Abstract
Background
The effects of ethanol on brain function are thought to be due in part to alterations in the activity of ion channels that regulate synaptic activity. Results from previous studies from this lab and others have shown that ethanol inhibits the function of the N-methyl-D-aspartate (NMDA) receptors, a calcium-permeable ion channel activated by the neurotransmitter glutamate. Factors that alter the acute sensitivity of NMDA receptors to ethanol may be critical in determining how neurons and neuronal networks respond to the presence of ethanol. In this study, we have examined the effect of physiologically relevant concentrations of magnesium on the ethanol sensitivity of recombinant NMDA receptors and how ethanol inhibition under these conditions is influenced by the NR3A subunit.
Methods
Recombinant cDNAs encoding NMDA receptor subunits were expressed in human embryonic kidney (HEK) 293 cells. Whole-cell patch-clamp electrophysiology was used to measure currents induced by rapid application of glutamate in the absence and presence of ethanol.
Results
In magnesium-free recording solution, ethanol inhibited glutamate-mediated currents in cells transfected with NMDA receptor subunits. The magnitude of ethanol inhibition was significantly enhanced when recordings were carried out in media containing 1 mM magnesium. This effect was reversible and required magnesium-sensitive receptors. Magnesium did not enhance ethanol inhibition of glycine-activated NR1/NR3A/NR3B receptors. However, NR3A co-expression prevented the enhancement of ethanol's inhibitory effect on receptors composed of NR2A but not NR2B subunits.
Conclusions
These results suggest that under physiological conditions, NR3A may be an important regulator of the acute ethanol sensitivity of brain NMDA receptors
Keywords: electrophysiology, addiction, glutamate, HEK cells
N-methyl-D-aspartate receptors are heteromeric ion channels activated by glutamate and related excitatory amino acids (reviewed by Dingledine et al., 1999). They are expressed throughout the brain and are particularly enriched on dendritic spines, specialized neuronal compartments housing structural and functional elements involved in excitatory neurotransmission. NMDA receptors are particularly important in regulating neuronal plasticity, the ability of synapses to undergo reversible changes in signaling efficacy thought to be important in mediating many forms of learning and memory. While changes in synaptic efficacy are likely to be important in the behavioral adaptation during normal experience-dependent learning, alterations in NMDA receptor activity by drugs or disease may be counter-productive and could contribute to the persistence of maladaptive behaviors that characterizes the addictive state (Hyman and Malenka, 2001).
In recent years, a growing body of evidence has suggested that changes in glutamate-mediated signaling may be especially important in mediating some of the actions of ethanol (Chandler, 2003). Both native and recombinant NMDA receptors are reversibly inhibited by acute application of ethanol (Lovinger et al., 1989; Mirshahi and Woodward, 1995) while long-term exposure to ethanol induces marked changes in the synaptic localization and function of NMDA receptor subunits (Carpenter-Hyland et al., 2004). These results suggest the acute ethanol sensitivity of NMDA receptors may be an important factor involved in the development of alcohol dependence.
The magnitude of ethanol's inhibition of NMDA receptors is influenced by several factors including subunit composition (Jin and Woodward, 2006), interaction with cytoskeletal elements (Anders et al., 2000) and the developmental status of the brain (Lovinger, 1995).In general, NMDA receptors in adolescent brains appear more sensitive to inhibition by ethanol (Swartzwelder et al., 1995) and in some brain areas this appears to be correlated with the level of expression of NR2B subunits that are highest during early periods of development (Lovinger, 1995; but see Popp et al., 1999). One factor that has received only modest attention in the search for conditions that regulate ethanol sensitivity of NMDA receptors is the divalent cation magnesium.
Most in vitro studies that measure NMDA receptor function routinely use magnesium-free solutions to avoid the well-known voltage-dependent block of receptor current by this ion. However, in some studies, the ethanol inhibition of NMDA-mediated responses was reported to be significantly enhanced when experiments were conducted with physiological (~1 mM) levels of magnesium (Chandler et al., 1994; Martin et al., 1991; Morrisett et al., 1991). In those studies, the subunit makeup of the neuronal NMDA receptors was not known. Both NR2A and NR2B containing receptors show a high sensitivity to magnesium block while those containing NR2C or NR2D are less affected (Monyer et al., 1994; Stern et al., 1994). Magnesium block of NMDA receptors is also influenced by the presence of the modulatory NR3 subunit that shows both regional and developmentally regulated profiles of expression. In this study, we tested whether the ethanol inhibition of recombinant NMDA receptors is also enhanced by extracellular magnesium and whether this effect is regulated by the NR3A subunit.
Materials and Methods
Molecular Biology, Cell Culture and Transfection
The NMDA receptor cDNAs used in these experiments were kindly provided by the following individuals: NR1-1a and NR2A (Drs. S. Nakanishi, Kyoto, Japan), NR2B and NR2D (Dr. P. Seeburg, Max Planck Institute for Medical Research, Heidelberg, Germany), NR3A (Dr. S. Lipton, Burnham Institute for Medical Research, La Jolla, CA) and NR2A(H128S) (J. Neyton, Paris, France). To identify cells that expressed the NR3 subunit, the coding region for NR3A was subcloned into the green fluorescent protein expression vector, pGFP-N3 as previously described (Smothers and Woodward, 2003). Human embryonic kidney (HEK) 293 cells were obtained from ATCC (Manassas, VA). Cells were maintained in feeder flasks containing serum-supplemented DMEM in a humidified incubator supplied with 5% CO2 and were split weekly. For recordings, cells were plated onto poly-ornithine coated 35 mm dishes and transfected with plasmids encoding various NMDA receptor subunits using Lipofectamine 2000 (Invitrogen, Inc.) according to the manufacturer's recommendation. A cDNA encoding enhanced green fluorescent protein (eGFP) was included in the transfection mixture Plasmids were used at a ratio of 1:1:1 unless otherwise indicated. In some experiments, a cDNA plasmid encoding an NR3A-GFP fusion protein was used instead of eGFP. Following transfection, the NMDA antagonist AP5 (200 µM) was added to the media to prevent glutamate-mediated excitotoxicity (Cik et al., 1994). AP5 was removed by extensive washing prior to recording.
Electrophysiology
Dishes containing transfected cells were mounted on the stage of an Olympus IX50 inverted microscope and perfused with extracellular recording solution at 1–2 ml/min. The recording solution contained (in mM); NaCl (135), KCl (5.4), CaCl2 (1.8), HEPES (5), glucose (10), (pH adjusted to 7.4 and osmolarity adjusted to 310–325 mOsm with sucrose. Patch pipettes (2–5 mOhms) were pulled from borosilicate glass (1.5 × 0.86 mm) and filled with internal solution containing (in mM); CsCl (140), HEPES (10), MgCl2 2, K2ATP (4), EGTA 2.5, and TEA 2.0 (pH adjusted to 7.2 with KOH). Transfected cells were identified by eGFP fluorescence and whole-cell voltage clamp recordings were carried out at room temperature using an Axon 200B microamplifier (Molecular Devices, Union City, CA). Cells were initially held at −60 mV to monitor seal breakthrough and then stepped to −30 mV for recording. Whole-cell capacitance and series resistance were compensated for and access resistance was monitored over the course of the experiment. Cells with unstable holding currents were not used for analysis. NMDA receptor currents were evoked using a Warner FastStep multi-barrel perfusion system to switch between normal extracellular solution to one containing agonist (glutamate (10 µM) plus glycine (10 µM)) or agonist plus ethanol (25–100 mM). Each cell tested was exposed to duplicate solutions containing agonist (− and + ethanol) with or without added magnesium. The order of solutions was alternated between cells. Data were filtered at 1–2 kHz and acquired at 5 kHz using an Instrutech ITC-16 digital interface (Instrutech Corp., Port Washington, NY) controlled by IgorPro software (Wavemetrics, Lake Oswego, OR) running the Pulse control acquisition module. Data were analyzed offline using Axograph software (Axograph, Sydney, New South Wales, Australia). Agonist-evoked currents were baseline subtracted and amplitudes were measured during the last 0.5 seconds of agonist application when currents had reached steady-state levels. Ethanol inhibition was calculated using the formula (1-(IGiutamate+Etoh/Icontroi)) × 100, where IGlutamate+Eioh represents the response to co-application of agonist + ethanol, and Icontrol represents the mean of two responses to agonist, one before and one after the co-application of ethanol. Ethanol was purchased from Aaper Alcohol and Chemical Company (Shelbyville, KY) while all other chemicals were purchased from Sigma Chemical Company (St. Louis, MO).
Data Analysis
Data are expressed as mean ± SEM and were analyzed by paired t-test using Prism 4.0 software (Graphpad Software, San Diego, CA).
Results
NMDA receptor subunits were expressed in HEK293 cells and whole-cell patch-clamp electrophysiology was used to monitor currents evoked during application of glutamate and glycine. In each cell tested, the effects of ethanol on NMDA receptor currents were determined in the absence and presence of extracellular magnesium. During these recordings, cells were held at −30 mV, a membrane potential at which inward currents are near maximal in the presence of 1 mM magnesium (Monyer et al., 1992).
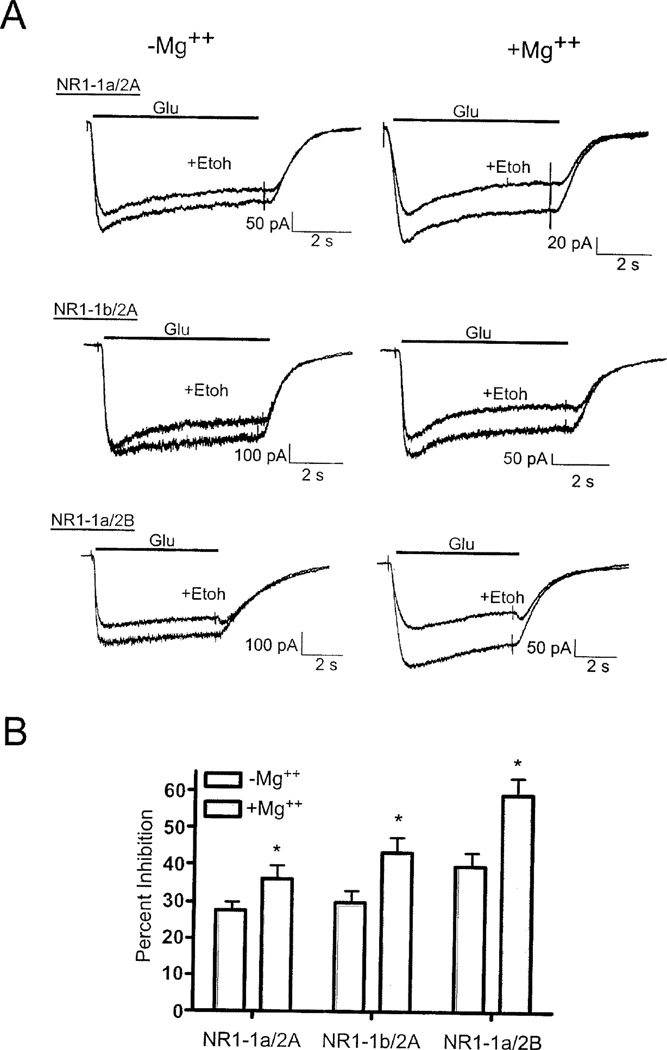
In the absence of Mg++, ethanol (100 mM), inhibited glutamate-activated currents in cells expressing NR1-1a/2A subunits by approximately 28% (Figure 1A). The inhibition increased to approximately 35% in the presence of 1 mM magnesium. Since NR1/2A receptors display a voltage-independent block by low nanomolar concentrations of zinc, we performed several experiments to test whether the enhanced ethanol inhibition of NR1/2A receptors may have been due to contaminating levels of zinc. First, the NR1-1b splice variant was substituted for NR1-1a. NR1-1b subunits contain an alternatively spliced N-terminal cassette that markedly attenuates inhibition by zinc and other modulators (Low et al., 2003). In these experiments, ethanol inhibited NR1-1b/2A receptors by approximately 29% in the absence of magnesium (Figure 1B). Inhibition increased to 42% in the presence of magnesium. A similar increase in ethanol inhibition was observed in cells expressing NR1-1a and a zinc-insensitive NR2A(H128S) mutant (data not shown). We then tested the effects of magnesium on the ethanol sensitivity of NR2B containing receptors. NR1/NR2B receptors also show a high sensitivity to magnesium block but are insensitive to nanomolar concentrations of zinc found in most extracellular solutions. In the absence of magnesium, ethanol inhibited NR1-1a/2B receptors by approximately 40%. Inhibition increased to nearly 60% in the presence of 1 mM magnesium (Figure 1C). The effect of magnesium on ethanol inhibition also was observed at lower concentrations of ethanol. For example, in NR1-1a/NR2B receptors, magnesium increased the degree of ethanol inhibition (mean ± SEM) at both 25 mM (zero magnesium, 4.7% ± 2.1; in 1 mM magnesium, 11.1% ± 2.6; N=5) and 50 mM ethanol (zero magnesium, 11.9% ± 1.9; in 1 mM magnesium, 38.9% ± 7.4; N=6). Together, these results suggest that physiologically relevant levels of magnesium enhance the magnitude of ethanol inhibition of recombinant NMDA receptors.
Figure 1.
Magnesium enhances the ethanol inhibition of recombinant NMDA receptors expressed in HEK 293 cells. A) Representative traces from cells expressing NR1-1a/2A, NR1-1b/2A or NR1-1a/2B receptors during exposure to 10 µM glutamate (with 10 µM glycine, solid line) in the absence or presence of ethanol (100 mM). Pairs of traces for each subunit combination were collected from the same cell in the absence (left) and presence (right) of 1 mM MgCl2. B) Summary of ethanol inhibition of NMDA receptors in the absence and presence of magnesium. Bars indicate percent inhibition of steady-state currents by ethanol and are mean (±SEM) from 7–8 cells for each subunit combination. Symbol (*); value significantly different from corresponding control, p < 0.05, paired t-test.
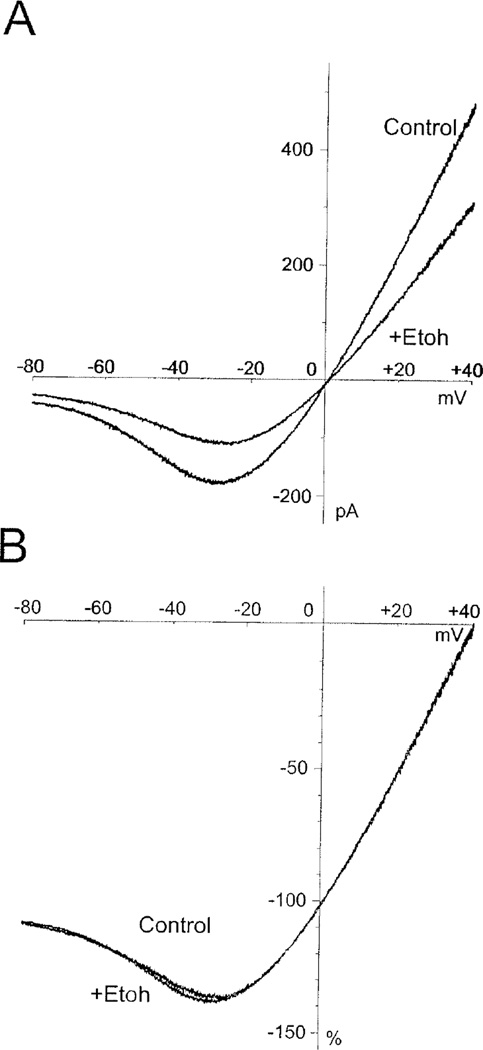
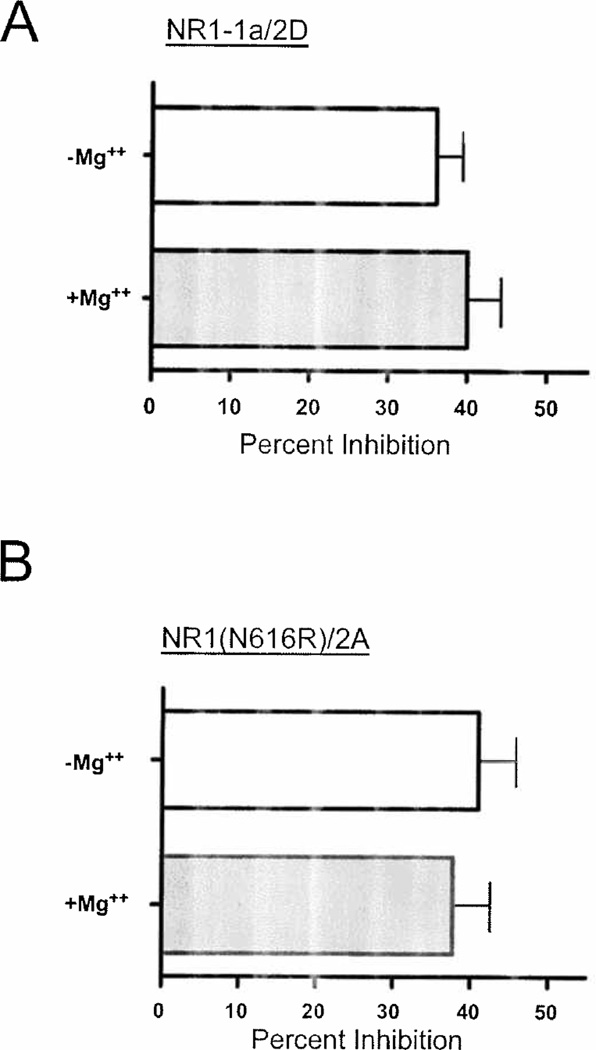
One question that arises from these observations is whether they reflect a magnesium-dependent increase in ethanol inhibition or an ethanol-induced increase in magnesium inhibition. Several experiments were conducted to try and address this issue. First we tested whether ethanol alters the voltage-dependence of magnesium inhibition of the receptor. In these experiments, a current-voltage (IV) relationship was generated by ramping the membrane potential of NR1/2A transfected cells between −80 mV and +40 mV during continuous application of glutamate and glycine. Figure 2A shows the well-known J-shaped current-voltage relationship for NMDA receptors recorded in 1 mM magnesium. Under these conditions, peak inward currents occurred at approximately −30 mV. When co-applied with glutamate and glycine, ethanol (100 mM) reduced whole-cell currents but had no significant effect on the overall characteristics of the IV curve. This is most clearly seen in Fig 2B that shows IV curves in the absence and presence of ethanol after normalizing currents in each cell to the peak current measured at +40 mV. The resulting superimposed curves suggest that ethanol does not significantly affect the voltage-dependence of magnesium block of NMDA receptors. We next tested the effects of ethanol on receptors with diminished sensitivity to magnesium block. If intrinsic sensitivity to magnesium is required for enhanced ethanol inhibition, eliminating this sensitivity should reduce or abolish the effect. To test this hypothesis, we measured the ethanol inhibition of NR1/2D receptors that are naturally much less sensitive to magnesium block (Monyer et al., 1994). As shown in Figure 3A, under magnesium-free conditions, ethanol inhibited NR1-1a/2D receptors by approximately 34%. In the presence of 1 mM magnesium, ethanol inhibition of NR1/2D receptors was not significantly enhanced. To further test whether a magnesium sensitive NMDA receptor is required to demonstrate enhanced ethanol inhibition, a mutant NR1 subunit that is insensitive to magnesium was used. Magnesium block of NMDA receptor current is eliminated when asparagine (N) 616 in the TM2 domain of the NR1 subunit is replaced with arginine (Sakurada et al., 1993). Cells transfected with NR1(N616R) and the NR2A subunits were inhibited by approximately 40% by 100 mM ethanol in the absence of magnesium (Figure 3B). When 1 mM magnesium was present, the degree of ethanol inhibition was unchanged. Taken together with the data obtained with NR2D receptors, these results suggest that enhanced ethanol inhibition requires a magnesium-sensitive NMDA receptor.
Figure 2.
Effects of ethanol on the current-voltage relationship of NMDA receptors. A) HEK 293 cells expressing NR1-1a/2A were stimulated with 10 µM glutamate (with 10 µM glycine) and membrane potential was ramped between −80 mV and +40 mV. Lines represents the average (N=6) leak-subtracted current in the presence of 1 mM magnesium obtained in the absence and presence of ethanol (100 mM). B) For each cell tested in panel A, currents were normalized to the peak current obtained at +40 mV. Each line represents the average normalized IV curve from 6 different cells.
Figure 3.
Ethanol inhibition of magnesium-insensitive NMDA receptors is not altered by magnesium. A) Summary figure showing percent inhibition of NR1-1a/2D receptors by 100 mM ethanol in the absence or presence of 1 mM MgCl2. B) Summary figure showing percent inhibition of NR1(N616R)/2A receptors by 100 mM ethanol in the absence or presence of 1 mM MgCl2. Values are mean (± SEM) from 6–9 cells.
In addition to NR1 and NR2 subunits, the NMDA receptor family also contains the NR3A and NR3B subunits. These subunits co-assemble with and influence the function of heteromeric NR1/NR2 receptors and can also form novel glycine-activated channels when combined with the NR1 subunit (Smothers and Woodward, 2007; Sucher et al., 1995). NR1/NR3 channels show a linear IV curve in the presence and absence of magnesium suggesting that they are magnesium-insensitive. In previous studies from this laboratory, we reported that the ethanol inhibition of heteromeric NR1/NR2 receptors was not significantly altered by the NR3A subunit and that glycine-activated NR1/NR3 receptors were modestly inhibited by ethanol (Smothers and Woodward, 2003; Smothers and Woodward, 2007). However, those studies were conducted in a magnesium-free recording solution. Thus, in the present study, we re-examined whether the ethanol inhibition of heteromeric NR1/NR2 and NR1/NR3 receptors is altered by magnesium.
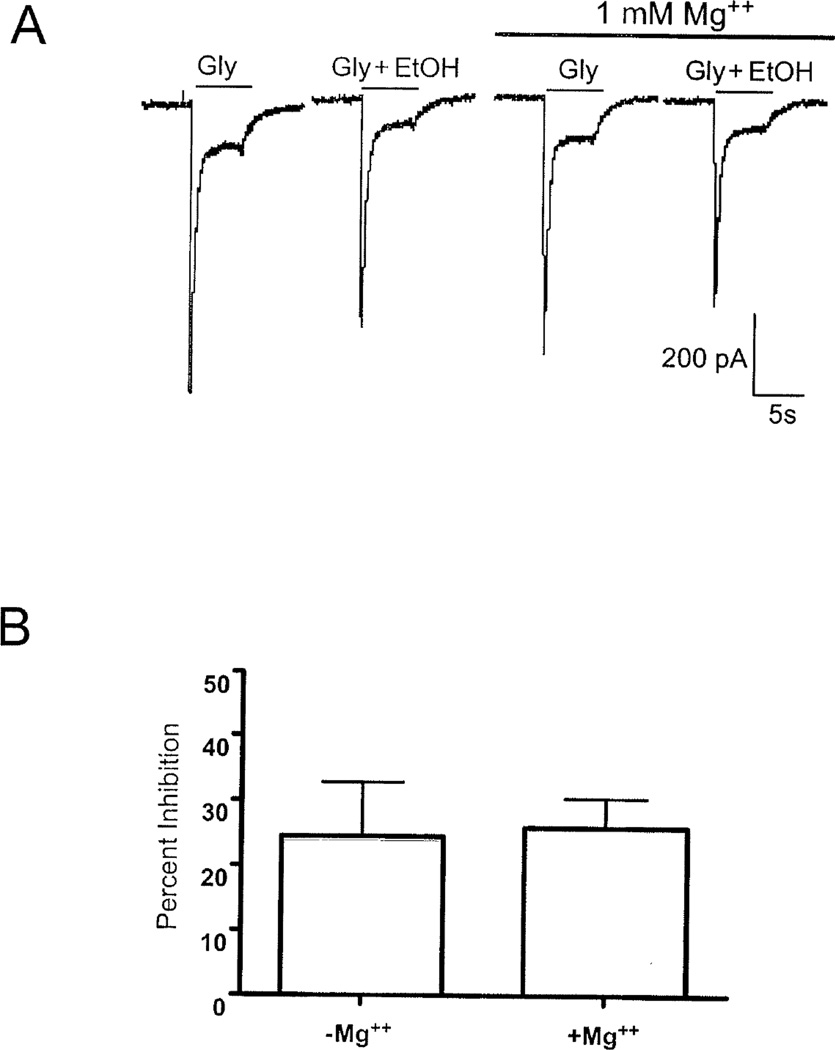
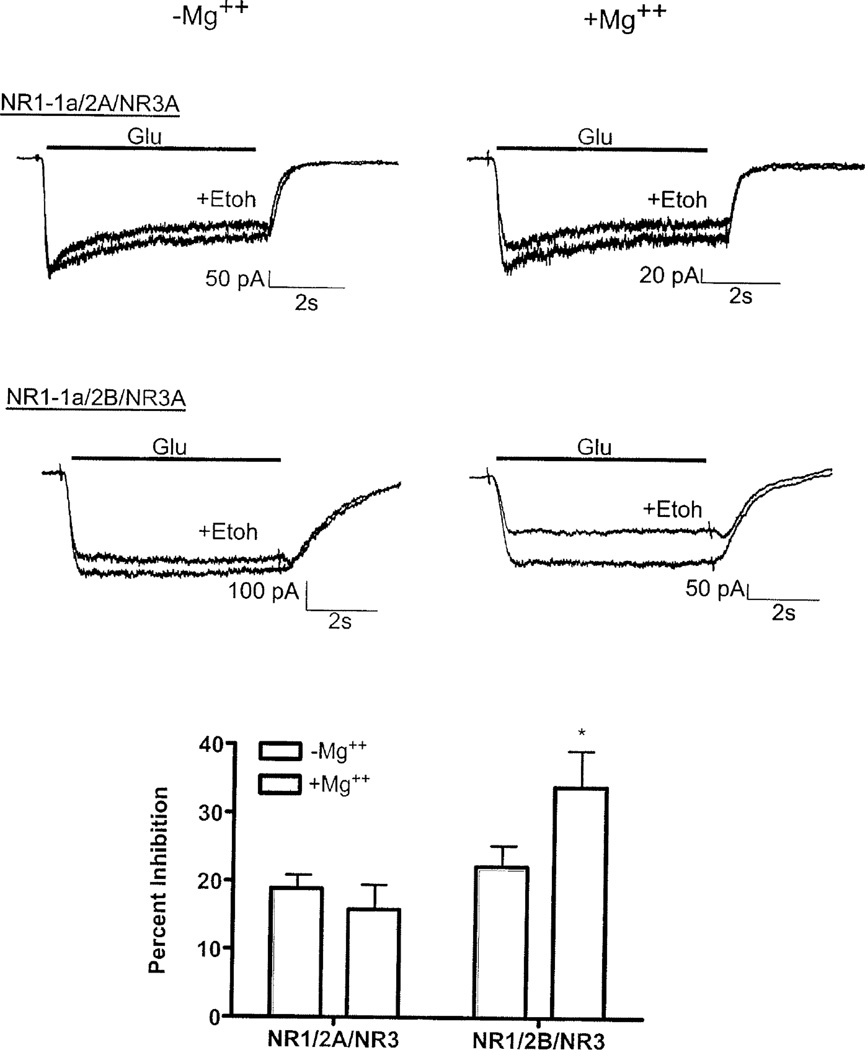
Consistent with previous findings (Smothers and Woodward, 2007), HEK 293 cells transfected with NR1/NR3A/NR3B subunits showed robust inward currents during application of glycine (Figure 4A). These currents were inhibited by approximately 20% during co-application of glycine and 100 mM ethanol. Unlike NR1/NR2 receptors, the degree of ethanol inhibition of NR1/NR3A/NR3B receptors was not further enhanced by 1 mM magnesium (Figure 4B). We then determined ethanol inhibition in cells transfected with NR1, NR2 and NR3A subunits. In these experiments, an NR3A-GFP fusion protein was used to verify expression. In cells transfected with NR3A-GFP and either NR1-1a/2A or NR1-1a/2B subunits, ethanol (100 mM) inhibited currents by approximately 20–22% in magnesium-free buffer (Figure 5); values similar to that previously reported (Smothers and Woodward, 2006). The ethanol inhibition of NR1/2B/NR3A-GFP receptors was significantly increased to 35% in the presence of 1 mM magnesium. In contrast, there was a slight reduction in the degree of ethanol inhibition of NR1-1a/2A/NR3A-GFP receptors when recordings were carried out in magnesium-containing buffer (Figure 5).
Figure 4.
Ethanol inhibition of NR1/NR3A/NR3B receptors is not altered by magnesium. A) Traces show the response of a single HEK 293 cell transfected with NR1/NR3A/NR3B subunits during exposure to 100 µM glycine (± 100 mM ethanol) in the absence and presence of 1 mM MgCl2. B) Summary figure showing percent inhibition by 100 mM ethanol of NR1/NR3A/NR3B receptors in the absence and presence of magnesium. Values are mean (± SEM) from 13–16 cells.
Figure 5.
Effect of the NR3A subunit on ethanol inhibition of NMDA receptors. A) Representative traces from cells expressing NR1-1a/NR2A/NR3A-GFP or NR1-1a/NR2B/NR3A-GFP during exposure to 10 µM glutamate (with 10 µM glycine, solid line) in the absence or presence of ethanol (100 mM). Pairs of traces for each subunit combination were collected from the same cell in the absence (left) and presence (right) of 1 mM MgCl2. B) Summary of ethanol inhibition of NMDA receptors in the absence and presence of magnesium. Bars indicate percent inhibition of steady-state currents by ethanol and are mean (±SEM) from 8–10 cells for each subunit combination. Symbol (*); value significantly different from corresponding control, p < 0.05, paired t-test.
Discussion
The major findings of the present study demonstrate that ethanol inhibition of NR1/NR2A and NR1/NR2B recombinant NMDA receptors is enhanced when physiological levels of extracellular magnesium are included in the recording buffer. This effect is regulated by the NR3A subunit in an NR2 subunit dependent fashion. These findings add to our understanding of the acute ethanol sensitivity of NMDA receptors and suggest a previously unknown role for NR3A receptors.
Previous studies examining ethanol's inhibition of NMDA receptors have generally recorded under conditions of reduced or zero magnesium (Lovinger et al., 1989; Mirshahi and Woodward, 1995). This is because at the holding potentials used in these experiments (eg., −60 to −70 mV), NMDA receptor current is largely blocked by physiologically relevant concentrations (1–2 mM) of magnesium. Removing magnesium from these solutions allows neurons or transfected cells to be held at their normal resting membrane potential and maintains a large electrical driving force and maximal NMDA current amplitudes. However, early studies by Morrisset and colleagues suggested that the magnitude of ethanol inhibition of NMDA responses from neurons was significantly enhanced by magnesium. For example, Martin et al. (1991) showed that ethanol was approximately two-fold more potent at inhibiting NMDA-induced depolarization of CA1 pyramidal neurons when recordings were performed in the presence of 1 mM magnesium. This effect was specific for NMDA responses as ethanol inhibition of AMPA or kainate-mediated depolarizations were unaffected by magnesium. In a follow-up study, Morrisett et al. (1991) demonstrated that the ethanol inhibition of extracellular field potentials in CA1 hippocampus was also enhanced when physiologically relevant levels of magnesium were present. Results from additional experiments carried out in both of these studies suggested that ethanol and magnesium were likely acting at distinct sites to inhibit channel function. In addition to these findings, a similar enhancement of ethanol inhibition of NMDA responses by magnesium was reported for neurons isolated from the basolateral amygdala (Calton et al., 1999). In that study, ethanol inhibition of electrically evoked NMDA-mediated currents was attenuated when magnesium levels in the bathing solution were reduced from 1 mM to 0.3 mM. Despite these findings, not all studies have reported that magnesium has a significant effect on the ethanol inhibition of neuronal NMDA responses (Lovinger et al., 1989; Peoples et al., 1997; Woodward and Gonzales, 1990). This suggests that additional factors are likely to be important in determining the magnitude and/or reproducibility of this effect.
In the present study, the magnesium-dependent increase in ethanol inhibition of recombinant NMDA receptors was observed with NR1/NR2A and NR1/NR2B receptors that both show a high sensitivity to magnesium. No additional increase in ethanol inhibition was observed in receptors with low sensitivity to magnesium such as those containing the NR2D subunit or a magnesium-insensitive NR1 subunit. The ability of magnesium to potentiate ethanol inhibition of recombinant NR2A or NR2B containing receptors is in contrast with a previous study conducted using Xenopus oocytes (Chu et al., 1995). In that study, magnesium had no effect on the ethanol inhibition of either magnesium-sensitive (NR1/NR2A or NR1/NR2B) or insensitive (NR1/NR2C) NMDA receptors. While the reason for this apparent discrepancy is not known, differences in the experimental conditions between the two studies may underlie these results. For example, in the Chu et al. (1995) study, the concentrations of magnesium used to test for changes in ethanol inhibition were relatively low ranging from 3 to 12.5 µM. These values are well below the concentration used in the present study (1 mM) and the 0.5–3 mM concentrations used by Morrisett et al. (1991) and Martin et al. (1991). As levels of magnesium in cerebrospinal fluid are near 1 mM and are fairly tightly regulated, these results suggest under normal conditions, ethanol inhibition of NMDA responses in vivo may be greater than that estimated under standard magnesium-free recording conditions.
Although magnesium enhanced the ethanol inhibition of recombinant NMDA receptors in the present study, this effect was most noticeable with NR2B receptors. During the first two weeks of post-natal development in rodents, most neurons in the forebrain show greater expression of NR2B versus NR2A subunits (Williams et al., 1993). NR2A subunit expression rises dramatically following this time and most neurons in the adult brain show robust expression of both NR2 subtypes. To date, there have been no studies that have systematically investigated the relationship between NR2B expression and ethanol sensitivity using physiologically relevant concentrations of magnesium. However, using zero magnesium recording conditions, several studies have examined ethanol inhibition of NMDA receptor currents during development. Results from these studies show that in some brain regions (CA1 of the hippocampus), NMDA receptor ethanol sensitivity declines along with an age-dependent decrease in the antagonist efficacy of NR2B selective antagonists (Lovinger, 1995). However, in cerebellar granule neurons, NMDA responses do not show changes in ethanol sensitivity despite a similar reduction in the effects of NR2B antagonists (Popp et al., 1998). These results suggest that factors other than differences in NR2 subunit expression are likely to be involved in determining the overall ethanol sensitivity of NMDA receptors. Results from the present study suggest that the NR3A subunit may be one such factor.
NR3 subunits are a novel class of NMDA receptor proteins and are widely expressed throughout the central and somatic nervous systems (Ciabarra et al., 1995; Sucher et al., 1995). They share some sequence identity with NR1 subunits and bind glycine, but not glutamate, with high affinity. NR3 subunits associate with NR1/NR2 receptors to reduce the magnesium sensitivity and calcium permeability of these receptors. In the absence of NR2 subunits, NR1/NR3 receptors form functional receptors and generate glycine-dependent currents (Chatterton et al., 2002; Madry et al., 2007; Smothers and Woodward, 2007). Consistent with our previous findings (Smothers and Woodward, 2003), ethanol inhibited glycine-activated currents in cells expressing NR1/NR3A/NR3B subunits. As expected for these magnesium-insensitive receptors, this effect was not altered in the presence of 1 mM magnesium. However, when NR3A was co-expressed with NR1/NR2A receptors, the magnesium-dependent increase in ethanol inhibition was prevented. This effect was more pronounced for NR2A containing receptors as magnesium still enhanced ethanol inhibition of cells transfected with NR1/NR2B/NR3A subunits. These results suggest that in vivo, NR3A subunits may differentially regulate the overall ethanol sensitivity of neuronal NMDA receptors. This effect would be expected to be most noticeable in areas that show high expression of both NR2A and NR3A subunits. Initial studies using in situ hybridization showed that while NR3A mRNA was highly expressed during development, expression was virtually absent in adult animals except for the nucleus of the olfactory tract (Sucher et al., 1995). NR2A shows an opposite pattern of expression with low levels during development and widespread expression in the adult brain (Monyer et al., 1994; Petralia et al., 1994). However, subsequent studies using a monoclonal antibody to detect NR3A protein showed that in rodents, NR3A subunits, while highly expressed during development continued to be expressed in discrete brain areas during adulthood (Wong et al., 2002). In primates, NR3A also shows a robust expression in the adult brain suggesting an important role for this subunit in neuronal function (Mueller and Meador-Woodruff, 2005). Regions that show high levels of NR3A in both adult rodents and primates include layer V of the prefrontal cortex and specific nuclei of the thalamus, hypothalamus, hippocampus, amygdala, midbrain and cerebellum (Mueller and Meador-Woodruff, 2005; Wong et al., 2002). Interestingly, areas of the dorsal and ventral striatum show little NR3A expression in adult animals. As all of these areas also express high levels of NR1 and NR2 subunits, these results suggest that in vivo, the ethanol inhibition of NR1/NR2A receptors may be significantly affected by the presence of the NR3A subunit.
In conclusion, the results of the present study suggest that the ethanol sensitivity of NMDA receptors in the intact brain may be greater than previously thought. They also suggest that the magnitude of inhibition may be influenced by brain regional differences the expression of NR2 and NR3 subunits.
Acknowledgments
This work was supported by funds provided by NIH grant AA09986 and K0200238 to J.J.W.
References
- Anders DL, Blevins TL, Smothers CT, Woodward JJ. Reduced ethanol inhibition of N-methyl-D-aspartate receptors by deletion of the NR1 CO domain or overexpression of a-actinin-2 proteins. Journal of Biological Chemistry. 2000;275(20):15019–15024. doi: 10.1074/jbc.275.20.15019. [DOI] [PubMed] [Google Scholar]
- Calton JL, Wilson WA, Moore SD. Reduction of voltage-dependent currents by ethanol contributes to inhibition of NMDA receptor-mediated excitatory synaptic transmission. Brain Res. 1999;816(1):142–148. doi: 10.1016/s0006-8993(98)01144-5. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24:7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ. Ethanol and brain plasticity: receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol Ther. 2003;99(3):311–326. doi: 10.1016/s0163-7258(03)00096-2. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Guzman NJ, Sumners C, Crews FT. Magnesium and zinc potentiate ethanol inhibition of N-methyl-D-aspartate-stimulated nitric oxide synthase in cortical neurons. J. Pharmacol. Exp. Ther. 1994;271:67–75. [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415(6873):793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- Chu B, Anantharam V, Treistman SN. Ethanol inhibition of recombinant heteromeric NMDA channels in the presence and absence of modulators. J. Neurochem. 1995;65:140–148. doi: 10.1046/j.1471-4159.1995.65010140.x. [DOI] [PubMed] [Google Scholar]
- Ciabarra AM, Sullivan JM, Gahn LG, Pecht G, Heinemann S, Sevarino KA. Cloning and characterization of chi-1: A developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J. Neurosci. 1995;15:6498–6508. doi: 10.1523/JNEUROSCI.15-10-06498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cik M, Chazot PL, Stephenson FA. Expression of NMDAR1-1a (N598Q)/NMDAR2A receptors results in decreased cell mortality. Eur. J. Pharmacol. Mol. Pharmacol. 1994;266:R1–R3. doi: 10.1016/0922-4106(94)90146-5. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2(10):695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Jin C, Woodward JJ. Effects of 8 different NR1 splice variants on the ethanol inhibition of recombinant NMDA receptors. Alcohol Clin Exp Res. 2006;30(4):673–679. doi: 10.1111/j.1530-0277.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Developmental decrease in ethanol inhibition of N-methyl-D-aspartate receptors in rat neocortical neurons: Relation to the actions of ifenprodil. J. Pharmacol. Exp. Ther. 1995;274:164–172. [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Low CM, Lyuboslavsky P, French A, Le P, Wyatte K, Thiel WH, Marchan EM, Igarashi K, Kashiwagi K, Gernert K, Williams K, Traynelis SF, Zheng F. Molecular determinants of proton-sensitive N-methyl-D-aspartate receptor gating. Mol Pharmacol. 2003;63(6):1212–1222. doi: 10.1124/mol.63.6.1212. [DOI] [PubMed] [Google Scholar]
- Madry C, Mesic I, Bartholomaus I, Nicke A, Betz H, Laube B. Principal role of NR3 subunits in NR1/NR3 excitatory glycine receptor function. Biochem Biophys Res Commun. 2007;354(1):102–108. doi: 10.1016/j.bbrc.2006.12.153. [DOI] [PubMed] [Google Scholar]
- Martin D, Morrisett RA, Bian XP, Wilson WA, Swartzwelder HS. Ethanol inhibition of NMDA mediated depolarizations is increased in the presence of Mg2+ Brain Res. 1991;546:227–234. doi: 10.1016/0006-8993(91)91486-k. [DOI] [PubMed] [Google Scholar]
- Mirshahi T, Woodward JJ. Ethanol sensitivity of heteromeric NMDA receptors: Effects of subunit assembly, glycine and NMDAR1 Mg2+-insensitive mutants. Neuropharmacology. 1995;34:347–355. doi: 10.1016/0028-3908(94)00155-l. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties for four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: Molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Martin D, Oetting TA, Lewis DV, Wilson WA, Swartzwelder HS. Ethanol and magnesium ions inhibit N-methyl-D-aspartate-mediated synaptic potentials in an interactive manner. Neuropharmacology. 1991;30:1173–1178. doi: 10.1016/0028-3908(91)90162-5. [DOI] [PubMed] [Google Scholar]
- Mueller HT, Meador-Woodruff JH. Distribution of the NMDA receptor NR3A subunit in the adult pig-tail macaque brain. J Chem Neuroanat. 2005;29(3):157–172. doi: 10.1016/j.jchemneu.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Peoples RW, White G, Lovinger DM, Weight FF. Ethanol inhibition of N-methyl-D-aspartate-activated current in mouse hippocampal neurones: whole-cell patch-clamp analysis. Br. J. Pharmacol. 1997;122:1035–1042. doi: 10.1038/sj.bjp.0701483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. The NMDA receptor subunits NR2A and NR2B show histological and ultrastructural localization patterns similar to those of NR1. J. Neurosci. 1994;14:6102–6120. doi: 10.1523/JNEUROSCI.14-10-06102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp RL, Lickteig R, Browning MD, Lovinger DM. Ethanol sensitivity and subunit composition of NMDA receptors in cultured striatal neurons. Neuropharmacology. 1998;37:45–56. doi: 10.1016/s0028-3908(97)00186-x. [DOI] [PubMed] [Google Scholar]
- Popp RL, Lickteig RL, Lovinger DM. Factors that enhance ethanol inhibition of N-methyl-D-aspartate receptors in cerebellar granule cells. J. Pharmacol. Exp. Ther. 1999;289(3):1564–1574. [PubMed] [Google Scholar]
- Sakurada K, Masu M, Nakanishi S. Alteration of Ca2+ permeability and sensitivity to Mg2+ and channel blockers by a single amino acid substitution in the N-methyl-D-aspartate receptor. J. Biol. Chem. 1993;268:410–415. [PubMed] [Google Scholar]
- Smothers CT, Woodward JJ. Effect of the NR3 subunit on ethanol inhibition of recombinant NMDA receptors. Brain Res. 2003;987(1):117–121. doi: 10.1016/s0006-8993(03)03315-8. [DOI] [PubMed] [Google Scholar]
- Smothers CT, Woodward JJ. Pharmacological characterization of glycine-activated currents in HEK 293 cells expressing N-methyl-D-aspartate NR1 and NR3 subunits. J Pharmacol Exp Ther. 2007;322(2):739–748. doi: 10.1124/jpet.107.123836. [DOI] [PubMed] [Google Scholar]
- Stern P, Cik M, Colquhoun D, Stephenson FA. Single channel properties of cloned NMDA receptors in a human cell line: Comparison with results from Xenopus oocytes. J. Physiol. (Lond.) 1994;476:391–397. doi: 10.1113/jphysiol.1994.sp020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucher NJ, Akbarian S, Chi CL, Leclerc CL, Awobuluyi M, Deitcher DL, Wu MK, Yuan JP, Jones EG, Lipton SA. Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. J. Neurosci. 1995;15:6509–6520. doi: 10.1523/JNEUROSCI.15-10-06509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb Ml. Differential sensitivity of NMDA receptor-mediated synaptic potentials to ethanol in immature versus mature hippocampus. Alcoholism: Clinical and Experimental Research. 1995;19(2):320–323. doi: 10.1111/j.1530-0277.1995.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Williams K, Russell SL, Shen YM, Molinoff PB. Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron. 1993;10:267–278. doi: 10.1016/0896-6273(93)90317-k. [DOI] [PubMed] [Google Scholar]
- Wong HK, Liu XB, Matos MF, Chan SF, Perez-Otano I, Boysen M, Cui J, Nakanishi N, Trimmer JS, Jones EG, Lipton SA, Sucher NJ. Temporal and regional expression of NMDA receptor subunit NR3A in the mammalian brain. J Comp Neurol. 2002;450(4):303–317. doi: 10.1002/cne.10314. [DOI] [PubMed] [Google Scholar]
- Woodward JJ, Gonzales RA. Ethanol inhibition of N-methyl-D-aspartate-stimulated endogenous dopamine release from rat striatal slices: Reversal by glycine. J. Neurochem. 1990;54:712–715. doi: 10.1111/j.1471-4159.1990.tb01931.x. [DOI] [PubMed] [Google Scholar]