences in the time between randomisation and baseline readings (ie, initiation of treatment); however, the time differences were small in the context of this slowly progressive disease in comparison with the length of follow-up, and as a result the risk of bias is small.
Recruitment was very slow and the trial was designed over 14 years ago. However, we believe that the trial results are still relevant, not least because few RCTs of drugs for this disease have been done, with none in the subset of patients with deteriorating excretory renal function.
One aspect of the slow recruitment was the changes in methods that became established during the trial, both for estimation of excretory kidney function and assessment of proteinuria. We used one consistent method for the calculation of primary endpoints. We did not use gold-standard methods of measuring kidney function such as isotope clearance studies. These methods are expensive and invasive, and although our trial depended on estimations, we believe it is relevant to everyday clinical practice since we used standard estimation techniques that are used in nephrology practice worldwide.
In this study we assessed ciclosporin monotherapy and noted that the drug's nephrotoxicity, even at blood concentrations advocated in a previous RCT,12,15 was harmful in the severely affected subset that we selected. Some physicians advocate use of ciclosporin together with prednisolone, but we know of no good evidence that this combination reduces renal toxicity.
We need to follow up these patients for even longer to absolutely assess the value of the delay to end-stage renal disease that we noted in the prednisolone and chlorambucil group and to gather longer-term data for delayed adverse effects in the two intervention groups, especially lymphomas and other malignancies.
Acknowledgments
Acknowledgments
The trial was largely funded by two grants from the Medical Research Council (MRC; grant reference G9721265). Before MRC funding was obtained, a small unrestricted grant from Novartis supported the purchase of trial record books and some other trial materials. Additional contributions to continuation funding came from Kidney Research UK and the Renal Association. The drug treatments, investigations, and clinic visits were not funded by the trial, they were all provided as part of routine clinical management.
Contributors
The trial was conceived and designed by members of the UK Renal Association Clinical Trials Committee's glomerulonephritis subgroup, chaired by PWM (the principal investigator), who was the sole applicant on funding applications to the UK Medical Research Council. All authors helped to write the report and commented on the manuscript. AH analysed the data and advised on statistical issues at the time of the trial write up. TLC was the trial administrator; obtained the data; and prepared communications with participating centres, the data monitoring committee, and funders. MML and CF were research nurses responsible for recruitment and return of data. DA, JF, GJG, DRWJ, DO'D, MB-J, and PWM designed the trial. PWM took overall responsibility for communications during the trial, analysed the data, and wrote the first draft of the report.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Hofstra JM, Wetzels JF. Introduction of a cyclophosphamide-based treatment strategy and the risk of ESRD in patients with idiopathic membranous nephropathy: a nationwide survey in the Netherlands. Nephrol Dial Transplant. 2008;23:3534–3538. doi: 10.1093/ndt/gfn350. [DOI] [PubMed] [Google Scholar]
- 2.National Kidney and Urologic Diseases Information Clearinghouse (NKUDIC) Kidney Disease Statistics for the United States. http://kidney.niddk.nih.gov/kudiseases/pubs/kustats/ (accessed Sept 9, 2012).
- 3.Donadio JV, Jr, Torres VE, Velosa JA. Idiopathic membranous nephropathy: the natural history of untreated patients. Kidney Int. 1988;33:708–715. doi: 10.1038/ki.1988.56. [DOI] [PubMed] [Google Scholar]
- 4.Mathieson PW, Rees AJ. A critical review of treatment for membranous nephropathy. Adv Nephrol Necker Hosp. 1991;20:151–174. [PubMed] [Google Scholar]
- 5.Davison AM, Cameron JS, Kerr DN, Ogg CS, Wilkinson RW. The natural history of renal function in untreated idiopathic membranous glomerulonephritis in adults. Clin Nephrol. 1984;22:61–67. [PubMed] [Google Scholar]
- 6.Winearls CG, Sanderson F. Treatment of aggressive idiopathic membranous glomerulonephritis. Q J Med. 1994;87:199–201. [PubMed] [Google Scholar]
- 7.Mathieson P. Treating progressive and indolent MGN. Q J Med. 1998;91:167. doi: 10.1093/qjmed/91.2.167. [DOI] [PubMed] [Google Scholar]
- 8.Beck LH, Jr, Salant DJ. Membranous nephropathy: recent travels and new roads ahead. Kidney Int. 2010;77:765–770. doi: 10.1038/ki.2010.34. [DOI] [PubMed] [Google Scholar]
- 9.Beck LH, Jr, Bonegio RG, Lambeau G. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanescu HC, Arcos-Burgos M, Medlar A. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med. 2011;364:616–626. doi: 10.1056/NEJMoa1009742. [DOI] [PubMed] [Google Scholar]
- 11.Ponticelli C, Zucchelli P, Passerini P. A randomized trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med. 1989;320:8–13. doi: 10.1056/NEJM198901053200102. [DOI] [PubMed] [Google Scholar]
- 12.Cattran DC, Greenwood C, Ritchie S. A controlled trial of cyclosporine in patients with progressive membranous nephropathy. Canadian Glomerulonephritis Study Group. Kidney Int. 1995;47:1130–1135. doi: 10.1038/ki.1995.161. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 14.Mathieson PW, Maidment CGH, Turner AN, Evans DJ, Rees AJ. Prednisolone and chlorambucil treatment in idiopathic membranous nephropathy with deteriorating renal function. Lancet. 1988;2:869–872. doi: 10.1016/s0140-6736(88)92470-1. [DOI] [PubMed] [Google Scholar]
- 15.Medicines and Healthcare products Regulatory Agency Clinical trials for medicines: safety reporting—SUSARs and ASRs. www.mhra.gov.uk//howweregulate/medicines/licensingofmedicines/clinicaltrials/safetyreporting-SUSARsandASRs/index.htm (accessed Oct 2, 2012).
- 16.Cattran DC, Reich HN, Kim SJ, Troyanov S. Have we changed the outcome in membranous nephropathy? A propensity study on the role of immunosuppressive therapy. Clin J Am Soc Nephrol. 2011;6:1591–1598. doi: 10.2215/CJN.11001210. [DOI] [PubMed] [Google Scholar]
- 17.Cattran DC, Appel GB, Hebert LA, for the North America Nephrotic Syndrome Study Group Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int. 2001;59:1484–1490. doi: 10.1046/j.1523-1755.2001.0590041484.x. [DOI] [PubMed] [Google Scholar]
- 18.Alexopoulos E, Papagianni A, Tsamelashvili M, Leontsini M, Memmos D. Induction and long-term treatment with cyclosporine in membranous nephropathy with the nephrotic syndrome. Nephrol Dial Transplant. 2006;21:3127–3132. doi: 10.1093/ndt/gfl360. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Zhang YM, Qu Z, Zhao MH, Liu G. Low-dose cyclosporine treatment in Chinese nephrotic patients with idiopathic membranous nephropathy: an uncontrolled study with prospective follow-up. Am J Med Sci. 2010;339:532–536. doi: 10.1097/MAJ.0b013e3181d9f00b. [DOI] [PubMed] [Google Scholar]
- 20.Ponticelli C, Altieri P, Scolari F. A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol. 1998;9:444–450. doi: 10.1681/ASN.V93444. [DOI] [PubMed] [Google Scholar]
- 21.Hofstra JM, Wetzels JF. Alkylating agents in membranous nephropathy: efficacy proven beyond doubt. Nephrol Dial Transplant. 2010;25:1760–1766. doi: 10.1093/ndt/gfq017. [DOI] [PubMed] [Google Scholar]
- 22.Jha V, Ganguli A, Saha TK. A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol. 2007;18:1899–1904. doi: 10.1681/ASN.2007020166. [DOI] [PubMed] [Google Scholar]
- 23.Cravedi P, Sghirlanzoni MC, Marasà M, Salerno A, Remuzzi G, Ruggenenti P. Efficacy and safety of rituximab second-line therapy for membranous nephropathy: a prospective, matched-cohort study. Am J Nephrol. 2011;33:461–468. doi: 10.1159/000327611. [DOI] [PubMed] [Google Scholar]
- 24.Fervenza FC, Abraham RS, Erickson SB, for the Mayo Nephrology Collaborative Group Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol. 2010;5:2188–2198. doi: 10.2215/CJN.05080610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuccori M, Focosi D, Blandizzi C. Inclusion of rituximab in treatment protocols for non-Hodgkin's lymphomas and risk for progressive multifocal leukoencephalopathy. Oncologist. 2010;15:1214–1219. doi: 10.1634/theoncologist.2010-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schieppati A, Perna A, Zamora J, Giuliano GA, Braun N, Remuzzi G. Immunosuppressive treatment for idiopathic membranous nephropathy in adults with nephrotic syndrome. Cochrane Database Syst Rev. 2004;4:CD004293. doi: 10.1002/14651858.CD004293.pub2. [DOI] [PubMed] [Google Scholar]
- 27.International Society of Nephrology Kidney Disease—Improving Glocal Outcomes (KDIGO) clinical practice guideline for glomerulonephritis. Chapter 7: idiopathic membranous nephropathy. Kidney Int Suppl. 2012;2:186–197. doi: 10.1038/kisup.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]


