Abstract
Context:
Growth of endometriotic lesions in rodent model of endometriosis is inhibited by resveratrol, a natural polyphenol with antiproliferative and antiinflammatory properties, and simvastatin, an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) activity.
Objective:
The objective of the investigation was to study the mechanism of action of resveratrol and its interactions with simvastatin, focusing on cholesterol biosynthesis and HMGCR gene expression and protein activity in primary cultures of human endometrial stromal (HES) cells.
Methods:
HES cells were obtained from healthy volunteers. Biosynthesis of cholesterol was assessed by measuring the conversion of [14C]acetate to [14C]cholesterol. HMGCR mRNA transcripts were quantified by real-time PCR, protein expression by Western blot analysis, and enzyme activity by measuring the conversion of [3-14C]3-hydroxy-3-methyl-glutaryl-coenzyme A to [14C]mevalonic acid lactone in HES cell microsomes.
Results:
Resveratrol inhibited cholesterol biosynthesis, HMGCR mRNA, and enzyme activity. Simvastatin inhibited cholesterol biosynthesis and enzyme activity but increased HMGCR mRNA and protein expression. Resveratrol potentiated the inhibitory effects of simvastatin on cholesterol biosynthesis and HMGCR enzyme activity and abrogated the stimulatory effects of simvastatin on HMGCR mRNA transcripts and protein expression.
Conclusions:
Resveratrol inhibits key steps of the mevalonate pathway by mechanisms that are partly complementary to and partly comparable with simvastatin via reducing both expression and activity of HMGCR. A combination of resveratrol and simvastatin may be of potential clinical relevance to development new treatments of human endometriosis.
The mevalonate pathway consists of a series of reactions starting from acetyl-coenzyme A and leading to the formation of several biologically important substances including substrates of isoprenylation [farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP)], coenzyme Q, dolichol, and cholesterol (1, 2). The rate-limiting step in the mevalonate pathway involves conversion of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) to mevalonate by HMG-CoA reductase (HMGCR). Statins are competitive inhibitors of this enzyme.
Previously we have shown that statins inhibit growth of human endometrial stromal (HES) cells, alter their cytoskeleton, and induce apoptosis (3, 4). These findings are of potential relevance to development of novel treatments of endometriosis, a condition characterized by growth of endometrial tissues outside the uterus. Endometriosis is one of the most common and debilitating disorders affecting women of reproductive age, and it contributes to chronic pelvic pain and infertility. We found that one of the statins, simvastatin, greatly reduces growth of human endometriotic implants in a nude mouse model of endometriosis (5).
However, use of statins is associated with potential adverse effects including a recently demonstrated increase in the risk of development of type 2 diabetes (6). Hence, there is an urgent need to identify new treatments of endometriosis that would either replace statins or potentiate their beneficial effects to reduce their dosage and minimize their adverse effects.
Resveratrol is a natural polyphenol and a promising nutritional supplement that has been shown to reduce insulin resistance in women; thus, resveratrol may decrease the risk of development of type 2 diabetes (7). Studies in various biological systems indicate that resveratrol has potential anticarcinogenic, antiinflammatory, antioxidant, and cardioprotective properties (8–10). Recently we have demonstrated that resveratrol, like simvastatin, is effective in reducing the number and the size of lesions established by human endometrial tissues in a nude mouse model of endometriosis (11).
In the present study, we embarked on the evaluation of the mechanisms of action of resveratrol on endometrial stromal cells, focusing on their actions on the mevalonate pathway and especially on the expression and activity of HMGCR. Herein we demonstrate for the first time that resveratrol inhibits cholesterol synthesis by reducing the expression and the activity of HMGCR in human endometrial stromal cells. We also show that resveratrol potentiates the inhibitory effects of simvastatin on the mevalonate pathway via mechanisms partly complementary and partly comparable with simvastatin.
Materials and Methods
Chemicals
Na [2–14C]acetate (50–60 Ci/mmol) and [1α, 2α (n)-3H]cholesterol (43 Ci/mmol) were purchased from Amersham Pharmacia Biotech Inc (Piscataway, New Jersey). Hydroxy-3-methylglutaryl coenzyme A, DL-3-[glutaryl-3-14C] (52.3 mCi/mmol), and collagenase B were purchased from PerkinElmer (Boston, Massachusetts). Deoxiribunuclease was obtained from Worthington Biochemical Corp (Lakewood, New Jersey), and all culture media, SDS-PAGE reagents, real-time PCR human primers for HMGCR and 18S were obtained from Life Technologies/Gibco/Applied Biosystems/Invitrogen (Pleasanton, California). Human fibronectin-coated plates were purchased from BD Biosciences (Bedford, Massachusetts), and HMG-CoA reductase polyclonal antibody was obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, California); goat antirabbit IRDye 800 and goat antimouse IRDye 680 were purchased from LI-COR Biosciences (Lincoln, Nebraska). Simvastatin, resveratrol, and other chemicals and supplies were obtained from Sigma-Aldrich (St Louis, Missouri).
Human endometrial tissues
Human endometrial biopsies were obtained from 8 patients undergoing surgeries for benign gynecological conditions or healthy volunteers (aged 25–40 years). None of the subjects was using oral contraceptive pills or any other hormonal treatment for at least 3 months before endometrial biopsy. This study was approved by the institutional review board for the protection of human subjects from the University of California, Davis, and written consent was obtained from all subjects.
Cell culture and treatments
Biopsies were collected in phenol-red Hanks' balanced salt solution (Life Technologies/Gibco/Applied Biosystems/Invitrogen) and maintained at 4°C during transport. The HES cells were isolated as previously described (4). Briefly, excised tissue was minced (12), digested with collagenase B/deoxiribunuclease I, and passed through a 70-μm strainer (Sigma) followed by plating cells in T75 flasks and cultured at 37°C in humidified air and 5% carbon dioxide in phenol red-free DMEM/F12 with 1% antibiotic, 10% charcoal-dextran stripped fetal bovine serum, and 1 nM estradiol. The cells were then trypsinized, counted, and transferred to fibronectin-coated plates. Subsequently the culture media were changed to phenol red-free and serum-free DMEM/F12 supplemented with 1% antibiotic and 1 nM estradiol for 24 hours followed by the addition of treatments. The cells were incubated without (control) or with resveratrol (30–100 μM), simvastatin (0.1–10 μM), and their combination for 24–48 hours. The concentrations of these compounds were selected based on our previous studies (11, 12), and the above-mentioned time points were chosen based on our previous work evaluating invasiveness of HES cells (11).
Cholesterol synthesis assay
Cholesterol biosynthesis by HES cells was assessed in vitro by measuring the conversion of [14C]acetate to [14C]cholesterol in the presence of resveratrol (30–100 μM), simvastatin (0.1–10 μM), or their combination (resveratrol 30 μM + simvastatin 0.1 μM). The reaction was terminated after 24 hours of treatment by removing the medium and adding 0.25 mL of aqueous 50% KOH and 0.5 mL of ethanol containing approximately 100 000 cpm of [3H]cholesterol as an internal standard to correct for recovery during lipid extraction (13, 14). Then 50 μL of cell lysate was set aside for the protein assay, and the remaining aqueous ethanolic mixture was heated at 70°C for 1 hour under an atmosphere of N2. After cooling, 0.5 mL of distilled water and 50 μg of cholesterol in 25 μL of ethanol (2 μg/μL) were added (to aid in the detection of chromatographed cholesterol), and the alkaline solution was extracted 3 times with 3 mL of hexane containing 0.05% butylated hydroxytoluene. The upper hexane/lipid phases were transferred to a new tube and washed once with 2 mL of 0.1 N NaOH and once more with 2 mL of distilled water. The upper hexane fractions containing the nonsaponifiable lipids were transferred to a new tube and evaporated under a stream of N2 at 40°C, reconstituted with 120 μL of hexane/0.05% hydroxytoluene solution, and then separated by thin-layer chromatography (TLC) in benzene-ethyl acetate (9:1) on prewashed TLC plates. Cholesterol bands were identified by using 50 μg cholesterol and visualized by primuline under UV light. Afterward, individual bands were cut and transferred into scintillation vials for radioactivity measurements of [14C]cholesterol and [3H]cholesterol.
HMGCR activity assay
HMGCR activity was determined on purified HES cell microsomes by measuring the conversion of [3-14C]HMG-CoA to [14C]mevalonic acid lactone, as described by Wilce and Kroon (15) with slight modifications. Briefly, the reaction mixture containing 30–45 μg of microsomal protein normalized to 40 μL were preincubated without (control), with resveratrol (30 μM) or simvastatin (0.1 μM) and their combination, plus 10 μL of 25 mM nicotinamide adenine dinucleotide phosphate, 200 mM glucose-6-phosphate, 40 μL of 200 mM potassium phosphate (pH 7.4), and 1 μL (1 U/μL) of glucose-6-phosphate dehydrogenase for 15 minutes at 37°C. Then 11 μL of substrate (500 μL of 10 μCi [3-14C] HMG-CoA containing 46 μL of 2.5 mg/mL cold HMG-CoA) was added to initiate the reaction and incubated further for 60 minutes at 37°C. The reaction was stopped by adding 10 μL of 5 M HCl followed by the addition of 1 μL (1 mg/μL) of mevalonic acid lactone to serve as the internal standard and incubated for an additional 30 minutes at 37°C. The samples were centrifuged at 21 000 × g for 5 minutes, and 75 μL of the supernatant fraction was separated by TLC in chloroform-acetone (1:1) (Merck, Rahway, New Jersey). After drying the TLC plates, mevalonic acid lactone bands were visualized by iodine vapor staining, the bands were scraped into scintillation vials, and the radioactivity was determined using a scintillation counter.
Total RNA isolation, cDNA synthesis, and quantitative real-time PCR
Total RNA was isolated from HES cells using the MagMAX-96 total RNA isolation kit (Applied Biosystems, Foster City, California) and the KingFisher robot (Thermo Scientific, Vantaa, Finland). The nucleic acid concentration and purity was assessed using NanoDrop 1000 (Thermo Scientific, Pittsburgh, Pennsylvania) and first-strand cDNA synthesized from 100 ng of total RNA using the SuperScript III reverse transcriptase (Invitrogen, Carlsbad, California). Quantitative real-time PCRs were performed in triplicate using the ABI 7300 real-time PCR system (Applied Biosystems). The human primer sequences were as follows: HMGCR sense, 5′-GTCATTCCAGCCAAGGTTGT-3′ and antisense, 5′-GGGACCACTTGCTTCCATTA-3′; 18S sense, AAATAGCCTTTGCCATCACTG and antisense, ACGTTCCACCTCATCCTC. In addition, the following proprietary primers were obtained from QIAGEN (Germantown, Maryland): 3-hydroxy-3-methylglutaryl-coenzyme A lyase: (HMGCL), farnesyl pyrophosphate synthase (FPPS), squalene synthase (SS), and cholesterol 7α-monooxygenase (CH7AM). Proprietary primer for farnesyl pyrophosphate transferase (FPPT) was obtained from GeneCopoeia, Inc (Rockville, Maryland). Relative concentrations of mRNA of interest were normalized to 18S.
Western blot analysis
After 48 hours of treatments, HES cells were trypsinized, collected by centrifugation, and processed as previously described (4). Briefly, pelleted cells were resuspended in lysis buffer (Invitrogen) containing protease inhibitor cocktail (Roche, Indianapolis, Indiana) and sonicated twice for 30 seconds. The cell lysates were centrifuged at 13 000 rpm for 10 minutes at 4°C, and the supernatant fraction was collected and stored at −80°C. Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad, Hercules, California), following the manufacturer's instructions.
For SDS-PAGE analysis, 100 μg of protein was resolved in 12% sodium dodecyl sulfate-polyacrylamide gels and transferred to a nitrocellulose membrane (LI-COR). The blot containing the transferred protein was blocked with Odyssey blocking buffer (LI-COR Biosciences) for 1 hour at room temperature, followed by incubation with the HMGCR primary antibody (1:200) for 24 hours at 4°C and β-actin antibody (1:40 000) for 1 hour at room temperature. Membranes were washed 4 times (5 minutes each) in PBS + 0.05% Tween-20, followed by incubation with 680 and 800 infrared dye-labeled secondary antibodies (1:20 000). Blots were developed with the Odyssey infrared imaging system (LI-COR Biosciences). Band intensities were quantified using the Odyssey version 3.0 software (LI-COR Biosciences) and normalized to β-actin.
Statistical analysis
Group values were expressed as mean ± SEM of percentage control. Significant differences (P < .05) between groups were determined using JMP 9.0 software (SAS, Cary, North Carolina), by one-way ANOVA followed by post hoc pairwise comparisons of individual means. Normality of distribution was assessed by the Shapiro-Wilk W test. In the absence of normality, data were logarithmically transformed, and/or nonparametric testing (Kruskal-Wallis) was used when appropriate.
Results
Effects of simvastatin and resveratrol on cholesterol biosynthesis
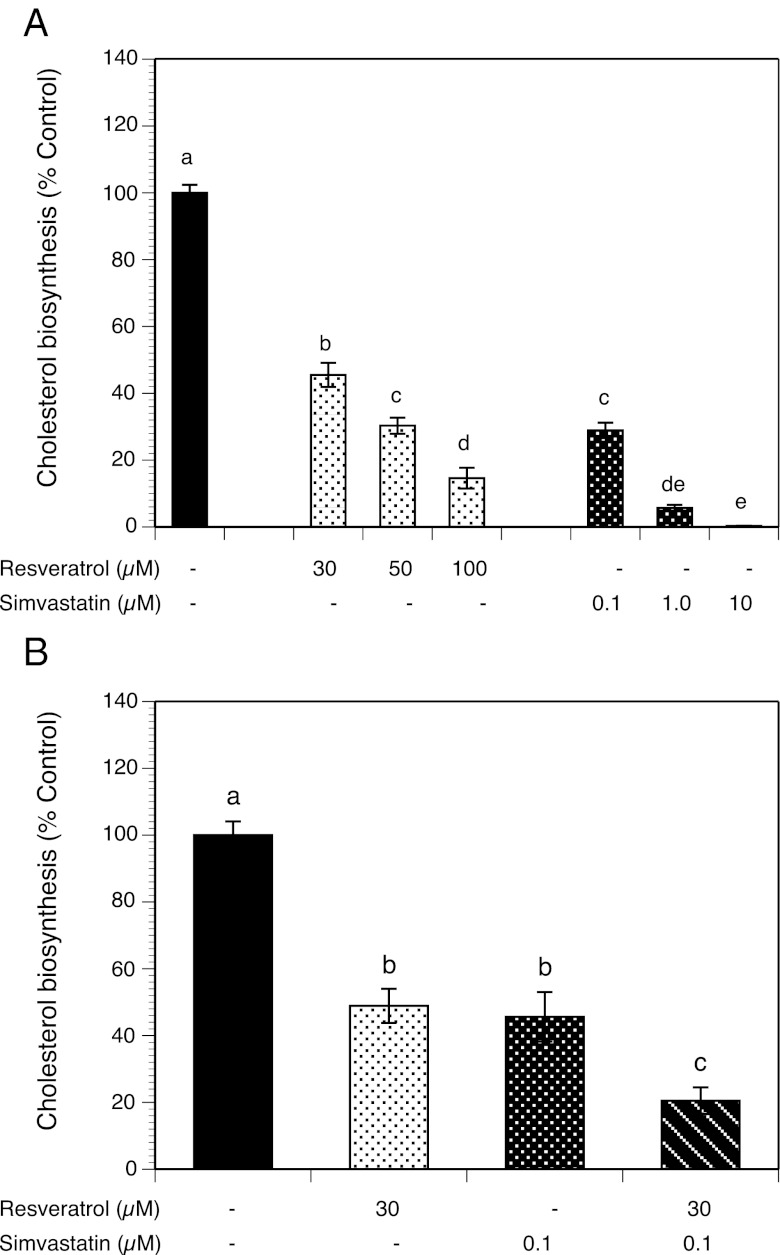
To determine the effects of simvastatin and resveratrol on cholesterol biosynthesis, HES cells were incubated with different concentrations of resveratrol (30–100 μM) or simvastatin (0.1–10 μM), and the conversion of [14C]acetate substrate to [14C]cholesterol was quantified. As illustrated in Figure 1A, resveratrol and simvastatin, each, induced a concentration-dependent inhibition after 24 hours of treatment compared with control. Simvastatin at concentrations of 0.1, 1, and 10 μM reduced cholesterol biosynthesis, respectively, by 71.1%, 94.3%, and 99.7%, (all at P < .001). Resveratrol at 30, 50, and 100 μM inhibited cholesterol biosynthesis, respectively, by 54.5%, 69.7%, and 85.4%, (all at P < .001), respectively. To determine the interactive effects of simvastatin and resveratrol, HES cells were treated without (control) or with simvastatin (0.1 μM) and resveratrol (30 μM). As shown in Figure 1B, resveratrol and simvastatin each independently inhibited cholesterol biosynthesis by about 50%, whereas in combination cholesterol biosynthesis was decreased by 80%. Thus, the combinatorial treatment with simvastatin and resveratrol led to a significant decrease compared with either treatment alone (P < .0005 and P = .0017, respectively).
Figure 1.
Effects of resveratrol and simvastatin on cholesterol biosynthesis. HES cells were cultured with 1 nM estradiol alone (control), with 30–100 μM resveratrol, or 0.1–10 μM simvastatin in the presence of [14C]acetate. Conversion of [14C]acetate to [14C]cholesterol was assessed after 24 hours of incubation. A, Dose responses to resveratrol and simvastatin. B, Effects of resveratrol and simvastatin alone and in combination. Each bar represents data from three different patients (4 replicates each) expressed as mean ± SEM. Means with no superscripts in common are significantly different (P < .001).
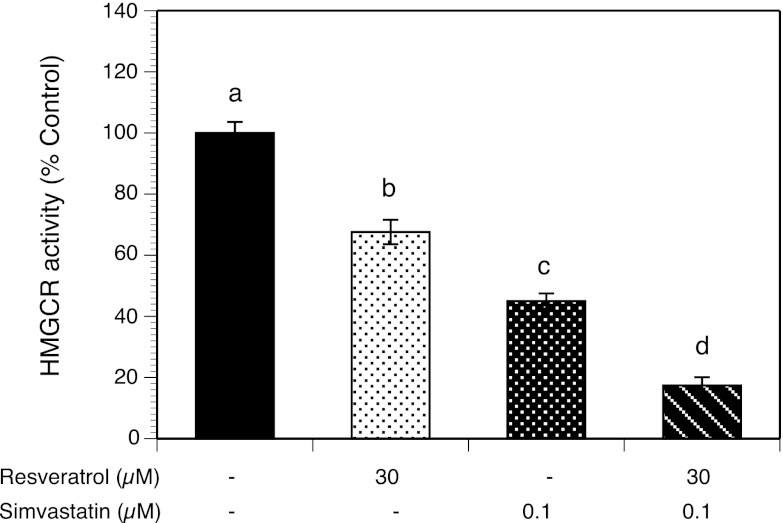
Synergistic effects of simvastatin and resveratrol on HMGCR enzyme activity
To determine the independent and interactive properties of simvastatin (0.1 μM) and resveratrol (30 μM) on HMGCR activity, we measured the conversion of [3–14C]HMG CoA to mevalonic acid in HES cell microsomes. As shown in Figure 2, resveratrol alone decreased activity by 32.4% (P < .0001), whereas simvastatin alone induced a decrease of 45.0% (P < .0001). A combination of resveratrol and simvastatin led to a greater inhibition than each agent alone decreasing HMGCR activity by 82.6% (P < .001).
Figure 2.
Effects of resveratrol and simvastatin on HMGCR enzymatic activity. Purified HES cell microsomes were incubated without additives (control), with 30 μM resveratrol alone, with 0.1 μM simvastatin, or with a combination of resveratrol and simvastatin in the presence of [3–14C]HMG CoA. Conversion to [14C]mevalonic acid was measured after 60 minutes of incubation at 37°C. Each bar represents data from 3 different patients (4 replicates each) expressed as mean ± SEM. Means with no superscripts in common are significantly different (P < .0001).
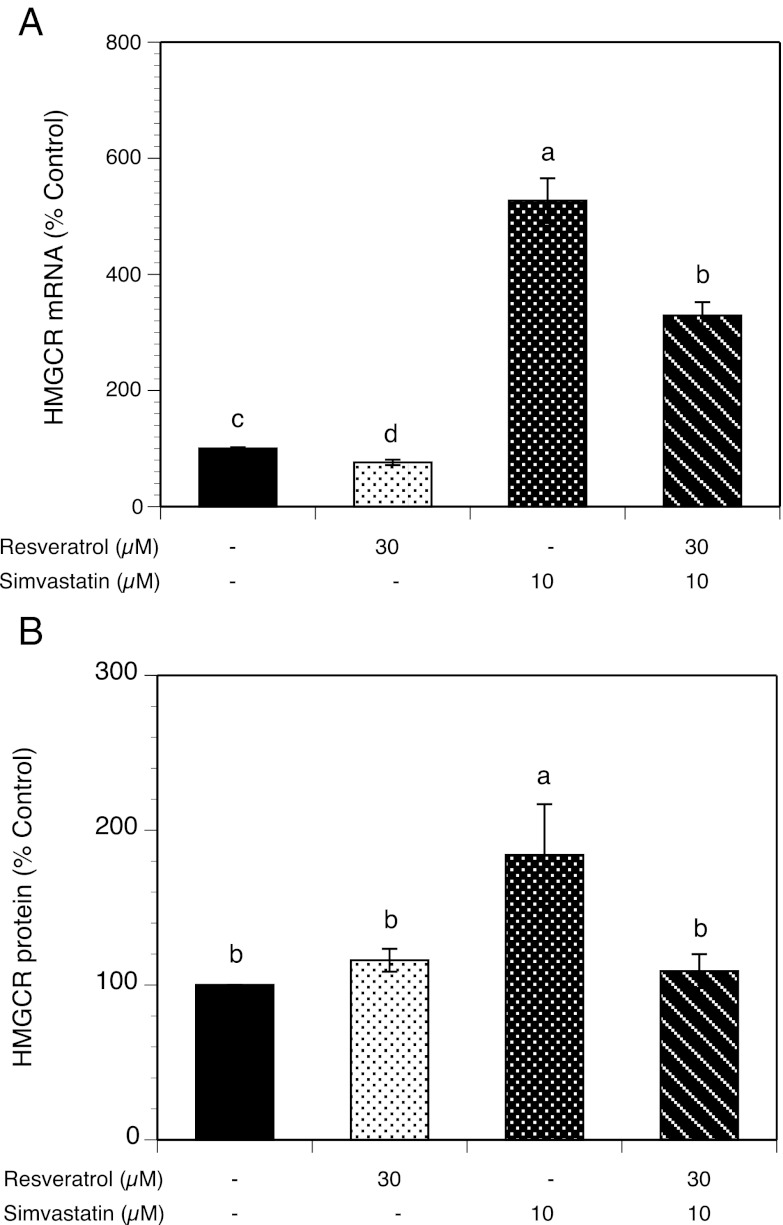
Effects of simvastatin and resveratrol on HMGCR mRNA and protein expression
To further study the molecular mechanism by which resveratrol and simvastatin inhibit cholesterol biosynthesis in HES cells, we determined HMGCR mRNA transcripts by quantitative real-time PCR and protein expression by Western blot analysis in the absence (control) or the presence of resveratrol (30 μM), simvastatin (10 μM), and their combination. As illustrated in Figure 3A, after 48 hours of treatment, resveratrol decreased mRNA expression by 24% (P < .02), whereas simvastatin increased transcripts by 5-fold (P < .0001). Resveratrol partly abrogated the effects of simvastatin by decreasing mRNA expression by 2-fold compared with simvastatin alone (P < .0002). Similar to HMGCR transcript concentration data, Figure 3B shows that simvastatin (10 μM) induced a 2-fold increased in HMGCR protein expression in HES cells (P < .005), whereas no significant effect was observed after exposure of cells to 30 μM resveratrol alone. Addition of resveratrol abrogated the effect of simvastatin and HMGCR protein expression was reduced to normal level (P < .05).
Figure 3.
Effects of resveratrol and simvastatin on HMGCR mRNA expression (A) and protein level (B). HES cells were cultured with 1 nM estradiol alone (control) or with 30 μM resveratrol, 10 μM simvastatin, or a combination of resveratrol and simvastatin. HMGCR mRNA transcripts normalized to 18S expression were quantified by real-time PCR after 48 hours of incubation. HMGCR protein normalized to β-actin expression was determined by Western blot analysis after 48 hours of incubation. Each bar represents data from 3 different patients (4 replicates each) expressed as mean ± SEM. Means without superscripts in common are significantly different (P < .02).
Effects of simvastatin and resveratrol on mRNA expression of other genes relevant to the mevalonate pathway
To evaluate the expression of other enzymes affecting the mevalonate pathway, additional determinations were made to assess the effects of 48 hours of treatments on HMGCL (conversion of 3-hydroxy-3-methylglutaryl-coenzyme A to acetyl-coenzyme A and acetoacetate), FPPS (conversion of geranyl pyrophosphate to farnesyl pyrophosphate), FPPT (farnesylation of proteins), SS (squalene synthesis), and CH7AM (catabolism of cholesterol). As presented in Table 1, simvastatin significantly up-regulated mRNA expression of FPPS and SS but had no effect on HMGCL and FPPT. Resveratrol alone had no significant effect, but it induced partial reversal of the stimulatory effects of simvastatin on FPPS and SS. Expression of mRNA for CH7AM was undetectable in all experiments and all treatments.
Table 1.
Evaluation of mRNA Expression of Selected Genes Affecting the Mevalonate Pathway
| Gene Expression | Treatment |
P Value | |||
|---|---|---|---|---|---|
| Control | Resveratrol (30 μM) | Simvastatin (10 μM) | Resveratrol (30 μM) + Simvastatin (10 μM) | ||
| HMGCL | 100 ± 17a | 94 ± 2a | 122 ± 8a | 109 ± 4a | .09 |
| FPPS | 100 ± 5a | 83 ± 5a | 367 ± 50c | 232 ± 42b | <.001 |
| FPPT | 100 ± 7a,b | 83 ± 4a | 118 ± 5b,c | 131 ± 5c | <.001 |
| SS | 100 ± 4a,b | 82 ± 7a | 324 ± 60c | 205 ± 50b | <.001 |
Individual values represent relative amounts of mRNA transcripts normalized to 18S expression and are presented as a percentage of controls (means ± SEM) from 3 separate experiments. Means with no superscripts in common are significantly different (P < .05).
Discussion
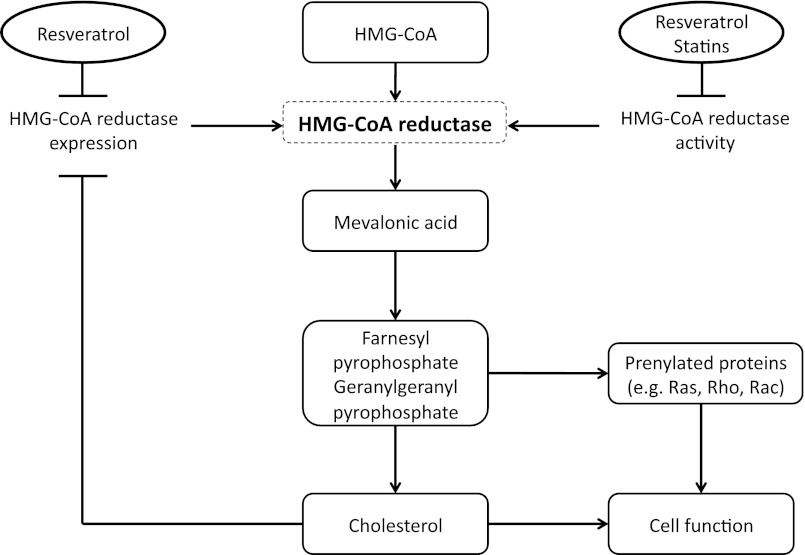
This study demonstrates for the first time that resveratrol inhibits cholesterol biosynthesis in HES cells in a dose-dependent fashion by 2 independent mechanisms: inhibition of HMGCR mRNA transcripts and a decrease of HMGCR enzymatic activity (Figure 4). The effects of resveratrol on enzymatic activity of HMCGR are independent of the effects on HMCGR expression because the assay of enzymatic activity evaluated acute effects of resveratrol added to a microsomal fraction of HES cells. We also demonstrate that simvastatin, a competitive inhibitor of HMGCR, decreases this enzyme's activity, but stimulates mRNA and protein expression, an observation consistent with statin-induced reduction of the negative feedback of products of the mevalonate pathway on HMGCR expression (16–19). Furthermore, we found that resveratrol potentiates the inhibitory effects of simvastatin on cholesterol biosynthesis and HMGCR activity and abrogates the stimulatory effects of simvastatin on HMGCR mRNA and protein expression.
Figure 4.
Proposed interactions of resveratrol and simvastatin in modulation of HMG CoA reductase gene expression and activity. Resveratrol inhibits HMGCR mRNA expression, whereas both resveratrol and simvastatin inhibit enzyme activity. HMGCR is a rate-limiting step of mevalonate pathway, and its products include isoprenoids: FPP and GGPP, which are required for isoprenylation of proteins involved in signal transduction pathways regulating cell proliferation, apoptosis, adhesiveness, invasiveness, and maintenance of cellular functions. Products of the mevalonate pathway exert a negative feedback on HMGCR expression.
These observations are comparable with our recent demonstration of resveratrol-induced inhibition of HMGCR in rat theca-interstitial cells (20), indicating that these effects occur in diverse tissues and in different species. Consistent with these findings, in vivo studies using hamsters fed high-fat diets show that resveratrol supplementation decreases serum cholesterol, hepatic HMGCR mRNA, and activity (21). Similarly, in apolipoprotein E-deficient mice, which exhibit excessive plasma cholesterol, even when maintained on a normal diet, resveratrol supplementation decreases plasma cholesterol and hepatic HMGCR activity (22). Mice fed high-fat diets supplemented with resveratrol had decreased fatty liver and organ pathology, increased the number of mitochondria and greater AMP-activated protein kinase (AMPK) activity, and improved insulin sensitivity (23). Other studies demonstrate that resveratrol exposure inhibits adipogenesis and diminishes lipid accumulation (24–26). In contrast, AMPK-deficient mice are resistant to the metabolic effects of resveratrol (27). Hence, it appears that the AMPK signaling pathway may play a role in mediation of the above actions of resveratrol. Indeed, resveratrol has been shown to be an effective activator of this pathway (28). Furthermore, in other studies, it has been demonstrated that AMPK induces reversible phosphorylation and deactivation of HMGCR (29, 30). Taken together, these data suggest that the increase in AMPK activity by resveratrol may lead to the down-regulation of HMGCR activity and thus contribute to decreased levels of products of the mevalonate pathway, including cholesterol.
Regulation of the mevalonate pathway involves compensatory mechanisms, whereby decreased levels of mevalonate and cholesterol induce a feedback up-regulation of gene transcription and mRNA translation of HMGCR (18, 19). Indeed, the effects of statins are partially blunted by this feedback, and as shown in the present study, simvastatin exposure of HES cells results in an increase of HMGCR transcription and protein expression. In contrast, resveratrol supplementation, despite inhibition of the mevalonate pathway, does not induce a compensatory increase of HMGCR expression; furthermore, resveratrol counteracts the stimulatory effects of simvastatin on HMGCR both at the level of mRNA and protein. This unique activity of resveratrol is likely an important contributor to the observed potentiation of inhibitory effects of simvastatin on the mevalonate pathway. Inhibition of the mevalonate pathway leads to reduction in the levels of its intermediate products such as substrates for protein isoprenylation: FPP and GGPP, which play an important role in the regulation of HES cell proliferation, apoptosis, motility, adhesiveness, and invasiveness (4, 12).
In parallel to the effects on mRNA expression of HGMCR, we also observed that simvastatin up-regulated mRNA expression of 2 other genes involved in the mevalonate pathway: FPPS and SS. These effects were partly abrogated by resveratrol, suggesting that the inhibition of cholesterol synthesis by a combination of simvastatin and resveratrol may be partly due to modulation on expression of genes encoding enzymes regulating intermediate steps of the mevalonate pathway.
The present findings, demonstrating additive effects of resveratrol and simvastatin on the inhibition of the mevalonate pathway in HES cells, are of potential clinical relevance in view of the growing evidence that statins may provide an effective treatment for endometriosis. Endometriosis is characterized by ectopic attachment, growth, and invasiveness of endometrial cells in association with inflammation and increased oxidative stress (31). Statins appear to affect each of these processes (12). Antiproliferative and proapoptotic actions of statins on HES cells have been demonstrated. We have shown that mevastatin and simvastatin inhibit synthesis of DNA and decrease the number of viable HES cells (3). In other studies, lovastatin reduced growth in an in vitro model of endometriosis, whereas simvastatin decreased proliferation of cells obtained from endometriomas (32, 33). Furthermore, statins exhibited both antiinflammatory and immunomodulatory properties, decreasing mediators and markers of inflammation (ie, C-reactive protein, TNF-α, interleukins, and monocyte chemoattractant protein-1) (34–36). However, use of statins, especially at higher doses, is associated with significant adverse effects and risks, including rhabdomyolysis and the recently demonstrated increase in type 2 diabetes mellitus (6).
In contrast to adverse effects of statins on glucose metabolism, resveratrol lowers plasma insulin and normalizes hyperglycemia in streptozotocin-nicotinamide-induced experimental diabetic rats (37). More importantly, recent clinical trials indicate that resveratrol improves insulin sensitivity and reduces oxidative stress in type 2 diabetic patients (38) and improves insulin sensitivity and postprandial plasma glucose in subjects with impaired glucose tolerance (7). Consequently, the present observations provide support to consideration for the use of resveratrol in conjunction with statins to attain effective inhibition of the mevalonate pathway and hopefully reduces at least some of the risks associated with the use of statins. The addition of resveratrol may also facilitate lowering the dose of statin and therefore potentially further reducing side effects and risks. These concepts will require validation from in vivo studies. Furthermore, the present observations may be relevant to disorders other than endometriosis in which the use of statins may benefit from the addition of resveratrol.
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through Cooperative Agreement U54 HD052668 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Disclosure Summary: A.J.D. received grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (April 1, 2007, to the present).The other authors have declared that no conflict of interest exists.
Footnotes
- AMPK
- AMP-activated protein kinase
- CH7AM
- cholesterol 7α-monooxygenase
- FPP
- farnesyl pyrophosphate
- FPPS
- FPP synthase
- FPPT
- FPP transferase
- GGPP
- geranylgeranyl pyrophosphate
- HES
- human endometrial stromal
- HMGCL
- 3-hydroxy-3-methylglutaryl-coenzyme A lyase
- HMG-CoA
- 3-hydroxy-3-methyl-glutaryl-coenzyme A
- HMGCR
- HMG-CoA reductase
- SS
- squalene synthase
- TLC
- thin-layer chromatography.
References
- 1. Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430 [DOI] [PubMed] [Google Scholar]
- 2. Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–199 [DOI] [PubMed] [Google Scholar]
- 3. Piotrowski PC, Kwintkiewicz J, Rzepczynska IJ, et al. Statins inhibit growth of human endometrial stromal cells independently of cholesterol availability. Biol Reprod. 2006;75:107–111 [DOI] [PubMed] [Google Scholar]
- 4. Sokalska A, Wong DH, Cress A, et al. Simvastatin induces apoptosis and alters cytoskeleton in endometrial stromal cells. J Clin Endocrinol Metab. 2010;95:3453–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruner-Tran KL, Osteen KG, Duleba AJ. Simvastatin protects against the development of endometriosis in a nude mouse model. J Clin Endocrinol Metab. 2009;94:2489–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women's Health Initiative. Arch Intern Med. 2012;172:144–152 [DOI] [PubMed] [Google Scholar]
- 7. Crandall JP, Oram V, Trandafirescu G, et al. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci 2012;67(12):1307–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang H, Zhang L, Kuo J, et al. Resveratrol-induced apoptotic death in human U251 glioma cells. Mol Cancer Ther. 2005;4:554–561 [DOI] [PubMed] [Google Scholar]
- 9. Kundu JK, Shin YK, Kim SH, Surh YJ. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-κB in mouse skin by blocking IκB kinase activity. Carcinogenesis. 2006;27:1465–1474 [DOI] [PubMed] [Google Scholar]
- 10. Sharma S, Chopra K, Kulkarni SK, Agrewala JN. Resveratrol and curcumin suppress immune response through CD28/CTLA-4 and CD80 co-stimulatory pathway. Clin Exp Immunol. 2007;147:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bruner-Tran KL, Osteen KG, Taylor HS, Sokalska A, Haines K, Duleba AJ. Resveratrol inhibits development of experimental endometriosis in vivo and reduces endometrial stromal cell invasiveness in vitro. Biol Reprod. 2011;84:106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sokalska A, Cress A, Bruner-Tran KL, et al. Simvastatin decreases invasiveness of human endometrial stromal cells. Biol Reprod. 2012;87:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ho YK, Faust JR, Bilheimer DW, Brown MS, Goldstein JL. Regulation of cholesterol synthesis by low density lipoprotein in isolated human lymphocytes. Comparison of cells from normal subjects and patients with homozygous familial hypercholesterolemia and abetalipoproteinemia. J Exp Med. 1977;145:1531–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Popjak G, Clarke CF, Hadley C, Meenan A. Role of mevalonate in regulation of cholesterol synthesis and 3-hydroxy-3-methylglutaryl coenzyme A reductase in cultured cells and their cytoplasts. J Lipid Res. 1985;26:831–841 [PubMed] [Google Scholar]
- 15. Wilce PA, Kroon PA. Assay of 3-hydroxy-3-methylglutaryl coenzyme A reductase. In: Converse CA, Skinner ER, eds. Lipoprotein analysis—a practical approach. Oxford, UK: I.R.L. Press; 1992:203–214 [Google Scholar]
- 16. Bergstrom JD, Bostedor RG, Rew DJ, Geissler WM, Wright SD, Chao YS. Hepatic responses to inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase: a comparison of atorvastatin and simvastatin. Biochim Biophys Acta. 1998;1389:213–221 [DOI] [PubMed] [Google Scholar]
- 17. Conde K, Roy S, Freake HC, Newton RS, Fernandez ML. Atorvastatin and simvastatin have distinct effects on hydroxy methylglutaryl-CoA reductase activity and mRNA abundance in the guinea pig. Lipids. 1999;34:1327–1332 [DOI] [PubMed] [Google Scholar]
- 18. Edwards PA, Lan SF, Tanaka RD, Fogelman AM. Mevalonolactone inhibits the rate of synthesis and enhances the rate of degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in rat hepatocytes. J Biol Chem. 1983;258:7272–7275 [PubMed] [Google Scholar]
- 19. Nakanishi M, Goldstein JL, Brown MS. Multivalent control of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mevalonate-derived product inhibits translation of mRNA and accelerates degradation of enzyme. J Biol Chem. 1988;263:8929–8937 [PubMed] [Google Scholar]
- 20. Wong DH, Villanueva JA, Cress AB, Sokalska A, Ortega I, Duleba AJ. Resveratrol inhibits the mevalonate pathway and potentiates the antiproliferative effects of simvastatin in rat theca-interstitial cells. Fertil Steril. 2011;96:1252–1258 [DOI] [PubMed] [Google Scholar]
- 21. Cho IJ, Ahn JY, Kim S, Choi MS, Ha TY. Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem Biophys Res Commun. 2008;367:190–194 [DOI] [PubMed] [Google Scholar]
- 22. Do GM, Kwon EY, Kim HJ, et al. Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem Biophys Res Commun. 2008;374:55–59 [DOI] [PubMed] [Google Scholar]
- 23. Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rayalam S, Della-Fera MA, Yang JY, Park HJ, Ambati S, Baile CA. Resveratrol potentiates genistein's antiadipogenic and proapoptotic effects in 3T3-L1 adipocytes. J Nutr. 2007;137:2668–2673 [DOI] [PubMed] [Google Scholar]
- 25. Park HJ, Yang JY, Ambati S, et al. Combined effects of genistein, quercetin, and resveratrol in human and 3T3-L1 adipocytes. J Med Food. 2008;11:773–783 [DOI] [PubMed] [Google Scholar]
- 26. Szkudelska K, Szkudelski T. Resveratrol, obesity and diabetes. Eur J Pharmacol. 635:1–8 [DOI] [PubMed] [Google Scholar]
- 27. Um JH, Park SJ, Kang H, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 59:554–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ajmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G833–G842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clarke PR, Hardie DG. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990;9:2439–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gibson DM, Parker RA, Stewart CS, Evenson KJ. Short-term regulation of hydroxymethylglutaryl coenzyme A reductase by reversible phosphorylation: modulation of reductase phosphatase in rat hepatocytes. Adv Enzyme Regul. 1982;20:263–283 [DOI] [PubMed] [Google Scholar]
- 31. Santanam N, Murphy AA, Parthasarathy S. Macrophages, oxidation, and endometriosis. Ann NY Acad Sci. 2002;955:183–198; discussion 119–200, 396–406 [DOI] [PubMed] [Google Scholar]
- 32. Esfandiari N, Khazaei M, Ai J, et al. Effect of a statin on an in vitro model of endometriosis. Fertil Steril. 2007;87:257–262 [DOI] [PubMed] [Google Scholar]
- 33. Nasu K, Yuge A, Tsuno A, Narahara H. Simvastatin inhibits the proliferation and the contractility of human endometriotic stromal cells: a promising agent for the treatment of endometriosis. Fertil Steril. 2009;92:2097–2099 [DOI] [PubMed] [Google Scholar]
- 34. Ando H, Takamura T, Ota T, Nagai Y, Kobayashi K. Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. J Pharmacol Exp Ther. 2000;294:1043–1046 [PubMed] [Google Scholar]
- 35. Shishehbor MH, Brennan ML, Aviles RJ, et al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108:426–431 [DOI] [PubMed] [Google Scholar]
- 36. Dje N'Guessan P, Riediger F, Vardarova K, et al. Statins control oxidized LDL-mediated histone modifications and gene expression in cultured human endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29:380–386 [DOI] [PubMed] [Google Scholar]
- 37. Palsamy P, Subramanian S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed Pharmacother. 2008;62:598–605 [DOI] [PubMed] [Google Scholar]
- 38. Brasnyo P, Molnar GA, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 106:383–389 [DOI] [PubMed] [Google Scholar]