Abstract
Context:
Optimizing effective prevention and treatment of type 2 diabetes in youth is limited by incomplete understanding of its pathophysiology and how this varies across ethnicities with high risk.
Objective:
The aim of this study was to examine the contribution of visceral adipose tissue (VAT), hepatic fat fraction (HFF), and pancreatic fat fraction (PFF) to prediabetes in overweight/obese African American (AA) and Latino youth.
Design and Setting:
We conducted a cross-sectional study in an academic pediatric care facility.
Subjects:
A total of 148 healthy, overweight/obese adolescents (56 AA, 92 Latino; 72 males, 76 females; age, 15.5 ± 1.2 y; BMI z-score, 2.1 ± 0.5) participated in the study. They were normal glucose tolerant (n = 106) and prediabetic (n = 42), based on fasting glucose of 100–125 mg/dL and/or 2-hour glucose of 140–199 mg/dL, and/or hemoglobin A1C 6.0–6.4%.
Main Outcome Measures:
We measured sc abdominal adipose tissue, VAT, HFF, and PFF by 3-Tesla magnetic resonance imaging and measured total body fat by dual-energy x-ray absorptiometry.
Results:
Adolescents with prediabetes had 30% higher HFF (P = .001) and 31% higher PFF (P = .042), compared to those with normal glucose tolerance after controlling for age, sex, pubertal stage, ethnicity, total percentage body fat, and VAT. Logistic regression showed that PFF predicted prediabetes in AAs and HFF predicted prediabetes in Latinos, with the odds of having prediabetes increased by 66% for every 1% increase in PFF in African Americans, and increased by 22% for every 1% increase in HFF in Latinos.
Conclusion:
These data demonstrate that ectopic fat phenotypes associated with prediabetes are established by adolescence. Ethnic differences in the deposition of ectopic fat in adolescents with prediabetes may differ, with pancreatic fat in AAs, vs hepatic fat in Latino adolescents, being associated with diabetes risk.
Pediatric obesity rates in the United States show a well-defined disparity by race and ethnicity, whereby 41.2% of African American and 42.4% of Latino youth 12–19 years old are ≥ the 85th percentile for body mass index (BMI), compared to 30.0% in Caucasians (1). These high obesity rates in minority youth are paralleled by higher incidence and prevalence rates of type 2 diabetes mellitus (T2DM) in African American and Latino populations (2, 3). The role of ectopic fat accumulation (ie, nonsubcutaneous adipose fat storage) in explaining the increased diabetes risk has gained considerable attention, due in part to the increasing prevalence of nonalcoholic fatty liver disease in children (4). However, few studies have assessed the relationship between high insulin resistance, ectopic fat stores, and diabetes risk in minorities, such as African American and Latino pediatric populations (5, 6). Latino children have a strong inverse relationship between visceral fat and insulin sensitivity, independent of total body fat (7). Tracking these children longitudinally showed that increasing visceral fat stores were predictive of persistent prediabetes (8). In a separate multiethnic cohort of obese children, it was shown that increased hepatic fat was associated with insulin resistance and prediabetes (9), independent of visceral and intramyocellular fat (10). These studies provide early insight into the potential role of visceral and hepatic fat stores in diabetes risk, but the pancreatic fat depot remains understudied. One recent study in children showed that pancreatic fat accumulation was associated with the metabolic syndrome and impaired insulin response (11). Further study is clearly needed to clarify the relationships between ectopic fat depots and metabolic disease risk.
The few studies that have aimed at differentiating the ectopic fat phenotypes that may contribute to risk of T2DM in minority children and adolescents have focused on visceral and hepatic fat, but none have addressed pancreatic fat deposition and its relationship to T2DM risk. In order to address this question, this study was designed to assess the relationship between ectopic fat depots and prediabetes, using advanced 3-dimensional (3-D) magnetic resonance imaging (MRI) methodology. Our objective was to examine the differences in visceral, hepatic, and pancreatic fat deposition in prediabetic vs normal glucose-tolerant overweight African American and Latino adolescents. The specific hypotheses were that: 1) hepatic and pancreatic fat deposition, independent of visceral and general adiposity, would be higher in those subjects with prediabetes than those with normal glucose tolerance (NGT); and 2) the patterns of visceral, hepatic, and pancreatic fat deposition in youth with prediabetes vs those with NGT would be different in African Americans compared to Latinos.
Subjects and Methods
Participants
This report presents the main outcomes analysis for the Diabetes Risk due to Ectopic Adiposity in Minority Youth (DREAM) study, a cross-sectional investigation into relationships between ectopic fat distribution and T2DM risk in overweight minority adolescents. Overweight or obese, but otherwise healthy African American and Latino-American adolescent males and females (n = 148) were recruited from greater Los Angeles County and met the following inclusion criteria: 1) either African American (all four grandparents of Afro-American descent) or Latino ethnicity (all four grandparents of Latin-American descent); 2) age 14–17 years; and 3) age- and gender-adjusted BMI ≥ 85th percentile based on the standards of the Centers for Disease Control and Prevention. Children were excluded if they had a previous major illness, including type 1 or 2 diabetes, took medications, or had a condition known to influence body composition, insulin action, or insulin secretion. Four participants were diagnosed with T2DM during the screening visit and were therefore excluded from the study. Participants and their parents provided written informed consent. The study was approved by the Institutional Review Board of the University of Southern California (USC), Health Sciences Campus, Los Angeles.
Procedures
All participants attended 2 visits at the Clinical Trials Unit (CTU) at the USC University Hospital. On the first visit, participants received a comprehensive medical history and physical examination by a licensed health care provider. Pubertal stage was determined (by physical examination) as breast stage for girls and pubic hair stage for boys (12). Clinical staff collected blood pressure in a seated position in triplicate and performed a 2-hour oral glucose tolerance test (OGTT). Blood was drawn in the fasting state and then at 30, 60, and 120 minutes after oral glucose challenge (1.75 g oral glucose solution/kg body weight to a maximum 75 g). Within approximately 2 months (median, 24 d) after the outpatient visit, participants were admitted in the late afternoon for their second overnight inpatient visit at the USC CTU. Participants were served dinner and a snack before 8 pm, after which only water was permitted overnight. At 6:30 am the following morning, a 13-sample insulin-modified frequently sampled iv glucose tolerance test (FSIVGTT) was performed as follows. Intravenous catheters were placed in the antecubital fossae of both arms, one for infusions and one for sampling. After 2 fasting blood samples were taken at −15 and −5 minutes, glucose (0.3 g/kg body weight) was administered at time 0 over a 1-minute period. Subsequent blood samples were collected at 2, 4, 8, and 19 minutes. Insulin (0.02 U/kg body weight, Humulin R; Eli Lilly, Indianapolis, Indiana) was administered iv at 20 minutes, followed by blood sample collection at 22, 30, 40, 50, 70, 100, and 180 minutes. Total body composition was determined at either visit, based on availability of the participant and staff, by dual-energy x-ray absorptiometry using a Hologic QDR 4500 W (Hologic, Bedford, Massachusetts).
All subjects underwent MRI scanning on a commercial 3-Tesla MRI system (Excite HD; GE Healthcare, Waukesha, Wisconsin) equipped with an 8-channel abdominal coil array. The MRI pulse sequence consisted of a chemical-shift technique known as IDEAL (GE Healthcare). This approach generated separate water and fat datasets of the anatomy on a voxel-wise basis. The multibreath-hold, 3-D whole-abdomen scan produced several quantitative endpoints including visceral and sc adipose tissue (VAT and SAT; measured in liters) and hepatic and pancreatic fat fractions (HFF and PFF; measured as the percentage of fat found within organ tissue). After MRI data acquisition, the image postprocessing, tissue segmentation, and analysis were all performed off-line on a dedicated workstation by a single analyst using a commercial semiautomated software tool (SliceOmatic; Tomovision, Inc., Montreal, Québec, Canada). This approach has been described in detail in literature and has been validated against other modalities (13–16).
Assays
Glucose was assayed using a Yellow Springs Instruments analyzer (YSI Inc., Yellow Springs, Ohio) that uses a membrane-bound glucose oxidase technique. Insulin was assayed using an automated enzyme immunoassay (Tosoh AIA 600 II analyzer; Tosoh Bioscience, Inc., South San Francisco, California; sensitivity, 0.31 μU/mL; intraassay coefficient of variation, 2.9%; interassay coefficient of variation, 5.8%). Hemoglobin A1C was measured by HPLC (Tosoh 11c 2.2 HLC-723; Tosoh, Tokyo, Japan), an assay approved by the International Federation of Clinical Chemistry Working Group (IFCC-WG) on A1C standardization. The interassay coefficients of variation were 0.76 and 0.57% for A1C of 6.2 and 10.3%, respectively.
Calculation of insulin resistance and secretion parameters
Glucose and insulin from the FSIVGTT were entered into MINMOD software (version 6.02; RN Bergman, Los Angeles, California) for calculation of whole-body insulin sensitivity (SI), the acute insulin response to glucose (AIRg; the area under the plasma insulin curve between 0 and 10 min, an early phase secretion in response to iv glucose bolus), and the disposition index (DI; the product of SI and AIRg, a measure of the ability of the islet cells to secrete insulin normalized to the degree of insulin resistance—ie, an assessment of β-cell function) (17). Homeostasis model of assessment–insulin resistance (HOMA-IR) was calculated as: [fasting glucoseOGTT (mg/dL) × fasting insulinOGTT (μU/mL)]/405.
Categorization of participants into prediabetes and NGT groups
The 148 subjects were divided for the primary analysis into 2 groups based on results from the OGTT visit: NGT (n = 106; fasting glucose < 100 mg/dL, 2-h glucose < 140 mg/L, and A1C < 6.0%) or prediabetic (n = 42; fasting glucose ≥ 100 mg/dL, and/or 2-h glucose of 140–199 mg/dL, and/or A1C 6.0–6.4%). The A1C criteria are based on the recommendations of the International Expert Committee (18) and our prior work demonstrating compromised β-cell function in overweight Latino teens with A1C between 6.0 and 6.4% (19).
Statistical analysis
Tests of normality (Q-Q plots, Shapiro-Wilkes test) were used to assess the distribution of all continuous variables. Non-normal variables were transformed as follows: 1) log-transformation: HFF, PFF, SI, AIRg, DI, HOMA-IR, and fasting insulin; and 2) square-root transformation: VAT and SAT. Adjusted means were back-transformed for figures. For descriptive purposes, a one-way ANOVA was used to determine mean physical and metabolic characteristics by prediabetes status. Unadjusted means ± SD were reported in Table 1. To test our first hypothesis, a one-way analysis of covariance was used to test for differences in ectopic fat deposition in those with and without prediabetes while adjusting a priori covariates of age, pubertal stage, sex, ethnicity, total percentage body fat and VAT, where appropriate. Adjusted means ± SE are shown in Figure 1. To test for ethnic differences in ectopic fat distribution by prediabetes status, we first performed unadjusted 2-way ANOVA, followed subsequently by 2-way ANCOVA, adjusting for a priori covariates of age, pubertal stage, sex, and total percentage body fat. Analyses that used HFF or PFF as the dependent variable also included VAT as a covariate. If a P value for an interaction was P < .10, the analyses were then further explored after stratifying by African American or Latino ethnicity. To determine the primary fat depot predictors of prediabetes in each ethnicity, multiple logistic regression was employed using age, sex, total percentage fat, VAT, SAT, HFF, and PFF as predictors. In post hoc analyses of the subset of adolescents with prediabetes, we examined the relationship between HFF and PFF with insulin secretion (AIR). Data were analyzed using SPSS for Mac version 18.0 (IBM Inc, Chicago, Illinois), with an a priori significance level of P < .05.
Table 1.
Physical and Metabolic Characteristics by Prediabetes Status
| NGT | Prediabetes | |
|---|---|---|
| n | 106 | 42 |
| Age, y | 16.0 ± 1.2 | 16.1 ± 1.1 |
| Gender (male/female), n | 51/55 | 21/21 |
| Pubertal stage, n | ||
| 1–3 | 2 | 3 |
| 4 | 18 | 10 |
| 5 | 86 | 29 |
| Height, cm | 166.7 ± 9.5 | 165.8 ± 8.8 |
| Weight, kg | 90.3 ± 19.5 | 96.0 ± 22.9 |
| Adiposity measures | ||
| BMI, kg/m2 | 32.2 ± 5.4 | 35.0 ± 7.8a |
| BMI z-score | 1.96 ± 0.47 | 2.11 ± 0.57a |
| Total % fat | 36.5 ± 8.1 | 37.1 ± 7.4 |
| Total fat mass, kg | 31.6 ± 9.7 | 34.8 ± 12.9a |
| Total lean mass, kg | 52.5 ± 11.6 | 55.3 ± 10.6 |
| Ectopic fat depots | ||
| SAT, L | 7.2 ± 3.3 | 8.4 ± 4.2 |
| VAT, L | 1.5 ± 1.0 | 2.0 ± 1.4 |
| HFF, % | 3.9 ± 2.9 | 8.3 ± 9.2c |
| PFF, % | 3.3 ± 2.0 | 4.5 ± 3.0b |
| Metabolic parameters | ||
| Fasting glucose, mg/dL | 84.7 ± 5.9 | 89.7 ± 7.7c |
| 2-h glucose, mg/dL | 110.9 ± 17.3 | 133.4 ± 26.1c |
| Hemoglobin A1C, % | 5.5 ± 0.3 | 5.9 ± 0.3c |
| HOMA-IR | 3.1 ± 2.2 | 4.6 ± 3.0b |
| SI, × 10−4 min−1/μU/mL | 1.84 ± 1.10 | 1.28 ± 0.86c |
| AIRg, μU/mL × 10 min | 1519 ± 1002 | 1722 ± 1484 |
| DI | 2210 ± 1154 | 1641 ± 989b |
Data are expressed as unadjusted means ± SD.
P < .05;
P < .01;
P < .001.
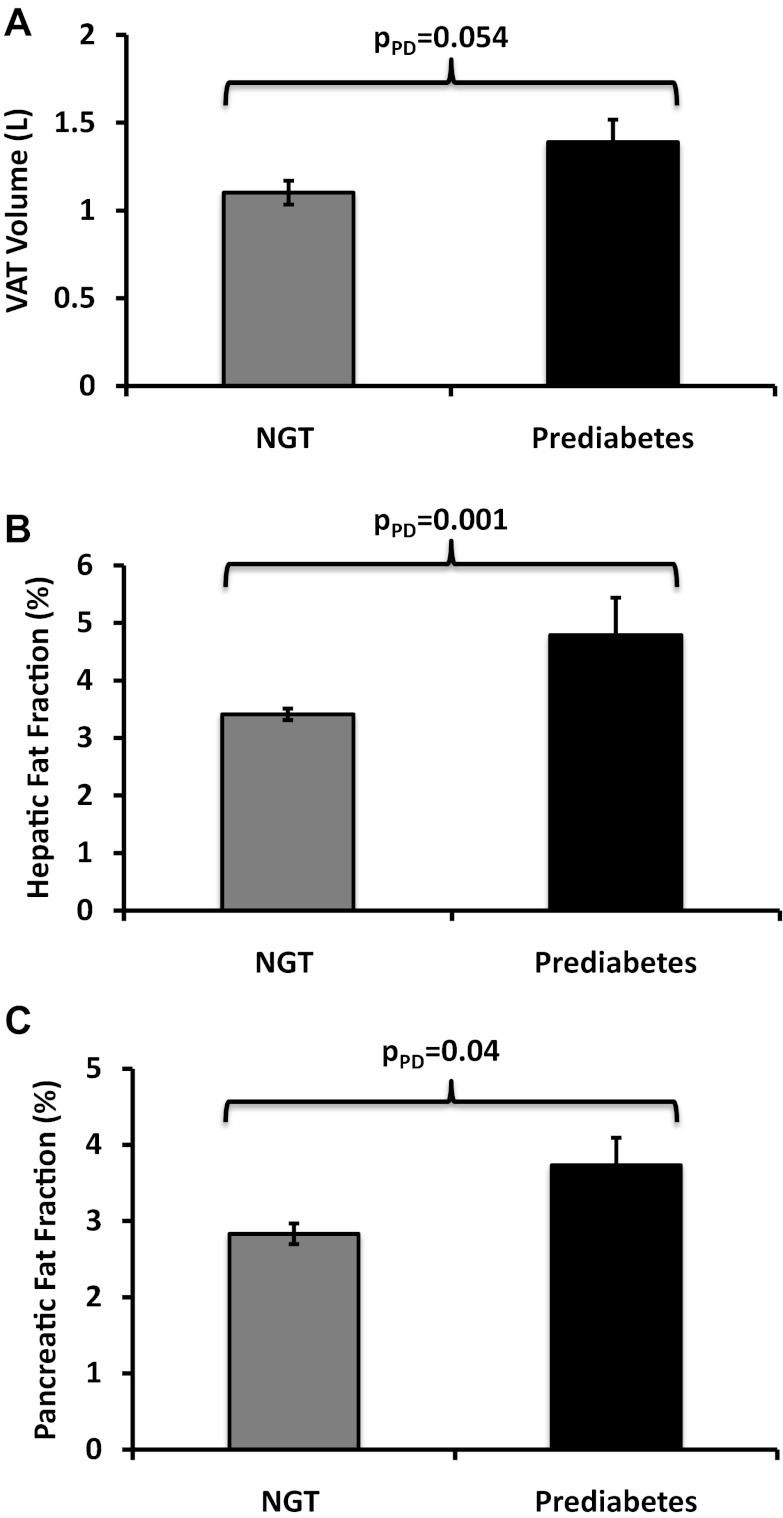
Figure 1.
VAT volume (A) and HFF (B) and PFF (C) by NGT and prediabetes groups. Data shown are adjusted means ± SE.
Results
Physical and metabolic characteristics in participants by prediabetes status are shown in Table 1. There were no significant differences between groups in age, gender, pubertal stage, height, weight, total percentage fat, SAT, or VAT. Participants with prediabetes had a higher BMI (P = .03), BMI z-score (P = .04), total fat mass (P = .04), HFF (P < .001), and PFF (P = .004) than participants with NGT. Prediabetic adolescents also had higher fasting glucose, 2-hour glucose, A1C, and HOMA-IR, whereas SI and DI were lower compared to the NGT group (all P values < .01).
The adjusted distributions of visceral and ectopic fat stores by prediabetes status are illustrated in Figure 1, A–C. Prediabetic adolescents had borderline higher VAT when compared to those with NGT (P = .054; Figure 1A) after controlling for a priori covariates of age, sex, pubertal stage, ethnicity, and total percentage fat. When compared to adolescents with NGT, prediabetic adolescents had 30% higher HFF (P = .001; Figure 1B) and 31% higher PFF (P = .042; Figure 1C), after controlling for age, sex, pubertal stage, ethnicity, total percentage body fat, and VAT.
We next explored ethnic differences in ectopic fat deposition related to prediabetes status. As shown in previous literature, there were main effects of ethnicity on ectopic fat depots, whereby Latinos had higher VAT (mean ± SE, 2.0 ± 0.13 vs 1.1 ± 0.6 L; P < .001) and HFF (6.4 ± 0.7 vs 3.2 ± 0.3%; P < .001) than their African American counterparts, whereas total percentage fat, total fat mass, SAT, and PFF did not differ by ethnicity (all P > .05, unadjusted analyses). Although Latino adolescents had higher VAT when compared to African American adolescents (P < .001), the magnitude of VAT deposition between those with prediabetes and NGT did not differ by ethnicity after controlling for covariates of age, sex, pubertal stage, and total percentage body fat (adjusted, pinteraction = 0.73). HFF was not related to ethnicity (adjusted, P = .12), and degree of HFF between those with prediabetes and NGT also did not differ by ethnicity (adjusted, pinteraction = 0.25) after adjusting for age, sex, pubertal stage, total percentage body fat, and VAT. Although PFF was not related to ethnicity (adjusted, P = .32), the relationship between PFF and prediabetes status was different in African Americans compared to Latinos (adjusted, pinteraction = 0.06). When stratified by ethnicity, African Americans with prediabetes had a 63% higher PFF than those with NGT (4.2 ± 1.5 vs 2.6 ± 0.6%; P = .006), whereas there was no difference between prediabetes and NGT in Latinos (3.2 ± 1.5 vs 3.2 ± 0.6%; P = .79) after controlling for age, sex, pubertal stage, total percentage body fat, and VAT.
To gain further understanding of the predictors of prediabetes, we employed multiple logistic regression using predictors of age, sex, total percentage body fat, VAT, SAT, HFF, and PFF in African Americans and Latinos (Table 2). This analysis revealed that pancreatic fat independently predicted prediabetes in African Americans, whereas hepatic fat independently predicted prediabetes in Latinos. Specifically, for every 1% increase in PFF, the odds of having prediabetes increased by 66% in African Americans (95% confidence interval [CI], 1.08–2.56; P = .02); in Latinos, for every 1% increase in HFF, the odds of having prediabetes increased by 22% (95% CI, 1.06–1.41; P = .01; Table 2).
Table 2.
Multiple Logistic Regression: Predictors of Prediabetes by Ethnicity
| Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| Model 1: African Americans | |||
| Age | 1.00 | 0.94–1.06 | .92 |
| Sex | 0.39 | 0.05–2.85 | .36 |
| Total % fat | 1.00 | 1.00–1.00 | .86 |
| VAT | 1.28 | 0.77–2.1 | .34 |
| SAT | 0.42 | 0.05–3.45 | .42 |
| HFF | 1.19 | 0.83–1.71 | .34 |
| PFF | 1.66 | 1.08–2.56 | .02 |
| Model 2: Latinos | |||
| Age | 1.03 | 0.99–1.07 | .19 |
| Sex | 0.95 | 0.31–2.94 | .93 |
| Total % fat | 1.00 | 1.00–1.00 | .62 |
| VAT | 0.93 | 0.72–1.21 | .59 |
| SAT | 0.99 | 0.48–2.04 | .98 |
| HFF | 1.22 | 1.06–1.41 | .01 |
| PFF | 1.07 | 0.84–1.36 | .57 |
Boldface type indicates significant P values.
Discussion
In a group of overweight and obese African American and Latino adolescents, our primary objective was to examine differences in ectopic fat distribution in prediabetes vs NGT controls using advanced MRI techniques. We found that ectopic fat in pancreatic and hepatic depots was higher in minority adolescents with prediabetes when compared to those without prediabetes, independent of total body fat and visceral adiposity. Although this is not the first paper to describe a relationship between hepatic fat and prediabetes, this is the first study to report a relationship between pancreatic fat and prediabetes in any adolescent population. We also found ethnic differences in ectopic fat distribution as it relates to prediabetes status, such that pancreatic fat independently predicted prediabetes in African American adolescents, whereas in Latino adolescents, hepatic fat was the sole predictor.
It is generally well accepted that insulin resistance and progression to T2DM are strongly associated with obesity. Among the competing theories that attempt to explain this relationship are the Randle/portal theory (20, 21) and the “ectopic fat storage syndrome” (4). The former theory postulates that excess free fatty acids released from the highly insulin-resistant VAT spills into the liver via the portal vein, leading to excess hepatic adiposity and subsequently leading to increased insulin resistance. In contrast, the ectopic fat storage syndrome emerged as a new paradigm whereby Ravussin and Smith (4) proposed that an increase in fat cell size may be due to the inability of the adipose tissue mass to proliferate and differentiate, leading to adipocyte hypertrophy. Therefore, these enlarged adipocytes cannot accommodate increased energy flux or dietary fat intake, which then leads to triglyceride accumulation in the surrounding organs (ie, ectopic adiposity). In the current study, we observed higher pancreatic fat in African Americans and higher hepatic fat in Latinos with prediabetes when compared to those without prediabetes. Given that our findings were independent of VAT, our results support the ectopic fat syndrome theory, as opposed the Randle/portal theory, but it does not indicate causality. However, others have shown that ectopic adiposity could precede and cause insulin resistance. A landmark article by Samuel Klein's laboratory suggests that ectopic fat accumulation may result in a redirecting of free fatty acid uptake from the adipose tissue to surrounding organs, such as the liver and pancreas, and that ectopic fat, not visceral fat, is the cause of metabolic complications (22). Because our results do not dictate causality, longitudinal studies with larger sample sizes would be necessary to clarify the exact role of these specific ectopic fat depots in the progression from NGT to T2DM in minority adolescents.
The causal relationship between fatty liver and hyperglycemia has not been fully elucidated. However, given the liver's metabolic roles, many have suggested that fatty liver and subsequent hepatic alterations precede metabolic disorders. It is possible that hepatic triglyceride content impairs hepatic insulin sensitivity, thereby decreasing the ability of insulin to suppress endogenous glucose production. This has been shown in studies with adults and dogs (23, 24). In this study, we show that in both African American and Latino overweight adolescents, even a relatively small amount of increased hepatic fat such as seen in the African American group, was associated with prediabetes. This suggests that fatty liver may precede T2DM. However, there is a new body of evidence that shows that genetic hypobetalipoproteinemia is strongly related to increased hepatic fat and that fatty liver does not necessarily cause insulin resistance (25–27). Collectively, the literature shows that further research to clarify the causality of these relationships is necessary.
In our study, we cannot say with certainty that the measured pancreatic fat is located in the islets. However, studies in obese Zucker rats have shown the fat content of the whole pancreas paralleled the amount of fat found in the islets, which may play a role in observed lipid-mediated β-cell destruction (28, 29). Lipotoxicity leading to pancreatic β-cell apoptosis has been hypothesized as a plausible mechanism whereby increased triglycerides and their by-products, such as ceramide and other toxic metabolites, lead to β-cell destruction (30). In animal models, fatty acids have been hypothesized to promote β-cell hyperplasia and insulin hypersecretion during times of excess caloric intake (31), but over time, the increase in pancreatic lipid accumulation can lead to islet dysfunction and cell death (32, 33). Further evidence to support these findings in animals has been observed in human adults, where increased pancreatic triglyceride stores have been observed in impaired fasting glucose or impaired glucose tolerance (34, 35). However, conflicting results have been reported regarding the relationship between pancreatic fat deposition and insulin secretion. Szczepaniak et al (36) found a positive relationship between AIR and PFF, whereas other laboratories have reported pancreatic triglycerides to be directly related to obesity and inversely related to insulin secretion (34, 35, 37). In one of these studies, the inverse relationship between pancreatic fat and insulin secretion was shown only among those with impaired fasting glucose or impaired glucose tolerance (37). Therefore, we ran a post hoc analysis in our cohort of adolescents with prediabetes to assess whether this relationship of pancreatic fat and insulin secretion also existed. We found that among the African Americans with prediabetes, there was a trend toward an inverse relationship with pancreatic fat and AIRg during the FSIVGTT (r = −0.51; P = .09), but no similar relationship was observed in the prediabetic Latinos. Thus, in prediabetic African American adolescents, there is the suggestion that pancreatic fat is inversely related to early phase insulin secretion (AIRg). Hence, in African American youth, but not Latino youth, small increases in pancreatic fat may be a component to impaired insulin secretion when prediabetes is present.
This report has potential implications to lifestyle changes and interventions for minority populations. Intervention studies in obese/overweight children show that combined nutrition and strength-training interventions decrease liver fat (38–40), but they have not addressed possible decreases in pancreatic fat as a result of these interventions. Future studies should assess intervention effects on pancreatic fat, and in turn, insulin secretion.
There are a number of strengths in our study that are worth noting. To our knowledge, this is the first study to include the use of recently recommended A1C cutoffs (19) in the categorization of prediabetes groups to study differences in ectopic fat deposition and T2DM risk. By using the A1C criteria for T2DM risk, we were able to identify more youth at risk and therefore better define the ectopic fat phenotypes of adolescents with heightened diabetes risk—ie, prediabetes. Secondly, this work benefits from the use of state-of-the-art 3-D whole abdomen MRI techniques to more accurately measure pancreatic fat content in overweight adolescents, a metric that has been predominantly assessed with single-voxel magnetic resonance spectroscopy. Thirdly, using rigorous direct measures of insulin action and secretion allowed us to better determine relationships between ectopic fat stores and insulin sensitivity/secretion dynamics than would have been possible using coarser measures, such as fasting insulin or HOMA-IR alone. Our study limitations include the relatively small sample size in the African American prediabetes group (n = 13), which may have limited the power to detect ethnic differences in prediabetes vs NGT groups and to clearly define relationships between pancreatic fat and insulin secretion. Lastly, whereas the stringent ethnic criterion is on the one hand another strength of the study, it also prohibits generalization of our findings beyond the African American and Latino adolescent populations studied.
In conclusion, our study showed that without specific regard to ethnicity, overweight/obese minority adolescents with prediabetes had increased ectopic fat storage in the liver and pancreas when compared to those without prediabetes, independent of overall adiposity and visceral adiposity. The ethnic differences in ectopic fat phenotypes associated with prediabetes in minority youth suggest that pancreatic fat in African Americans, vs hepatic fat in Latino adolescents, may be driving T2DM risk. Further mechanistic studies will be needed to fully determine a causative role for ectopic fat storage in the pathogenesis of T2DM in minority overweight youth.
Acknowledgments
We thank the staff of the University of Southern California/Los Angeles County General Clinical Research Center/Clinical Trials Unit and the dedicated DREAM staff; Quintilia Ávila, MPH; and Michelle Muñevar, MPH; and the rest of the DREAM staff over the past 5 years. Our gratitude is especially extended to the participants and their families for their participation.
C.M.T.-C. and M.J.W. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This study is supported by National Institutes of Health (NIH) National Center on Minority Health and Health Disparities Grant P60MD002254 (to M.I.G. and M.J.W.). C.M.T.-C. is supported by an American Diabetes Association Postdoctoral Fellowship (7-10-MI-04).
This study was also supported in part by NIH Grant UL1TR000130.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AIRg
- acute insulin response to glucose
- BMI
- body mass index
- 3-D
- 3-dimensional
- DI
- disposition index
- FSIVGTT
- frequently sampled iv glucose tolerance test
- HFF
- hepatic fat fraction
- HOMA-IR
- homeostasis model of assessment–insulin resistance
- MRI
- magnetic resonance imaging
- NGT
- normal glucose tolerance
- OGTT
- oral glucose tolerance test
- PFF
- pancreatic fat fraction
- SAT
- sc adipose tissue
- SI
- insulin sensitivity
- T2DM
- type 2 diabetes mellitus
- VAT
- visceral adipose tissue.
References
- 1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dabelea D, Bell RA, D'Agostino RB, Jr, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724 [DOI] [PubMed] [Google Scholar]
- 4. Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann NY Acad Sci. 2002;967:363–378 [DOI] [PubMed] [Google Scholar]
- 5. Goran MI, Bergman RN, Cruz ML, Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care. 2002;25:2184–2190 [DOI] [PubMed] [Google Scholar]
- 6. Hasson RE, Adam TC, Davis JN, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab. 2010;95:4048–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cruz ML, Bergman RN, Goran MI. Unique effect of visceral fat on insulin sensitivity in obese Hispanic children with a family history of type 2 diabetes. Diabetes Care. 2002;25:1631–1636 [DOI] [PubMed] [Google Scholar]
- 8. Goran MI, Lane C, Toledo-Corral C, Weigensberg MJ. Persistence of pre-diabetes in overweight and obese Hispanic children: association with progressive insulin resistance, poor β-cell function, and increasing visceral fat. Diabetes. 2008;57:3007–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cali AM, Zern TL, Taksali SE, et al. Intrahepatic fat accumulation and alterations in lipoprotein composition in obese adolescents: a perfect proatherogenic state. Diabetes Care. 2007;30:3093–3098 [DOI] [PubMed] [Google Scholar]
- 10. D'Adamo E, Cali AM, Weiss R, et al. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care. 2010;33:1817–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maggio AB, Mueller P, Wacker J, et al. Increased pancreatic fat fraction is present in obese adolescents with metabolic syndrome. J Pediatr Gastroenterol Nutr. 2012;54(6):720–726 [DOI] [PubMed] [Google Scholar]
- 12. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu HH, Nayak KS, Goran MI. Assessment of abdominal adipose tissue and organ fat content by magnetic resonance imaging. Obes Rev. 2011;12:e504–e515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu HH, Kim HW, Nayak KS, Goran MI. Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity (Silver Spring). 2010;18:841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alabousi A, Al-Attar S, Joy TR, Hegele RA, McKenzie CA. Evaluation of adipose tissue volume quantification with IDEAL fat-water separation. J Magn Reson Imaging. 2011;34:474–479 [DOI] [PubMed] [Google Scholar]
- 16. Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2001;34(4):spcone [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–1015 [DOI] [PubMed] [Google Scholar]
- 18. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toledo-Corral CM, Vargas LG, Goran MI, Weigensberg MJ. Hemoglobin A1c above threshold level is associated with decreased beta-cell function in overweight Latino youth. J Pediatr. 2012;160(5):751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11:351–356 [DOI] [PubMed] [Google Scholar]
- 21. Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789 [DOI] [PubMed] [Google Scholar]
- 22. Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430–15435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028 [DOI] [PubMed] [Google Scholar]
- 24. Kim SP, Ellmerer M, Van Citters GW, Bergman RN. Primacy of hepatic insulin resistance in the development of the metabolic syndrome induced by an isocaloric moderate-fat diet in the dog. Diabetes. 2003;52:2453–2460 [DOI] [PubMed] [Google Scholar]
- 25. Visser ME, Lammers NM, Nederveen AJ, et al. Hepatic steatosis does not cause insulin resistance in people with familial hypobetalipoproteinaemia. Diabetologia. 2011;54:2113–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amaro A, Fabbrini E, Kars M, et al. Dissociation between intrahepatic triglyceride content and insulin resistance in familial hypobetalipoproteinemia. Gastroenterology. 2010;139:149–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schonfeld G, Yue P, Lin X, Chen Z. Fatty liver and insulin resistance: not always linked. Trans Am Clin Climatol Assoc. 2008;119:217–223; discussion 223–224 [PMC free article] [PubMed] [Google Scholar]
- 28. Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. β-Cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-β-cell relationships. Proc Natl Acad Sci USA. 1994;91:10878–10882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee Y, Hirose H, Zhou YT, Esser V, McGarry JD, Unger RH. Increased lipogenic capacity of the islets of obese rats: a role in the pathogenesis of NIDDM. Diabetes. 1997;46:408–413 [DOI] [PubMed] [Google Scholar]
- 30. van Raalte DH, van der Zijl NJ, Diamant M. Pancreatic steatosis in humans: cause or marker of lipotoxicity? Curr Opin Clin Nutr Metab Care. 2010;13:478–485 [DOI] [PubMed] [Google Scholar]
- 31. Milburn JL, Jr, Hirose H, Lee YH, et al. Pancreatic β-cells in obesity. Evidence for induction of functional, morphologic, and metabolic abnormalities by increased long chain fatty acids. J Biol Chem. 1995;270:1295–1299 [DOI] [PubMed] [Google Scholar]
- 32. Shimabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH. Lipoapoptosis in β-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem. 1998;273:32487–32490 [DOI] [PubMed] [Google Scholar]
- 33. Shimabukuro M, Wang MY, Zhou YT, Newgard CB, Unger RH. Protection against lipoapoptosis of β-cells through leptin-dependent maintenance of Bcl-2 expression. Proc Natl Acad Sci USA. 1998;95:9558–9561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heni M, Machann J, Staiger H, et al. Pancreatic fat is negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance: a nuclear magnetic resonance study. Diabetes Metab Res Rev. 2010;26:200–205 [DOI] [PubMed] [Google Scholar]
- 35. van der Zijl NJ, Goossens GH, Moors CC, et al. Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on β-cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metab. 2011;96:459–467 [DOI] [PubMed] [Google Scholar]
- 36. Szczepaniak LS, Victor RG, Mathur R, et al. Pancreatic steatosis and its relationship to β-cell dysfunction in humans: racial and ethnic variations. Diabetes Care. 2012;35(11):2377–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tushuizen ME, Bunck MC, Pouwels PJ, et al. Pancreatic fat content and β-cell function in men with and without type 2 diabetes. Diabetes Care. 2007;30:2916–2921 [DOI] [PubMed] [Google Scholar]
- 38. Santomauro M, Paoli-Valeri M, Fernandez M, et al. Non-alcoholic fatty liver disease and its association with clinical and biochemical variables in obese children and adolescents: effect of a one-year intervention on lifestyle. Endocrinol Nutr. 2012;59(6):346–353 [DOI] [PubMed] [Google Scholar]
- 39. Hasson RE, Adam TC, Davis JN, et al. Randomized controlled trial to improve adiposity, inflammation, and insulin resistance in obese African-American and Latino youth. Obesity (Silver Spring). 2011;20:811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koot BG, van der Baan-Slootweg OH, Tamminga-Smeulders CL, et al. Lifestyle intervention for non-alcoholic fatty liver disease: prospective cohort study of its efficacy and factors related to improvement. Arch Dis Child. 2011;96:669–674 [DOI] [PubMed] [Google Scholar]