Abstract
Context:
The concentration of intratesticular testosterone (IT-T) required for human spermatogenesis is unknown because spermatogenesis can persist despite the markedly reduced IT-T concentrations observed with LH suppression. Methods to lower IT-T further are needed to determine the relationship between IT-T and spermatogenesis.
Objective:
The objective of the study was to determine the effect of inhibiting the synthesis and metabolism of testosterone (T) on IT-T in gonadotropin-suppressed human testes.
Design/Setting/Patients:
Forty normal men participated in a blinded, placebo-controlled, randomized trial at an academic center.
Intervention/Outcome Measures:
All men were first administered the GnRH antagonist acyline to suppress LH. Forty-eight hours after acyline administration, subjects were randomly assigned to placebo, ketoconazole (to inhibit T synthesis) at 400 or 800 mg, dutasteride (to inhibit T metabolism) 2.5 mg, or anastrazole (to inhibit T metabolism) 1 mg, daily for 7 days (n = 8/group). Intratesticular steroid concentrations were measured 48 hours after acyline administration alone and again after 7 days of combination treatment.
Results:
After 7 days of combination treatment, the median IT-T (25th, 75th percentile) in the placebo group was 14 (8.0, 21.2) ng/mL. IT-T was reduced to 3.7 (2.5, 7.1) ng/mL in the ketoconazole 400 mg group and 1.7 (0.8, 4.0) ng/mL in the ketoconazole 800 mg group (P < .001 vs placebo for both comparisons). IT-T concentrations in the dutasteride and anastrazole groups were similar to placebo.
Conclusion:
Combining inhibition of steroidogenesis with gonadotropin suppression lowers IT-T more than gonadotropin suppression alone. This combination might be useful to determine the minimum IT-T concentration necessary for human spermatogenesis, information essential for developing male hormonal contraceptives.
Male hormonal contraceptive strategies, which rely on the administration of exogenous testosterone (T) to suppress the secretion of pituitary gonadotropins, achieve azoospermia in 60%–70% of men (1, 2). Combinations of progestins and T further suppress gonadotropins and achieve azoospermia in up to 90% of men. Nevertheless, some men fail to completely suppress spermatogenesis on these regimens (3–5). Understanding why some men fail to completely suppress spermatogenesis despite profound inhibition of gonadotropin secretion is a significant barrier to male hormonal contraceptive development.
One possible explanation for this failure is that some men on male hormonal contraceptives continue to have low concentrations of intratesticular (IT) testosterone that are permissive for spermatogenesis (6). The LH receptor knockout mouse may be a useful model for understanding this phenomenon because these animals produce functional sperm despite an absence of LH signaling (7). Interestingly, spermatogenesis in these animals can be completely abrogated by the addition of the androgen receptor antagonist flutamide, suggesting that the process is androgen dependent. Similarly, in the rat quantitatively and qualitatively normal spermatogenesis can occur despite dramatic reductions in IT-T (8). In male hormonal contraceptive studies, some men continue to maintain spermatogenesis despite profound gonadotropin suppression, which decreases IT-T by 95% (6). In these men, IT-T still exceeds serum T concentrations. As a result, the quantitative relationship between low concentrations of IT-T and spermatogenesis in humans has not been defined. From the standpoint of the development of male hormonal contraceptives, it is possible that greater reductions in IT-T may result in more consistent suppression of spermatogenesis and higher rates of azoospermia.
In addition to IT-T, intratesticular dihydrotestosterone (DHT) and estradiol (E2) may play a role in the maintenance of spermatogenesis in the low IT-T environment created by male hormonal contraceptive regimens (6, 9). For example, some men have a dramatic drop in sperm concentrations associated with the chronic administration of 5α-reductase inhibitors such as finasteride and dutasteride (10), suggesting that spermatogenesis in some men might depend on intratesticular DHT. Conversely, aromatase inhibitors that block the metabolism of T to E2 have been used for the treatment of male infertility (11–13), and high serum E2 concentrations have been associated with suppression of gonadotropins and spermatogenesis (14). Establishing a method for suppressing DHT and E2 concentrations within the testis could lead to a greater understanding of the differential roles of these hormones on spermatogenesis in men and might help in the development of more effective male hormonal contraceptives.
In our previous work, we have examined the effect of gonadotropin suppression on IT-T using the administration of acyline, a GnRH antagonist (15), coupled with testicular fine-needle aspiration to obtain intratesticular fluid for the measurement of IT-T (16–19). Acyline administration alone decreases IT-T from a median of 715 ng/mL (more than 100 times serum concentrations of testosterone) to 22 ng/mL, a 95%–97% reduction from baseline concentrations (19). In this study, we hypothesized that in the presence of profound gonadotropin suppression (induced by acyline), inhibition of testosterone synthesis with ketoconazole, an inhibitor of the 17,20 lyase enzyme, (20, 21) would suppress, and inhibition of the metabolism of testosterone, with the 5α-reductase inhibitor dutasteride or the aromatase inhibitor anastrozole, would increase IT-T, relative to gonadotropin suppression alone. Therefore, we conducted a prospective, randomized, blinded, placebo-controlled, 5-arm interventional study in normal men of the GnRH antagonist acyline alone or in combination with ketoconazole (at 2 doses), dutasteride, or anastrazole to determine the effect of these medications on the concentration of IT-T and other intratesticular steroids.
Subjects and Methods
Subjects
Healthy men, aged 18–50 years were recruited for this study using newspaper and online advertisements. All subjects provided written informed consent prior to the screening evaluation. Subjects were included if they had a normal history and physical examination [body mass index (BMI) 19–32 kg/m2], a normal andrological history, a normal testicular volume measured by Prader orchidometer, normal random cortisol, normal prostate-specific antigen, normal serum gonadotropins, and serum testosterone concentrations. Exclusion criteria included poor general health, history of liver disease or adrenal insufficiency, BMI greater than 32 kg/m2, abnormal blood test results including prostate-specific antigen greater than 4.0 ng/mL, active skin conditions that would prevent the use of testosterone gel, active alcohol or drug abuse, a history of testicular or scrotal surgery, chronic pain syndrome, use of glucocorticoids or medications that are contraindicated with ketoconazole use (including terfenadine, astemizole, cisapride, budesonide, felodipine, fluticasone, lovastatin, midazolam, sildenafil, or vardenafil), a known bleeding disorder, or the use of medications that affect bleeding time (such as aspirin or warfarin). All subjects agreed to use a reliable form of contraception during the study.
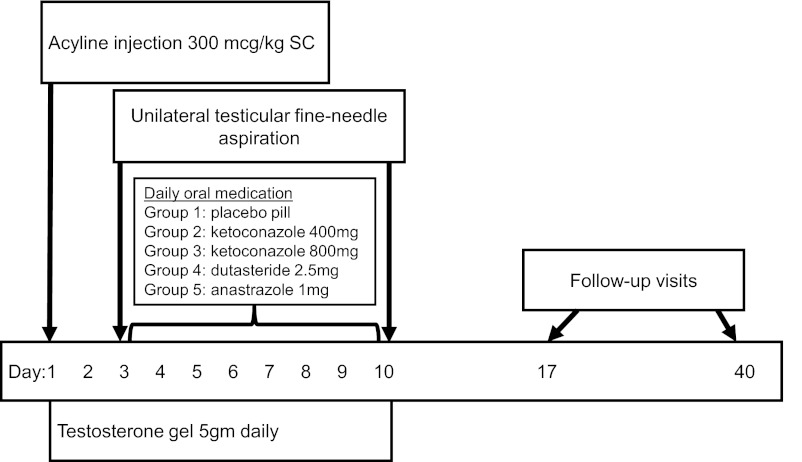
The study design is illustrated in Figure 1. Briefly, after enrollment, a baseline blood sample was obtained from all subjects for serum hormones and safety laboratory tests prior to administration of acyline (NeoMPS, San Diego, California) 300 μg/kg by sc injection. All subjects were administered T gel daily to maintain normal serum T concentrations throughout the treatment period. Subjects were instructed in the proper use and application of 1% testosterone gel, Testim (Auxilium Pharmaceuticals Inc, Malvern, Pennsylvania) 5 g daily for 10 days. In addition, the assessment of vital signs, adverse events, and concomitant medications occurred at every study visit.
Figure 1.
Study design. SC, subcutaneous.
Subjects returned on day 3 and were randomized to the side of unilateral testicular fine-needle aspirations (right vs left on day 3 vs day 10) by random number sequence. Local anesthesia was provided using 1% buffered lidocaine injected into the spermatic cord. We obtained a peripheral blood sample for quantification of serum hormones 2–10 minutes prior to the aspiration. After the procedure, all subjects were randomized using a random number sequence by the investigational pharmacists to 1 of 5 oral medication groups: group 1 received a placebo pill, group 2 received ketoconazole 400 mg (Teva Pharmaceuticals, Petah Tikva, Israel), group 3 received ketoconazole 800 mg, group 4 received dutasteride 2.5 mg (GlaxoSmithKline, London, United Kingdom), and group 5 received anastrazole 1 mg (AstraZeneca, London, United Kingdom), daily for 7 days. Subjects were blinded to their oral treatment group, except those in group 3, who required additional procedures at the day 10 and day 40 visits.
On day 10, we performed a testicular fine-needle aspiration of the testis contralateral to the testis aspirated on day 3 and obtained a peripheral blood sample for quantification of serum hormone concentrations 2–10 minutes prior to the aspiration. We have previously demonstrated a high correlation between intratesticular hormone concentrations in a given man; therefore, we aspirated the contralateral testis on day 10 to avoid repeat aspiration to the previously sampled testis within a short period of time (18, 19).
Subjects in group 3 receiving 800 mg ketoconazole daily completed a cosyntropin stimulation test immediately after the testicular aspiration on day 10 to assess for the possibility of adrenal suppression by high-dose ketoconazole. Subjects received 0.25 mg cosyntropin (Amphastar Pharmaceuticals Inc, Rancho Cucamonga, California) injected im into the deltoid muscle, followed by a repeat blood draw for serum cortisol 30 and 60 minutes after the injection. These subjects had a repeat cosyntropin stimulation test at the day 40 visit.
Subjects returned on day 17 and day 40 for a physical examination and blood sampling to confirm that their physical examinations, serum hormones, and safety laboratory measurements had returned to normal. The Institutional Review Board of the University of Washington approved this study protocol prior to study initiation. This trial was registered in advance [www.clinicaltrials.gov (National Clinical Trial number 01215292)].
Measurements
Testosterone, DHT, dehydroepiandrosterone (DHEA), androstenedione (ADD), and estradiol used for the standards were purchased from Cerilliant (Round Rock, Texas). Testosterone 16,16,17-d3 was purchased from Sigma-Aldrich Chemicals (Milwaukee, Wisconsin). DHT 16,16,17-d3 and estradiol 16,16,17-d3 were purchased from CDN Isotopes (Québec, Canada). DHEA 2,2,3,4,4,6-d6 was purchased from Cambridge Isotope Laboratory (Andover, Massachusetts). Androstenedione 19,19,19-d3 was purchased from BDG Synthesis (Wellington, New Zealand).
Testicular aspirates were immediately placed on ice, centrifuged at 300 × g, and the supernatant fluid was stored at −70°C. All testicular fluid and serum samples were assayed for T, DHT, ADD, DHEA, and estradiol by liquid chromatography-tandem mass spectrometry on an AB Sciex 5500 QTRAP mass spectrometer (AB Sciex, Framingham, Massachusetts) using a slight modification of our previously described method (18, 22). The intra- and interassay coefficients of variation were 3.5% and 7.7% for testosterone and were 3.5% and 6.3% for DHT. The assay sensitivities for serum and IT androgens (T, DHT, ADD, and DHEA) were less than 10 pg/mL.
For the estradiol assay, 25 μL of estradiol 16,16,17-d3 (2 ng/mL) diluted in methanol-water (1:1, vol/vol) was added to either 100 μL of serum or 100 μL of diluted testicular extract in a 13- × 100-mm screw cap glass tube. Using a screw cap with a polytetraflouroethylene-faced rubber liner, samples were briefly vortexed and allowed to sit at room temperature for 15 minutes prior to extraction twice with 2.5 mL of hexane-ethyl acetate (80:20, vol/vol) by slow rotational mixing for 15 minutes. Solvent phase was removed after centrifugation for 15 minutes (2800 rpm) and steroid extract evaporated to dryness in a Speedvac. Steroid extract was vortexed with an additional 0.3 mL of hexane-ethyl acetate (80:20, vol/vol) and evaporated to dryness in a Speedvac prior to derivatization. Extracted steroids were dissolved in 50 μL of sodium bicarbonate buffer (100 mmol/L, pH 10.5) and derivatized with 50 μL of dansyl chloride (1 g/L in acetone) in a capped 12- × 75-mm glass tube in a 60°C water bath for 10 minutes. After centrifugation for 5 minutes, the derivatized sample was transferred to autosampler vial with a clear glass conical 300 μL insert for liquid chromatography with tandem mass spectrometry analysis. For the measurement of serum estradiol, the lower limit of detection was 5 pg/mL and the intraassay coefficient of variation was 24%. Unfortunately, we were unable to accurately quantitate the concentration of intratesticular estradiol from our limited samples of intratesticular fluid. Therefore, only serum estradiol concentrations are presented.
Serum LH and FSH were quantified by immunofluorometric assay (6). Assays were run in duplicate and used 100 μL of serum. The sensitivity of the LH assay was 0.019 IU/L, and the intra- and interassay coefficients of variation for a midrange pooled value of 1.2 IU/L was 3.2% and 12.5%, respectively. The sensitivity of the FSH assay was 0.016 IU/L, and the intra- and interassay coefficients of variation were 2.9% and 6.1% for a midrange pooled value of 0.96 IU/L. Serum 17-hydroxyprogesterone (hypothesized to be increased in the setting of inhibition of the 17,20 lyase by ketoconazole) was measured in duplicate by RIA (Siemens Healthcare Diagnostics, Deerfield, Illinois) using 25 μL of serum with intra- and interassay coefficients of variation of 5.6% and 6.4%, respectively, and a lower limit of detection of less than 1 ng/mL. All samples for all subjects were batched and measured in a single assay.
Statistical analysis
Serum and intratesticular hormone concentrations from all 40 subjects were included in the final analysis. Due to nonnormal distribution, the data are expressed as medians and 25th and 75th percentiles. Comparisons of hormone concentrations between groups were performed using a Kruskal-Wallis ANOVA with a Wilcoxon rank-sum post hoc test. Comparisons of hormone concentrations within a group were made using a Wilcoxon signed-rank test. No corrections were made for multiple comparisons. All statistical analyses were performed using STATA version 10.0 (College Park, Texas). For all comparisons, an alpha <.05 was considered significant.
Results
Subjects
Fifty-two men were screened for the study and 46 met all inclusion criteria. Six subjects withdrew from the study prior to any procedures, and 40 were randomized to the study (n = 8/group). All 40 subjects who underwent randomization completed all of the study procedures. Of the 6 men who failed to meet the screening criteria for the study, 2 subjects had an abnormal genital examination, 1 subject had undergone a prior vasectomy, 1 subject exceeded the BMI criteria, 1 had previously undiagnosed severe hypertension, and 1 had an unstable psychiatric disorder. Of the 40 subjects randomized, 31 were Caucasian (27 non-Hispanic and 4 Hispanic), 4 were Asian, 3 were African American, 1 was a Pacific Islander, and 1 was an American Indian.
One serious adverse event occurred during the study, involving the development of a testicular hematoma and scrotal hematocele after a subject's second testicular aspiration procedure on day 10. This subject underwent operative evacuation of the hematocele and cauterization of a superficial testicular vessel. Follow-up ultrasounds showed complete resolution of the testicular hematoma and hematocele. The subject completed all study procedures and was included in the analysis. In addition, 18 subjects reported 21 nonserious adverse events (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). All adverse events resolved by study completion. Median testicular aspirate volume was 17 μL at day 3 and 20 μL at day 10 (P = .7). Testicular aspirate volume did not differ between treatment groups on day 3 or day 10 (P = .2 and P = .8, respectively) (Supplemental Table 2). Six of the 8 subjects randomized to the ketoconazole 800 mg daily group had an abnormal cosyntropin stimulation test (defined as peak serum cortisol < 18 ng/dL) on the day 10 visit. On day 10, the median (25th and 75th percentiles) serum cortisol was 13.5 (10.8, 15.5) ng/dL at 30 minutes and 14.6 (12.2, 17.8) ng/dL at 60 minutes after cosyntropin stimulation. All 8 subjects in this group had normal cosyntropin stimulation tests on day 40 (Supplemental Figure 1).
Intratesticular hormones
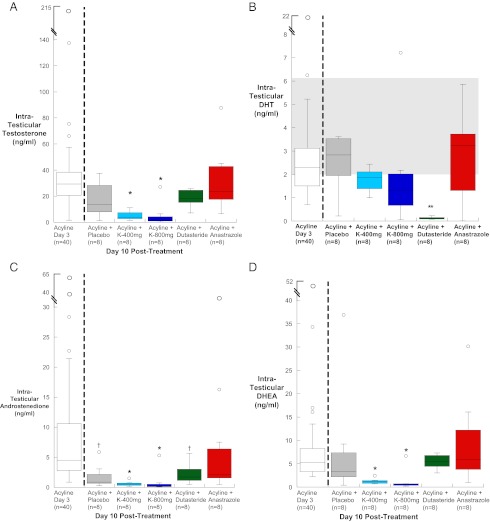
Forty-eight hours after acyline administration, IT-T in the group of 40 men was 30 (21, 39) ng/mL. This represents a 96% reduction from the baseline median IT-T concentration for normal men of 715 (486, 1000) ng/mL (15). There were no significant differences in IT-T between treatment groups on day 3 (data not shown). On day 10, after 7 days of medications, IT-T decreased further to a median of 14 (8.0, 21.2) ng/mL in the placebo group, 3.7 (2.6, 7.2) ng/mL in the 400 mg ketoconazole group, and 1.7 (0.9, 4) ng/mL in the 800 mg ketoconazole group (P < .001 for both comparisons with placebo) (Figure 2A). The IT-T concentrations were not significantly different in the groups receiving dutasteride or anastrazole compared with placebo and not significantly different from day 3 IT-T concentrations.
Figure 2.
A–D, Box plots of IT-T (A), IT-DHT (B), IT-ADD (C), and IT-DHEA (D) in gonadotropin-suppressed subjects on day 3 and day 10 by treatment group. Baseline median (25th and 75th percentiles) IT-T concentration for normal men is 715 (486, 1000) ng/mL (19). The baseline median IT-DHT concentration for normal men is shown in gray shaded area (19). The baseline median IT-ADD and IT-DHEA concentrations for normal men are 179 (88, 246) ng/mL and 162 (118, 253) ng/mL, respectively (23). K400, ketoconazole 400 mg; K800, ketoconazole 800 mg; * P < .05 compared with day 3 and all nonketoconazole treatment groups;**P < .05 compared with day 3 and all other treatment groups; †P < .05 compared with day 3.
IT-DHT decreased with acyline administration to a median concentration of 2.3 (1.5, 3.3) ng/ml, compared with a normal baseline median IT-DHT of 3.5 (2, 6.1) ng/mL (19). IT-DHT did not differ between treatment groups at day 3 (data not shown). After 7 days of medication, IT-DHT decreased by 95% in the group receiving dutasteride to a median of 0.12 (0.09, 0.15) ng/mL, which was significantly lower compared with all other treatment groups (P < .05) (Figure 2B).
IT-ADD decreased with acyline administration to a median concentration of 4.9 (2.8, 10.7) ng/mL, compared with an expected baseline of 179 (88, 246) ng/mL (23). IT-ADD did not differ between treatment groups after acyline administration at day 3 (data not shown), but after 10 days, IT-ADD decreased significantly compared with day 3 in all groups except for those receiving anastrazole. In addition, IT-ADD in the groups receiving ketoconazole were significantly suppressed as compared with the other oral treatment groups with a median of 0.5 (0.3, 0.7) ng/mL for the ketoconazole 400 mg group and 0.12 (0.1, 0.5) ng/mL for the ketoconazole 800 mg group (Figure 2C). There was no significant difference in the IT-ADD concentration at day 10 between the ketoconazole groups.
IT-DHEA decreased with acyline administration from normal median baseline concentration of 162 (118, 253) ng/mL (23) to 5.3 (3.3, 8.4) ng/mL 48 hours after receiving acyline. IT-DHEA did not differ between treatment groups at day 3 (data not shown). After 7 days of oral ketoconazole (both the 400 and 800 mg groups), IT-DHEA decreased significantly compared with day 3 and compared with treatment groups that did not receive ketoconazole to a median of 1.2 (0.8, 1.4) ng/mL in the 400 mg ketoconazole group and 0.5 (0.4, 0.7) ng/mL in the 800 mg ketoconazole group (Figure 2D). There was no significant difference in IT-DHEA between the ketoconazole groups at day 10.
Serum hormones
Serum gonadotropins decreased significantly with the administration of acyline. Median serum LH concentrations decreased to the lower limit of detection for all groups by 48 hours after acyline administration and remained less than 1 IU/L for subjects in all treatment groups at day 10 (Supplemental Figure 2A). Median serum FSH concentrations decreased to 1 IU/L for all treatment groups by 48 hours after acyline administration and continued to decrease significantly in all groups at day 10. In addition, serum FSH decreased significantly in the 2 groups receiving ketoconazole as compared with the other treatment groups (Supplemental Figure 2B).
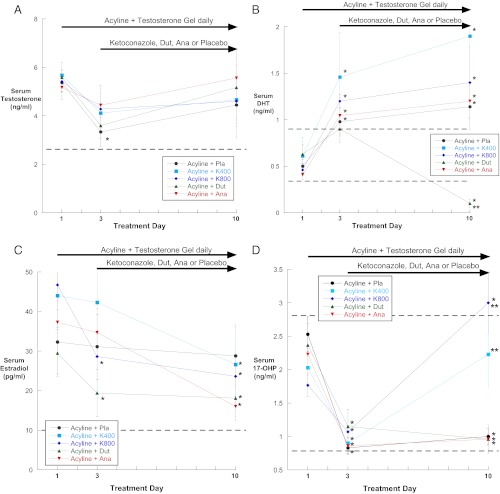
Baseline serum hormones and gonadotropins are presented in Table 1. There were no differences between treatment groups at baseline. Serum testosterone decreased from baseline in all groups after receiving acyline despite administration of testosterone gel, reaching statistical significance in the placebo group (Figure 3A). However, all subjects had normal serum testosterone concentrations on both study day 3 and day 10. In the groups receiving ketoconazole, there were no significant differences between the serum T and IT-T on day 10. In addition, no statistical correlation was found between serum T and IT-T at day 10 in any groups.
Table 1.
Baseline Characteristics and Serum Hormones of 40 Participants by Treatment Group [Median (25th, 75th Interquartile Range)]
| Acyline + Pla (n = 8) | Acyline + K400 (n = 8) | Acyline + K800 (n = 8) | Acyline + Dut (n = 8) | Acyline + Ana (n = 8) | All Subjects (n = 40) | |
|---|---|---|---|---|---|---|
| Age, y | 24 (21, 35.5) | 22.5 (20, 26) | 22 (20.5, 25.5) | 21.5 (20, 24) | 21.5 (20.5, 24.5) | 22 (20, 26) |
| BMI, kg/m2 | 24.1 (21.9, 26) | 22.5 (21.6, 25.6) | 24 (21.5, 25.3) | 24 (23.6, 26.3) | 25 (24, 27) | 24.3 (22.3, 25.8) |
| Serum hormones | ||||||
| T, ng/mL | 5.2 (4.6, 6.3) | 5.5 (5.2, 6.2) | 5.3 (4.7, 6.3) | 5.4 (4.4, 6.4) | 5.2 (4.2, 5.8) | 5.3 (4.8, 6.2) |
| DHT, ng/mL | 0.5 (0.37, 0.59) | 0.44 (0.35, 0.54) | 0.44 (0.35, 0.53) | 0.5 (0.43, 0.89) | 0.42 (0.3, 0.5) | 0.46 (0.36, 0.56) |
| E2, pg/mL | 28.3 (21.3, 39.6) | 39 (16.5, 65.8) | 39 (23.4, 69.4) | 24 (20.9, 30) | 39.4 (21.1, 51.2) | 28.3 (20.9, 53.9) |
| ADD, ng/mL | 0.76 (0.52, 1.12) | 0.7 (0.57, 1.1) | 0.69 (0.59, 0.76) | 0.75 (0.5, 1.1) | 0.65 (0.6, 0.81) | 0.67 (0.57, 0.95) |
| DHEA, ng/mL | 4.2 (3.3, 6.5) | 3.9 (2.7, 7.1) | 4.6 (2.8. 5.9) | 3.9 (3.2, 6.6) | 3.8 (2.8, 4.4) | 3.9 (2.9, 6.1) |
| 17-OHP, ng/mL | 7.2 (5.9, 9.5) | 6 (4.2, 7.9) | 4.8 (4.2, 6.7) | 6.1 (5.1, 9.1) | 6 (4.6, 9) | 6.1 (4.7, 8.1) |
| LH, IU/L | 4.3 (3.3, 5.2) | 5 (3.1, 6.6) | 5.8 (4.7, 7.4) | 4.7 (4.2, 5.4) | 4.9 (4.2, 6.6) | 4.8 (3.9, 6.4) |
| FSH, IU/L | 2.7 (2.3, 2.9) | 2.3 (1.5, 3.5) | 2.4 (1.7, 2.8) | 2.7 (1.8, 3.0) | 2.3 (1.4, 3) | 2.6 (1.7, 3) |
Abbreviations: Ana, anastrazole 1 mg; Dut, dutasteride 2.5 mg; K400, ketoconazole 400 mg; K800, ketoconazole 800 mg; Pla, placebo.
Figure 3.
A–D, Serum T (A), DHT (B), E2 (C), and 17-OHP (D) at baseline, 48 hours after acyline administration (day 3), and 1 week after oral medication administration (day 10) by treatment group. Dashed lines represent the normal reference range. Ana, anastrazole 1 mg; Dut, dutasteride 2.5 mg; K400, ketoconazole 400 mg; K800, ketoconazole 800 mg; Pla, placebo. *P < .05 compared with baseline; **P < .05 compared with all other treatment groups at day 10.
Serum DHT increased significantly in all subjects after acyline and testosterone gel administration and remained elevated compared with baseline in all groups except the group receiving oral dutasteride, in whom serum DHT was significantly reduced at day 10 (Figure 3B). Serum DHT did not differ between day 3 and day 10 for all other treatment groups. Serum estradiol decreased in all groups, but the group receiving anastrazole was not significantly lower compared with the other treatment groups (Figure 3C).
Serum 17-hydroxyprogesterone (17-OHP) decreased significantly in all treatment groups at 48 hours and then rose significantly in the 2 groups receiving ketoconazole as compared with the other treatment groups (Figure 2D). Serum androstenedione increased significantly in the dutasteride group, and DHEA was significantly decreased in the group receiving high-dose ketoconazole (Supplemental Figure 3, A and B), but otherwise there were no significant changes in androstenedione or DHEA during treatment.
Discussion
We have demonstrated that the combination of gonadotropin suppression with acyline and inhibition of testosterone biosynthesis with ketoconazole significantly decreases IT-T concentrations more than gonadotropin suppression alone. This finding suggests that Leydig cells continue to synthesize testosterone at low concentrations despite GnRH antagonist treatment and marked suppression of LH. Whether this testosterone synthesis occurs in response to very low concentrations of LH or is LH independent is unknown. Our previous studies and the work of others have shown that gonadotropin suppression decreases IT-T concentrations by 90%–95% from baseline (19, 24). Our current study demonstrates that the combination of acyline and ketoconazole, at a commonly used treatment dose, can reduce IT-T greater than 99%. Such concentrations of IT-T are at the low end of the normal range for serum testosterone and might be associated with significantly decreased androgen activity in the testes and greater suppression of spermatogenesis. In the future, to understand the relationship between intratesticular testosterone and spermatogenesis, we plan to study the combination of acyline and ketoconazole for 4–6 months in normal men. This study will include repeated analyses of semen and should help determine whether a threshold concentration necessary for spermatogenesis exists in man. Such a threshold has been suggested by experiments in rodents. For example, Zirkin et al (8) studied the relationship between IT-T and spermatogenesis and found that IT-T concentrations below 5% of baseline were unable to support spermatogenesis. Similar results were obtained by Singh and Handelsman using the gonadotropin-deficient hpg mouse model (25).
Both serum and IT-DHT were suppressed significantly in the group receiving dutasteride, but IT-DHT did not significantly suppress in the other groups and serum DHT concentrations actually increased significantly in the other groups, likely due to the use of testosterone gel, which is known to increase serum DHT due to 5α-reduction in the skin. This study is the first to demonstrate the dramatic effect of a 5α-reductase inhibitor, dutasteride, on IT-DHT concentrations in gonadotropin-suppressed normal men and contributes to our understanding of the impact of this drug on intratesticular hormone physiology in men. Unfortunately, we were unable to ascertain a similar effect on intratesticular estradiol due to technical difficulties performing high-quality measurements of estradiol on the limited sample volumes obtained by testicular aspiration. The idea that differences in intratesticular DHT may account for differences in the response to male hormonal contraceptives was first suggested in 1996 (9). However, the addition of a 5α-reductase inhibitor to male hormonal contraceptive regimens does not significantly improve the rates of suppression of spermatogenesis (26). In contrast, some men can have dramatic suppression of spermatogenesis with use of a 5α-reductase inhibitor alone (10). DHT appears to have a highly variable intraindividual effect on spermatogenesis, and the potential role for DHT in supporting spermatogenesis in men with low IT-T remains unknown.
In addition to suppressing IT-T, both doses of ketoconazole significantly suppressed IT-ADD and IT-DHEA. This finding is compatible with the known inhibition of the 17,20 lyase by ketoconazole. Similarly, both groups receiving ketoconazole exhibited significant increases in serum 17-OHP. Interestingly, despite the marked reduction in IT-ADD, we did not see a significant suppression of serum ADD concentrations with ketoconazole, possibly due to some conversion of the exogenously administered T gel back into ADD by 17-hydroxysteroid dehydrogenase.
Our study had some limitations. The omission of corrections for multiple comparisons in the statistical analysis increased the risk of a type I error. In addition, the short duration of treatment did not allow for determination of the impact of reducing IT-T on spermatogenesis and limited our ability to determine whether there may have been adverse effects associated with longer treatment. Lastly, progestogens are commonly used in male hormonal contraceptive regimens but were not included in our study. Therefore, we cannot comment on the effect of progestogens on IT-T. This will be the focus of future research.
The study protocol was well tolerated by most men, except for the subject who experienced bleeding from the testicular fine-needle aspiration, a rarely reported complication of this procedure (27). Ketoconazole has been reported to cause liver inflammation in 0.1%–1% of patients (28), yet we observed no evidence of liver inflammation in our subjects. Notably, however, the 800-mg dose mildly suppressed the response to cosyntropin stimulation, indicating a potential long-term safety concern. For this reason, dexamethasone is administered when ketoconazole is used in treatment of prostate cancer (29, 30), but this approach would be unacceptable for healthy men for the purposes of male contraception. In contrast to ketoconazole, which inhibits the 17,20 lyase, an inhibitor of type III 17β-hydroxysteroid dehydrogenase may provide more effective suppression of T synthesis without the need for concomitant glucocorticoid administration (32).
In summary, our results demonstrate that the combination of LH suppression and inhibition of T synthesis or metabolism can dramatically reduce intratesticular concentrations of sex steroids. In particular, combinations of acyline and ketoconazole may prove to be useful in future studies designed to determine the minimum concentration of IT-T necessary for human spermatogenesis, information essential for the further development of male hormonal contraceptives.
Supplementary Material
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through Cooperative Agreement U54 HD-42454 as part of the Cooperative Contraceptive Research Centers Program. M.Y.R. is supported, in part, by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant K12 HD053984. J.J.S.N. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases training grant 5T32 DK007247-35. A.M.M. is supported by the Department of Veterans Affairs.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADD
- androstenedione
- BMI
- body mass index
- DHEA
- dehydroepiandrosterone
- DHT
- dihydrotestosterone
- E2
- estradiol
- IT
- intratesticular
- 17-OHP
- 17-hydroxyprogesterone
- T
- testosterone.
References
- 1. World Health Organization Contraceptive efficacy of testosterone-induced azoospermia in normal men. World Health Organization Task Force on Methods for the Regulation of Male Fertility. Lancet. 1990;336:955–959 [PubMed] [Google Scholar]
- 2. Wu FC, Farley TM, Peregoudov A, Waites GM. Effects of testosterone enanthate in normal men: experience from a multicenter contraceptive efficacy study. World Health Organization Task Force on Methods for the Regulation of Male Fertility. Fertil Steril. 1996;65:626–636 [PubMed] [Google Scholar]
- 3. Anawalt BD, Bebb RA, Bremner WJ, Matsumoto AM. A lower dosage levonorgestrel and testosterone combination effectively suppresses spermatogenesis and circulating gonadotropin levels with fewer metabolic effects than higher dosage combinations. J Androl. 1999;20:407–414 [PubMed] [Google Scholar]
- 4. Page ST, Amory JK, Bremner WJ. Advances in male contraception. Endocr Rev. 2008;29:465–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ly LP, Liu PY, Handelsman DJ. Rates of suppression and recovery of human sperm output in testosterone-based hormonal contraceptive regimens. Hum Reprod. 2005;20:1733–1740 [DOI] [PubMed] [Google Scholar]
- 6. Page ST, Kalhorn TF, Bremner WJ, Anawalt BD, Matsumoto AM, Amory JK. Intratesticular androgens and spermatogenesis during severe gonadotropin suppression induced by male hormonal contraceptive treatment. J Androl. 2007;28:734–741 [DOI] [PubMed] [Google Scholar]
- 7. Zhang FP, Pakarainen T, Poutanen M, Toppari J, Huhtaniemi I. The low gonadotropin-independent constitutive production of testicular testosterone is sufficient to maintain spermatogenesis. Proc Natl Acad Sci USA. 2003;100:13692–13697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zirkin BR, Santulli R, Awoniyi CA, Ewing LL. Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology. 1989;124:3043–3049 [DOI] [PubMed] [Google Scholar]
- 9. Anderson RA, Wallace AM, Wu FC. Comparison between testosterone enanthate-induced azoospermia and oligozoospermia in a male contraceptive study. III. Higher 5α-reductase activity in oligozoospermic men administered supraphysiological doses of testosterone. J Clin Endocrinol Metab. 1996;81:902–908 [DOI] [PubMed] [Google Scholar]
- 10. Amory JK, Wang C, Swerdloff RS, et al. The effect of 5α-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J Clin Endocrinol Metab. 2007;92:1659–1665 [DOI] [PubMed] [Google Scholar]
- 11. Roth MY, Amory JK, Page ST. Treatment of male infertility secondary to morbid obesity. Nat Clin Pract Endocrinol Metab. 2008;4:415–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raman JD, Schlegel PN. Aromatase inhibitors for male infertility. J Urol. 2002;167:624–629 [DOI] [PubMed] [Google Scholar]
- 13. Reifsnyder JE, Ramasamy R, Husseini J, Schlegel PN. Role of optimizing testosterone before microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol. 2012;188:532–537 [DOI] [PubMed] [Google Scholar]
- 14. Finkelstein JS, Whitcomb RW, O'Dea LS, Longcope C, Schoenfeld DA, Crowley WF. Sex steroid control of gonadotropin secretion in the human male. I. Effects of testosterone administration in normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab. 1991;73:609–620 [DOI] [PubMed] [Google Scholar]
- 15. Herbst KL, Coviello AD, Page S, Amory JK, Anawalt BD, Bremner WJ. A single dose of the potent gonadotropin-releasing hormone antagonist acyline suppresses gonadotropins and testosterone for 2 weeks in healthy young men. J Clin Endocrinol Metab. 2004;89:5959–5965 [DOI] [PubMed] [Google Scholar]
- 16. Jarow JP, Chen H, Rosner TW, Trentacoste S, Zirkin BR. Assessment of the androgen environment within the human testis: minimally invasive method to obtain intratesticular fluid. J Androl. 2001;22:640–645 [PubMed] [Google Scholar]
- 17. Coviello AD, Matsumoto AM, Bremner WJ, et al. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab. 2005;90:2595–2602 [DOI] [PubMed] [Google Scholar]
- 18. Roth MY, Lin K, Amory JK, et al. Serum LH correlates highly with intratesticular steroid levels in normal men. J Androl. 2010;31:138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roth MY, Page ST, Lin K, et al. Dose-dependent increase in intratesticular testosterone by very low-dose human chorionic gonadotropin in normal men with experimental gonadotropin deficiency. J Clin Endocrinol Metab. 2010;95:3806–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pont A, Graybill JR, Craven PC, Galgiani JN, Dismukes WE, Reitz RE, Stevens DA. High-dose ketoconazole therapy and adrenal and testicular function in humans. Arch Intern Med. 1984;144:2150–2153 [PubMed] [Google Scholar]
- 21. Van Tyle JH. Ketoconazole. Mechanism of action, spectrum of activity, pharmacokinetics, drug interactions, adverse reactions and therapeutic use. Pharmacotherapy. 1984;4:343–373 [DOI] [PubMed] [Google Scholar]
- 22. Kalhorn TF, Page ST, Howald WN, Mostaghel EA, Nelson PS. Analysis of testosterone and dihydrotestosterone from biological fluids as the oxime derivatives using high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:3200–3206 [DOI] [PubMed] [Google Scholar]
- 23. Roth MY, Page ST, Lin K, et al. The effect of gonadotropin withdrawal and stimulation with human chorionic gonadotropin on intratesticular androstenedione and DHEA in normal men. J Clin Endocrinol Metab. 2011;96:1175–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McLachlan RI, O'Donnell L, Stanton PG, et al. Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men. J Clin Endocrinol Metab. 2002;87:546–556 [DOI] [PubMed] [Google Scholar]
- 25. Singh J, O'Neill C, Handelsman DJ. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 1995;136:5311–5321 [DOI] [PubMed] [Google Scholar]
- 26. Matthiesson KL, Stanton PG, O'Donnell L, et al. Effects of testosterone and levonorgestrel combined with a 5μ-reductase inhibitor or gonadotropin-releasing hormone antagonist on spermatogenesis and intratesticular steroid levels in normal men. J Clin Endocrinol Metab. 2005;90:5647–5655 [DOI] [PubMed] [Google Scholar]
- 27. Carpi A, Menchini Fabris FG, Palego P, et al. Fine-needle and large-needle percutaneous aspiration biopsy of testicles in men with nonobstructive azoospermia: safety and diagnostic performance. Fertil Steril. 2005;83:1029–1033 [DOI] [PubMed] [Google Scholar]
- 28. Bok RA, Small EJ. The treatment of advanced prostate cancer with ketoconazole: safety issues. Drug Saf. 1999;20:451–458 [DOI] [PubMed] [Google Scholar]
- 29. Harris KA, Weinberg V, Bok RA, Kakefuda M, Small EJ. Low dose ketoconazole with replacement doses of hydrocortisone in patients with progressive androgen independent prostate cancer. J Urol. 2002;168:542–545 [PubMed] [Google Scholar]
- 30. Taplin ME, Regan MM, Ko YJ, et al. Phase II study of androgen synthesis inhibition with ketoconazole, hydrocortisone, and dutasteride in asymptomatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7099–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.