Abstract
Background:
Although hypothyroidism is associated with increased morbidity, an association with increased mortality is still debated. Our objective was to investigate, at a nationwide level, whether a diagnosis of hypothyroidism influences mortality.
Methods:
In an observational cohort study from January 1, 1978 until December 31, 2008 using record-linkage data from nationwide Danish health registers, 3587 singletons and 682 twins diagnosed with hypothyroidism were identified. Hypothyroid individuals were matched 1:4 with nonhypothyroid controls with respect to age and gender and followed over a mean period of 5.6 years (range 0–30 years). The hazard ratio (HR) for mortality was calculated using Cox regression analyses. Comorbidity was evaluated using the Charlson score (CS).
Results:
In singletons with hypothyroidism, the mortality risk was increased (HR 1.52; 95% confidence interval [CI]: 1.41–1.65). Although the effect attenuated, hypothyroidism remained associated with increased mortality when evaluating subjects with a CS = 0 (HR 1.23; 95% CI: 1.05–1.44). In twin pairs discordant for hypothyroidism, the hypothyroid twin had excess mortality compared with the corresponding euthyroid cotwin (HR 1.40; 95% CI 0.95–2.05). However, after stratifying for zygosity, hypothyroidism was associated with excess mortality in dizygotic twin pairs (HR 1.61; 95% CI 1.00–2.58), whereas the association attenuated in monozygotic pairs (HR 1.06; 95% CI 0.55–2.05).
Conclusions:
Hypothyroidism is associated with an excess mortality of around 50%, which to some degree is explained by comorbidity. In addition, the finding of an association between hypothyroidism and mortality within disease discordant dizygotic but not monozygotic twin pairs indicates that the association between hypothyroidism and mortality is also influenced by genetic confounding.
Hypothyroidism is a common condition, with a prevalence of 1% to 2% (1, 2). The development of hypothyroidism is the result of complex interactions between a number of factors, such as gender (1), genetic predisposition (3), epigenetic factors (4), iodine intake (5), and cigarette smoking (6, 7). The vast majority of hypothyroid individuals either has chronic autoimmune thyroiditis or has had previous thyroid surgery or radioiodine treatment (1). Irrespective of its cause, hypothyroidism is linked with a number of metabolic changes such as hyperlipidemia (8) and coagulopathy (9) as well as endothelial dysfunction (10), hypertension (11), and other cardiovascular disorders (12). In addition, hypothyroid patients have reduced quality of life that persists when thyroid function has been restored (13). Whether hypothyroidism affects life expectancy remains to be clarified. Some studies report increased mortality in patients with hypothyroidism (14–16), whereas others demonstrate similar overall mortality compared with controls (8, 17–22).
As pointed out in our recent reviews (23, 24), the interpretation of the studies dealing with the relationship between hypothyroidism as well as hyperthyroidism and mortality is hampered by methodological shortcomings such as major differences in size of study populations and definition of phenotype. Moreover, there is a lack of appropriate control for comorbidity and genetic confounding (that is, mortality and hypothyroidism being influenced by shared genes) (23). Regarding the latter, twin studies have estimated a genetic liability between 60% and 80% for thyroid disorders (25) and a heritability of 20% to 30% for longevity (26, 27). As a consequence, if some genes linked to life expectancy are also involved in the development of hypothyroidism, the observed association between hypothyroidism and mortality could, at least partly, be due to shared genetic factors.
Denmark holds a long tradition for collecting data into well-established nationwide health and administrative registers (28). By virtue, they provide an excellent resource for epidemiologic research that allows longitudinal follow-up of individuals without any significant selection or loss of follow-up. From the complete registration of admission to hospitals, outpatient treatment, and treatment in general practice, hypothyroid individuals as well as controls can be ascertained with high accuracy. Moreover, comorbidity can be controlled for to a higher degree than previously. Herein we use these nationwide data to investigate whether a diagnosis of hypothyroidism is associated with increased mortality. Additionally, this is the first study using disease discordant twins, which allows control for genetic confounding by design (29).
Materials and Methods
Data sources
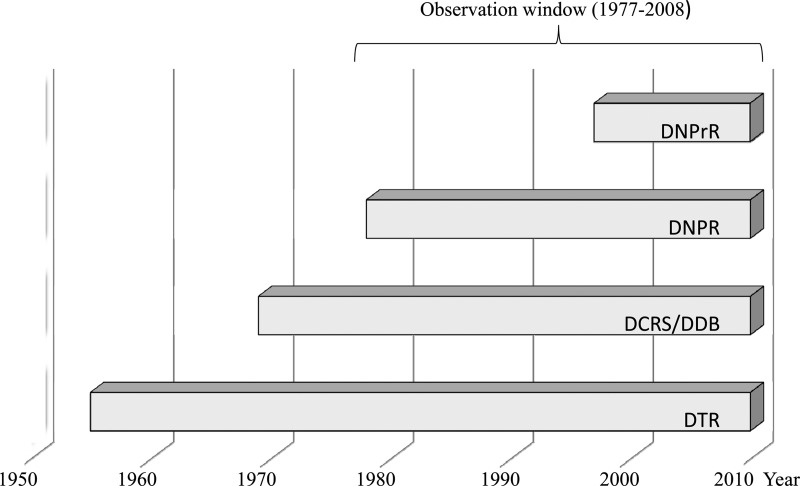
The calendar period covered by the individual data sources are shown in Figure. 1.
Figure 1.
Calendar period covered by different data sources. Abbreviations: DDB, The Danish Demographic Database; DTR, The Danish Twin Registry.
The Danish Civil Registration System (DCRS) and the Danish Demographic Database cover information on demographics, vital status, date of death, and residence of all persons living or having lived in Denmark from 1968 until 2008 (28). From these registers, we identified a random 5% sample of the Danish background population covering the birth cohorts 1870–2001.
The Danish Twin Registry is a nationwide and population-based register, established in 1954, and comprises nearly 150 000 twins born in Denmark between 1870 and 2001 (30). All twins in the register are ascertained independently of zygosity and disease.
The Danish National Patient Registry (DNPR) includes registrations of admission to hospitals (both primary and secondary diagnoses) since January 1, 1977. Outpatient admissions have been registered separately since January 1, 1995 (28). All registrations are according to the International Classification of Disease (ICD). The validity of the DNPR is very high and misclassification of hypothyroidism has been shown to occur in less than 2% of cases (31). The Danish National Prescription Registry (DNPrR) provides information on all prescriptions of drugs dispensed from Danish pharmacies since 1995 (28). Coding for medical products is according to the Anatomical Therapeutic Chemical (ATC) classification system. The register covers information on date of dispensing, ATC code, strength, and quantity (in defined daily doses). In Denmark, the national health security system covers all inhabitants and partially reimburses drug expenses. Data from the DNPrR are transmitted directly from the cash register in the pharmacy and are used in the calculation (made on an individual level) of the expenses reimbursed. In Denmark, Levothyroxine is sold solely as a prescribed drug. This, for our purpose, as in other Danish surveys, makes the register very valid (32).
All described databases are hosted at Statistics Denmark. DCRS is based on a unique 10-digit personal identification number (Det Centrale Personregister number) assigned to all persons living or having lived in Denmark. The Det Centrale Personregister number allows record linkage between all databases on an individual level.
Diagnosis of hypothyroidism
Information on thyroid status was drawn from DNPR (28) and DNPrR (28). To be classified as having hypothyroidism, subjects should be recorded in at least 1 of these registers. In DNPR, hypothyroidism was defined by ICD-8 and ICD-10 codes (244.00–244.03, 244.08, 244.09, and E03.2–E03.9, respectively). In DNPrR, hypothyroidism was defined by at least 2 dispensed prescriptions of thyroid hormone (ATC = H03A). To avoid misclassification, individuals diagnosed with thyroid cancer, congenital hypothyroidism, or pituitary hypothyroidism were excluded. Furthermore, cases were not allowed to be diagnosed with hyperthyroidism before the diagnosis of hypothyroidism. For individuals identified with hypothyroidism from both DNPR and DNPrR, the first date of registration was chosen as the date of diagnosis of hypothyroidism. To include only incident cases, individuals registered within the first year of registration in DNPR and DNPrR (1977 and 1995, respectively) were excluded.
Study population
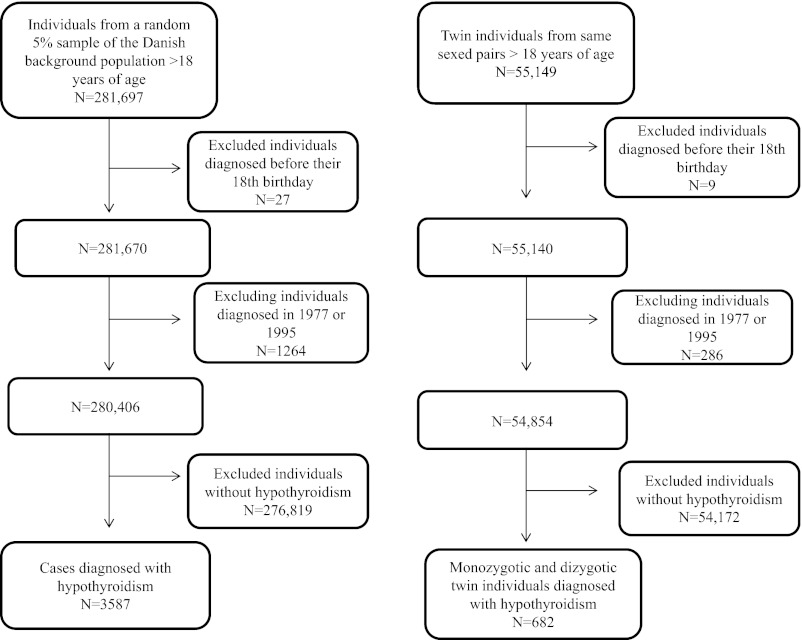
The 2 study populations (singletons and twins) were identified from DCRS (28) and the Danish Twin Registry (30). In this study the term singleton refers to individuals not born as twins. Participants were ascertained as shown in Figure 2. Individuals younger than 18 years of age were excluded and all participants were followed until death or December 31, 2008, whichever came first.
Figure 2.
Selection of study populations.
Mortality
Date of mortality originated from DCRS and the Danish Demographic Database (28).
Comorbidity
The Charlson score (CS) accounts for 19 disease groups (myocardial infarction, heart failure, vascular disease, cerebrovascular disease, dementia, chronic lung disease, rheumatic disease, gastric ulcer, liver disease, diabetes mellitus without complications, diabetes mellitus with complications, hemiplegia, kidney disease, cancer, cancer with metastases, lymphoma, leukemia, liver failure, and AIDS) by creating a weighted score on an individual level, to optimize the prediction of the 1-year mortality risk within each disease category (33). The CS has been validated and used in different diseases, including nonmalignant disorders (34, 35). In both cases and controls the CS was calculated from individual records in DNPR using relevant ICD codes and therefore covers all in-patient and outpatient treatments in a hospital setting. As many patients in Denmark with diabetes, cardiovascular disease (eg, hypertension), and pulmonary diseases (eg, chronic obstructive lung disease) are diagnosed and treated solely in primary care, we classified all users of antidiabetics, cardiovascular-related drugs, and drugs for obstructive airway disease (ATC codes A10, B01, C01, C03, C07, C08, C09, N02, and R03) as having diabetes, cardiovascular disease, and lung disease, respectively. For subjects with hypothyroidism, the CS reflects the time period from January 1, 1977 (start of DNPR) until the date of hypothyroidism. In controls, the CS covers the time period from the start of DNPR until the date of hypothyroidism of the corresponding case.
Data analyses
Group frequencies were compared with the Pearson χ2 test, whereas group means and medians were compared by a t test and the Mann-Whitney test, respectively. In the case of paired comparisons, the paired t test was used.
The relationship between hypothyroidism and mortality was evaluated by the Cox regression model. Age was chosen as the underlying time variable. In both cases and controls, person-years of follow-up were accumulated from the date of diagnosis of the case and terminated on the date of death or end of follow-up (December 31, 2008), whichever came first. In the analysis of the random 5% sample of the background population, 4 control subjects were randomly selected for each case, after the principles of density sampling (36), and matched for age and gender. To explore the impact of possible genetic confounding, we additionally performed intrapair analyses of the twin population in which the hypothyroid twin was matched with the corresponding euthyroid cotwin. In all analyses, the variable “pair” was used as a stratum variable, fixing the baseline hazard within a matched pair, while at the same time allowing this baseline hazard to vary freely between pairs. Subsequently, all analyses were adjusted for the history of comorbidity, as evaluated by the CS. All the data were stratified for the register from which they were identified, either DNPR or DNPrR, to evaluate potential differences. Finally, we performed analyses stratified according to the period of observation, defined by an early and a late study period, the distinction being before or after December 31, 1994.
Significant differences were defined as a P value <0.05 using two-tailed tests. All analyses were conducted using STATA version 11.0 (2009; Stata Corporation, College Station, Texas).
Results
Baseline characteristics of the study populations
Characteristics of the cases from the random 5% sample of the background population as well as cases from the twin cohort are presented in Table 1. There was no significant difference in gender distribution and CS between the singletons and the twin population. Subjects with hypothyroidism had significantly higher comorbidity than the controls (percentage of cases and controls with CS > 0: 60% and 46%, respectively, P < .001). This was especially due to cumulative prevalence of cardiovascular disease, which was significantly higher among cases (58%) than controls (46%) (P < .001). The hypothyroid individuals registered with a CS > 0 had a mean age of 62 years compared to 54 years in those with a CS = 0 (P < .001).
Table 1.
Baseline Characteristics of Individuals Identified With Hypothyroidism
| Random 5% Sample of Background Population | Twin Individuals From Same Sex Pairs | P Value | |
|---|---|---|---|
| Number | 3587 | 682 | |
| Females, % | 84% | 82% | .06 |
| Mean age at diagnosis of hypothyroidism, y | 60 | 58 | .005 |
| Study time prevalence of hypothyroidism in DNPRa or DNPrRb | 1.27% | 1.24% | .65 |
| Mean follow-up time, y | 5.6 | 5.5 | .78 |
| Number of individuals with a Charlson score of 0 (%) | 2202 (61%) | 404 (59%) | .29 |
The Danish National Patient Registry.
The Danish National Prescription Registry.
In total, 3587 singletons from the random 5% sample of the background population and 682 twin individuals from same sex twin pairs fulfilled the criteria for hypothyroidism (Figure 2). This corresponds to a prevalence of 1.27% (3587 of 281 670) and 1.24% (682 of 55 140) in singletons and twins, respectively. Among the 3587 singletons, 377 had hypothyroidism due to thyroid surgery. The prevalence of hypothyroidism increased with increasing quartiles of age (data not shown). Most singleton cases (2116 of 3587) as well as twin cases (434 of 682) were identified from DNPrR. About one fourth of singletons (994 of 3587) and twins (172 of 682) were registered in both DNPR and DNPrR. The mean follow-up time was 5.5 years in twins (range 0–29) and 5.6 years in singletons (range 0–30).
Standard Cox regression analyses
In the random 5% sample of the Danish background population, there was a significant excess mortality in hypothyroid compared with control individuals (hazard ratio [HR] 1.52; 95% confidence interval [CI]: 1.41–1.65). Excluding the 377 individuals with hypothyroidism due to surgery did not change the result (HR 1.55; 95% CI: 1.43–1.67). Adjustment and stratification for gender did not significantly change this finding (Table 2).
Table 2.
Hazard Ratio for Mortality in the Random 5% Sample From the Danish Background Population With Hypothyroidism
| Study Population | Number |
Hazard Ratio |
||
|---|---|---|---|---|
| Hypothyroid | Controls | Nonadjusted | Adjusteda | |
| All | 3587 [1062]b | 14348c [3771]b | 1.52 (1.41–1.65) | 1.38 (1.27–1.49) |
| Females | 3028 [848]b | 12112c [3094]b | 1.44 (1.32–1.57) | 1.33 (1.22–1.45) |
| Males | 559 [214]b | 2236c [677]b | 1.94 (1.62–2.32) | 1.62 (1.35–1.96) |
Adjusted for degree of comorbidity, before the diagnosis of hypothyroidism, using the Charlson score.
Number of deaths during the observation time.
Each hypothyroid individual is matched with 4 controls with respect to age and gender.
As summarized in Table 3, the data source had a major impact on the mortality risk estimates. Being recorded in DNPR resulted in significantly increased hazard ratios, irrespective of age. In contrast, the mortality of subjects with hypothyroidism identified via DNPrR was not significantly different from the control population. Stratification for age group, defined by being younger or older than the mean age of 59 years on the date of diagnosis, showed that the older individuals had the highest mortality risk associated with hypothyroidism. In addition, when restricting the analyses to the period either before or after 1995 (outpatient treatment is available in the register from 1995), we found no significant difference between the 2 periods either (data not shown).
Table 3.
Hazard Ratio for Mortality in a Register-Based Sample of Danes With Hypothyroidism
| Registry | Age Group | N, Cases | Age at Diagnosis | Hazard Ratio |
|
|---|---|---|---|---|---|
| Nonadjusted | Adjustedc | ||||
| All | ≤59 | 1681 | 44.8 | 1.31 (1.05–1.64) | 0.98 (0.77–1.26) |
| >59 | 1906 | 73.1 | 1.56 (1.43–1.69) | 1.43 (1.31–1.55) | |
| DNPRa 1977–2006 | ≤59 | 527 | 43.6 | 1.76 (1.34–2.30) | 1.30 (0.97–1.74) |
| >59 | 944 | 74.4 | 1.82 (1.65–2.01) | 1.62 (1.46–1.79) | |
| DNPrRb 1995–2007 | ≤59 | 1155 | 45.5 | 0.76 (0.50–1.15) | 0.57 (0.37–0.90) |
| >59 | 961 | 71.8 | 1.10 (0.95–1.29) | 1.07 (0.92–1.26) | |
Influence of age at diagnosis and type of registry. Separate evaluation of cases diagnosed in a DNPR or solely in b DNPrR, respectively, stratified by age group.
Adjusted for degree of comorbidity, before the diagnosis of hypothyroidism, using the Charlson score.
To evaluate the impact of comorbidity on the observed associations between hypothyroidism and mortality, we analyzed data stratified for CS. When restricting the analyses to subjects with a CS > 1, the HR was significantly increased (1.52; 95% CI: 1.23–1.88). However, when evaluating subjects with a CS = 0, the association attenuated (HR 1.23; 95% CI: 1.05–1.44).
Intrapair Cox regression analyses
The risk estimates based on the 451 twin pairs discordant for hypothyroidism are presented in Table 4. Overall, irrespective of gender and zygosity, there was a higher mortality in hypothyroid twins (HR 1.40; 95% CI: 0.95–2.05). Stratifying for zygosity showed that this persisted in dizygotic pairs discordant for hypothyroidism (HR 1.61; 95% CI: 1.00–2.58) but attenuated in monozygotic pairs (HR 1.06; 95% CI: 0.55–2.05).
Table 4.
Hazard Ratio for Mortality in Intra-Pair Analysis of Twin Pairs Discordant for Hypothyroidism
| Zygosity | Number |
Hazard Ratio | |
|---|---|---|---|
| Hypothyroid | Controls | ||
| Di- and monozygotic pairs | 451 [88]a | 451 [74]a | 1.40 (0.95–2.05) |
| Dizygotic pairs | 312 [61]a | 312 [48]a | 1.61 (1.00–2.58) |
| Monozygotic pairs | 139 [27]a | 139 [26]a | 1.06 (0.55–2.05) |
Number of deaths during the observation time.
Discussion
Previous studies are inconsistent as to whether a diagnosis of hypothyroidism is associated with an excess mortality (8, 14–22). In the present study, we analyzed this both in a random 5% sample of the Danish background population and in a nationwide sample of Danish twins. In our national dataset we found that hypothyroidism was associated with a 52% excess mortality. However, this attenuated when stratifying for comorbidity and in the analyses of the disease discordant monozygotic twin pairs. This scenario supports the finding that the increased mortality, observed in subjects with hypothyroidism, may, at least to some degree, be due to genetic confounding (29) or related to comorbidity. In the present study, subjects diagnosed with hypothyroidism had an increased burden of comorbidity compared with the controls. Comorbidity, especially cardiovascular disease, has a major impact on mortality. In addition, comorbidity may even facilitate the development of hypothyroidism (ie, treatment with Lithium or Amiodarone) and thereby confound the results. Alternatively, hypothyroidism could influence the development of comorbidity (eg, hyperlipidemia and subsequently cardiovascular disease) (12). Therefore, it is absolutely mandatory to take the burden and timing of comorbidity into account when estimating the independent effect of hypothyroidism on mortality. In contrast to previous studies, we obtained register-based data on the history of comorbidity. Employing the validated CS (33) on discharge diagnoses from hospitals and outpatient clinics, and by adding the comorbidities treated in primary care, we achieved a more complete, but clearly not total, coverage of the history of comorbidity than hitherto reported (20, 22). Importantly, in studies based on administrative health care registers, like the present study, where the cases might be ascertained years after the first symptoms of disease, it is very difficult to obtain precise information concerning the timing of onset of disease. By using the first date of registration of comorbidity and hypothyroidism and by only including incident hypothyroid cases, we aimed at limiting the above issue. Adjusting for comorbidity attenuated mortality estimates, which decreased further in individuals with no comorbidity.
To evaluate the causality of the link between hypothyroidism and mortality, we additionally analyzed the association within twin pairs discordant for hypothyroidism. In these analyses the hypothyroid twin had an excess mortality compared to the corresponding euthyroid cotwin, indicating an independent effect of hypothyroidism on mortality. However, within disease discordant monozygotic pairs, the association between hypothyroidism and mortality attenuated considerably. According to the classical interpretation of twin data, these findings are in line with the association between hypothyroidism and mortality being explained, at least to some degree, by genetic factors involved in both hypothyroidism and mortality.
Intuitively, more severe conditions of hypothyroidism should be associated with a worse prognosis, in this case higher mortality. Unfortunately, the relationship between the severity of hypothyroidism and mortality has not been systematically evaluated (23). Moreover, the effect of treatment remains unanswered. A positive correlation between serum concentrations of TSH and mortality has been reported in 1 study (37). In addition, accepting that hypothyroid individuals seen at hospitals have more severe disease than nonreferred hypothyroid individuals (38), 2 large studies based on hospitalized patients (16) and patients treated in primary care (20), respectively, indicated a dose-response relationship between the degree of disease and mortality. Patients treated in primary care have been reported with no increased mortality compared to the euthyroid background population (20), while hypothyroid patients treated in a hospital setting have been shown to have a significantly increased mortality (16). In line with this, our data clearly demonstrate excess mortality when analyzing data from DNPR, despite the fact that all patients, hospitalized as well as seen in primary care, are most likely treated for their hypothyroidism and the effects of hypothyroidism should therefore be equally mitigated. This difference in mortality between individuals from DNPR and DNPrR may also be explained by a higher burden of comorbidity among patients treated in a hospital setting (as reflected by the DNPR), which may be the explanation for the older individuals' increase in mortality as well.
Although the validity of DNPR (31) and DNPrR (32) is high, the individuals' thyroid hormone levels may have varied considerably during the observation. Evidently, this shortcoming is not unique to our study. Importantly, the fact that outpatient treatment has only been recorded in the DNPR since 1995 implies that we cannot rule out differences in the prognosis of outpatients and inpatients, either due to more pronounced hypothyroidism or due to increased risk of comorbidity. However, the lack of difference in the risk estimates, when comparing individuals ascertained before or after 1995, suggests that this kind of selection bias is not crucial.
Our results are in line with some (14–16), but not all, previous studies (8, 17–22), which, as recently pointed out, are characterized by a number of limitations and pitfalls hampering the data interpretation (23). Most of the past surveys on hypothyroidism and mortality deal with study populations numbering under 100 cases (15–19, 21, 22). This may have resulted in inadequate statistical power, as Cox power calculations estimate a necessary sample size of 900 subjects, to show an increase in HR of 20%. Therefore, the strengths of our study include adequate statistical power, due to sample size, and ascertainment of participants from nationwide population-based registers. Additionally, we used standardized and validated procedures for evaluating the history of comorbidity, and the cases were followed for more than 5 years. A long-term follow-up is important in light of the well-accepted fact that the previously described changes (eg, atherosclerosis) develop over years, lead to morbidity, and potentially increased mortality, with a considerable time delay.
Importantly, our findings need to be interpreted in light of potential limitations. First, the lack of information regarding the cause of hypothyroidism, the inability to monitor Levothyroxine compliance, as well as lack of periodic measurements of thyroid function over time could have influenced our results. The magnitude and direction of this potential bias are unknown. Even had such data been available, patients would not have been randomized to treatment, but probably would have been treated based on the severity of disease. In observational studies, healthy adherence bias may contribute to more favorable outcomes with adherence to treatment. A recent study demonstrated that high adherence to placebo was associated with better clinical outcomes and decreased mortality (39). The impact of such possible bias is impossible to predict; however, by restricting our definition of hypothyroidism from the DNPrR to at least 2 dispensed prescriptions of Levothyroxine, we reduce this problem.
To us, it seems counterintuitive that hypothyroid patients, coded in databases, would be left untreated. Therefore, we assume that all individuals are treated for hypothyroidism. It follows that the excess mortality observed in subjects with hypothyroidism, to some extent, is due to genetic confounding and/or related to comorbidity.
Acknowledgments
Dr Laszlo Hegedüs is the recipient of an unrestricted research grant from the Novo Nordisk Foundation. This work was supported by the National Institute on Aging Grant P01 AG08761 (to K.C.).
The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure Summary: M.T., F.B., D.A., K.C., L.H., and T.H.B. have nothing to declare. M.T. is enrolled as a PhD student financed by The University of Southern Denmark in Odense and by The School of Endocrinology, University of Southern Denmark in Odense.
Footnotes
- ATC
- Anatomical Therapeutic Chemical
- CI
- confidence interval
- CS
- Charlson score
- DCRS
- Danish Civil Registration System
- DNPR
- The Danish National Patient Registry
- DNPrR
- The Danish National Prescription Registry
- HR
- hazard ratio.
References
- 1. Carle A, Laurberg P, Pedersen IB, et al. Epidemiology of subtypes of hypothyroidism in Denmark. Eur J Endocrinol. 2006;154:21–28 [DOI] [PubMed] [Google Scholar]
- 2. Vanderpump MP, Tunbridge WM. Epidemiology and prevention of clinical and sunclinical hypothyroism. Thyroid. 2002;12:839–847 [DOI] [PubMed] [Google Scholar]
- 3. Brix TH, Kyvik KO, Hegedüs L. A population-based study of chronic autoimmune hypothyroidism in Danish twins. J Clin Endocrinol Metab. 2000;85:536–539 [DOI] [PubMed] [Google Scholar]
- 4. Brix TH, Knudsen GP, Kristiansen M, Kyvik KO, Orstavik KH, Hegedüs L. High frequency of skewed X-chromosome inactivation in females with autoimmune thyroid disease: a possible explanation for the female predisposition to thyroid autoimmunity. J Clin Endocrinol Metab. 2005;90:5949–5953 [DOI] [PubMed] [Google Scholar]
- 5. Laurberg P, Cerqueira C, Ovesen L, et al. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab. 2010;24:13–27 [DOI] [PubMed] [Google Scholar]
- 6. Vestergaard R, Rejnmark L, Weeke J, et al. Smoking as a risk factor for Graves' disease, toxic nodular goiter, and autoimmune hypothyroidism. Thyroid. 2002;12:69–75 [DOI] [PubMed] [Google Scholar]
- 7. Brix TH, Hansen PS, Kyvik KO, Hegedüs L. Cigarette smoking and risk of clinically overt thyroid disease: a population-based twin case-control study. Arch Intern Med. 2000;160:661–666 [DOI] [PubMed] [Google Scholar]
- 8. Nyirenda MJ, Clark DN, Finlayson AR, et al. Thyroid disease and increased cardiovascular risk. Thyroid. 2005;15:718–724 [DOI] [PubMed] [Google Scholar]
- 9. Erem C. Thyroid disorders and hypercoagulability. Semin Thromb Hemost. 2011;37:17–26 [DOI] [PubMed] [Google Scholar]
- 10. Lekakis J, Papamichael C, Alevizaki M, et al. Flow-mediated, endothelium-dependent vasodilation is impaired in subjects with hypothyroidism, borderline hypothyroidism, and high-normal serum thyrotropin (TSH) values. Thyroid. 1997;7:411–414 [DOI] [PubMed] [Google Scholar]
- 11. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76–131 [DOI] [PubMed] [Google Scholar]
- 12. Biondi B, Klein I. Hypothyroidism as a risk factor for cardiovascular disease. Endocrine. 2004;24:1–13 [DOI] [PubMed] [Google Scholar]
- 13. Watt T, Groenvold M, Rasmussen AK, et al. Quality of life in patients with benign thyroid disorders. A review. Eur J Endocrinol. 2006;154:501–510 [DOI] [PubMed] [Google Scholar]
- 14. Goldman MB, Monson RR, Maloof F. Cancer mortality in women with thyroid disease. Cancer Res. 1990;50:2283–2289 [PubMed] [Google Scholar]
- 15. Maldonado LS, Murata GH, Hershman JM, et al. Do thyroid function tests independently predict survival in the critically ill? Thyroid. 1992;2:119–123 [DOI] [PubMed] [Google Scholar]
- 16. McQuade C, Skugor M, Brennan DM, Hoar B, Stevenson C, Hoogwerf BJ. Hypothyroidism and moderate subclinical hypothyroidism are associated with increased all-cause mortality independent of coronary heart disease risk factors: A PreCIS database study. Thyroid. 2011;21:837–843 [DOI] [PubMed] [Google Scholar]
- 17. Petersen K, Bengtsson C, Lapidus L, Lindstedt G, Nystrom E. Morbidity, mortality, and quality of life for patients treated with levothyroxine. Arch Intern Med. 1990;150:2077–2081 [PubMed] [Google Scholar]
- 18. Radacsi A, Kovács G, Bernard W, Feldkamp J, Horster FA, Szabolcs I. Mortality rate of chronically ill geriatric patients with subnormal thyrotropin concentration: a 2-yr follow up study. Endocrine. 2003;21:133–136 [DOI] [PubMed] [Google Scholar]
- 19. Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flynn RW, MacDonald TM, Jung RT, Morris AD, Leese GP. Mortality and vascular outcomes in patients treated for thyroid dysfunction. J Clin Endocrinol Metab. 2006;91:2159–2164 [DOI] [PubMed] [Google Scholar]
- 21. Alevizaki M, Synetou M, Xynos K, Alevizaki CC, Vemmos KN. Hypothyroidism as a protective factor in acute stroke patients. Clin Endocrinol (Oxf). 2006;65:369–372 [DOI] [PubMed] [Google Scholar]
- 22. Gussekloo J, ven Excel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591–2599 [DOI] [PubMed] [Google Scholar]
- 23. Thvilum M, Brandt F, Brix TH, Hegedüs L. A review of the evidence for and against increased mortality in hypothyroidism. Nat Rev Endocrinol. 2012;8:417–424 [DOI] [PubMed] [Google Scholar]
- 24. Brandt F, Green A, Hegedüs L, Brix TH. A critical review and meta-analysis of the association between hyperthyroidism and mortality. Eur J Endocrinol. 2011;165:491–497 [DOI] [PubMed] [Google Scholar]
- 25. Brix TH, Hegedüs L. Twin studies as a model for exploring the aetiology of autoimmune thyroid disease. Clin Endocrinol (Oxf). 2012;76:457–464 [DOI] [PubMed] [Google Scholar]
- 26. Herskind AM, McGue M, Holm NV, Sørensen TI, Harvald B, Vaupel JW. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870–1900. Hum Genet. 1996;97:319–323 [DOI] [PubMed] [Google Scholar]
- 27. Skytthe A, Pedersen NL, Kaprio J. Longevity studies in GenomEUtwin. Twin Res. 2003;6:448–454 [DOI] [PubMed] [Google Scholar]
- 28. Thygesen LC, Daasnes C, Thaulow I, Brønnum-Hansen H. Introduction to Danish (nationalwide) registers on health and social issues: Structure, access, legislation and archiving. Scand J Public Health. 2011;39:12–16 [DOI] [PubMed] [Google Scholar]
- 29. McGue M, Osler M, Christensen K. Causal interference and observational research: The utility of twins. Perspect Psychol Sci. 2010;5:546–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skytthe A, Kyvik KO, Holm NV, Christensen K. The Danish Twin Registry. Scand J Public Health. 2011;39:75–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thomsen AF, Kvist TK, Andersen PK, Kessing LV. Increased risk of affective disorder following hospitalization with hyperthyroidism—a register based study. Eur J Endocrinol. 2005;152:535–543 [DOI] [PubMed] [Google Scholar]
- 32. Cerquiera C, Knudsen N, Ovesen L, et al. Doubling in use of thyroid hormone replacement therapy in Denmark: association to iodization of salt? Eur J Epidemiol. 2011;26:629–635 [DOI] [PubMed] [Google Scholar]
- 33. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 34. Albertsen PC, Moore DF, Shih W, Lin Y, Li H, Lu-Yao GL. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29:1335–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Almagro P, Salvadó M, Garcia-Vidal C, et al. Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respiration. 2012;84:36–43 [DOI] [PubMed] [Google Scholar]
- 36. Rothman XJ, Greenland S, Lash TL. Case control studies. In: Seigafuse S, ed. Modern Epidemiology. Philadelphia, PA: Lippincott, Williams, Wilkins; 2008:111–127 [Google Scholar]
- 37. Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med. 2005;165:2460–2466 [DOI] [PubMed] [Google Scholar]
- 38. Carlé A, Laurberg P, Pedersen IB, et al. Mainly younger hypothyroid patients are referred to hospital—evidence for referral bias. J Clin Epidemiol. 2009;62:446–451 [DOI] [PubMed] [Google Scholar]
- 39. Curtis JR, Larson JC, Delzell E, et al. Placebo adherence, clinical outcomes, and mortality in the women's health initiative randomized hormone therapy trials. Med Care. 2011;49:427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]