Abstract
Context:
Active Cushing's disease (CD) confers a 4-fold increase in mortality and is associated with significant morbidities. Although excess mortality risk may persist even after CD treatment, predictors of risk in treated CD are not well understood.
Objective:
To identify predictors of mortality, cardiovascular (CV) disease, and recurrence after long-term follow-up among patients with treated CD.
Design, Setting, and Patients:
A retrospective chart review was conducted to evaluate patients with CD who underwent transsphenoidal adenectomy with a single surgeon.
Outcome Measures:
Patients were categorized based on disease response after initial treatment. Cox proportional hazard models identified predictors of mortality, recurrence, and CV outcomes in the overall cohort and each subgroup.
Results:
Three hundred forty-six subjects were included. Mean age was 39.9 years, and mean duration of follow-up was 6.3 years (range, 1 mo to 30 y). Duration of exposure to excess glucocorticoids, estimated by duration of symptoms before diagnosis until remission was achieved by any means, was 40.0 months. Multivariate analyses demonstrated that duration of glucocorticoid exposure elevated the risk of death (P = .038), as did older age at diagnosis (P = 0.0001) and preoperative ACTH concentration (P = .007). Among patients who achieved remission, depression increased the hazard of death (P < .01). Male sex, age at diagnosis, diabetes, and depression elevated the risk of CV disease (P < .05).
Conclusion:
Long-term follow-up of a large cohort of treated patients with CD identified several novel predictors of mortality. These data illustrate the importance of early recognition and treatment of CD. Long-term follow-up, with management of persistent comorbidities, is needed even after successful treatment of CD.
Cushing's disease (CD) is a rare condition that leads to excess endogenous glucocorticoid (GC) exposure, resulting from an ACTH-secreting pituitary corticotroph adenoma. The incidence of newly diagnosed CD is estimated to be 1.2 to 2.4 per million per year (1, 2). Untreated CD is associated with significant morbidity and mortality, as initially described by Harvey Cushing (3) and confirmed by a later study showing a 5-year survival rate of 50% (4). Significant morbidities, including hypertension (HTN), insulin resistance, type 2 diabetes, dyslipidemia, and abdominal obesity, contribute to the adverse cardiometabolic profile and subsequent disease burden (5). Short-term follow-up data suggest that cardiovascular (CV) disease confers a 4-fold increase in mortality among patients with persistent disease compared with control populations (1, 5, 6). Longer term studies following patients from 5 to more than 10 years after transsphenoidal surgery (TSS) have shown decreased survival rates among patients with persistent or recurrent disease compared with controls (7–11).
It is not known whether disease remission entirely reverses these morbidities and normalizes survival rates. Both normalized (2, 8, 9, 11) and decreased (7, 10) survival has been shown in CD remission compared with the general population. Several predictors of excess mortality in CD remission have been proposed, including duration of GC exposure, older age, HTN, and abnormal glucose metabolism, but data supporting these risk factors are limited. Indeed, there is need for accurate characterization of risk factors that contribute to increased morbidity and mortality among patients with treated CD to stratify patients and develop treatment strategies. Therefore, the aims of our study were to identify predictors of mortality, CV disease, and recurrence in a large cohort of patients with treated CD operated on by a single surgeon. Identifying these long-term predictors of adverse outcomes will provide further insight into persistent complications associated with the post-hypercortisolemic state and improve the care of patients with CD and other patients exposed to chronic excess GCs.
Patients and Methods
Patient population
We conducted a retrospective chart review of 367 consecutive CD patients who underwent transsphenoidal surgery (TSS) by a single surgeon (K.D.P.) operating at tertiary care centers in the Eastern United States between 1980 and 2011. Patients were excluded from the study if they had a final diagnosis confirming 1) ectopic CS (n = 1); 2) silent ACTH tumors (n = 4); or 3) lack of chart data definitively confirming a preoperative diagnosis of ACTH-dependent hypercortisolism (based on review of all clinical, biochemical, and/or radiographic data) (n = 16). Analyses were conducted on the remaining 346 patients (baseline characteristics shown in Table 1).
Table 1.
Preoperative Characteristics
| Characteristic | Total Cohort (n = 346) |
|---|---|
| Age at diagnosis, ya | 39.9 (7–77) |
| Female:maleb | 265:81 (77%) |
| Duration of follow-upa | 6.3 y (1 mo to 30 y) |
| Duration of GC exposure, moc,d | 40 (2 mo to 20 y) |
| Comorbiditiesb | |
| Hypertension | 248 (72%) |
| Diabetes | 95 (27%) |
| Depression | 76 (22%) |
| Psychosis | 6 (2%) |
| Osteoporosis | 67 (19%) |
| Coronary artery disease | 14 (4%) |
| Cerebrovascular accident | 4 (1%) |
| Dyslipidemia | 55 (16%) |
| MRI findingsb,e | |
| Microadenoma | 117 (34%) |
| Macroadenoma | 39 (11%) |
| No visible adenoma | 87 (25%) |
| Inhomogeneous pituitaryf | 67 (19%) |
| BIPSSb | 160 (46%) |
| Preoperative ACTH, pg/mLa | 84.2 (8–1000) |
| Preoperative UFC, μg/24 hc | 269.5 (18.1–8097) |
| Preoperative hemoglobin A1cb,g | 7.9 (4.6–19.6) |
| Preoperative systolic BP, mm Hgb,h | 146 (96–220) |
| Preoperative diastolic BP, mm Hgb,h | 89 (53–150) |
| Preoperative BMI, kg/m2b,i | 31.5 (17–51.6) |
| Preoperative low-density lipoprotein, mg/dLb,j | 130.8 (60–352) |
| Preoperative triglycerides, mg/dLb,k | 167 (40–1243) |
Data expressed as mean (range).
Data expressed as actual number (percentage of total).
Data expressed as median (range).
Estimated based on duration of symptoms before surgery until remission was achieved by any means.
MRI findings unavailable for 36 of 346 patients in total cohort.
Irregular pituitary gland vs small adenoma seen on MRI.
Data available for 68 patients.
Data available for 258 patients.
Data available for 107 patients.
Data available for 102 patients.
Data available for 131 patients.
Data collection
The demographic, clinical, laboratory, radiographic, pathological and treatment data available at the time of diagnosis and in postsurgical follow-up were incorporated into a database. Comorbidities at the time of diagnosis were recorded, including documentation of HTN, insulin resistance or diabetes, dyslipidemia, coronary artery disease, stroke, osteoporosis, and depression, as well as pretreatment metabolic parameters [hemoglobin A1c, lipid profile, blood pressure (BP), height, weight, and body mass index (BMI)], when available. Information about GC exposure was obtained, estimated by duration of symptoms before diagnosis until postoperative remission was achieved by any means (ie, TSS, radiation therapy, or adrenalectomy). Pre- and postoperative magnetic resonance imaging (MRI) findings were recorded in the database when available (defined as microadenoma, <10 mm; macroadenoma, >10 mm; no visible adenoma; or inhomogeneous pituitary). The number of surgeries performed for each patient, date, type of surgical procedure, and surgical pathology reports with histologic diagnosis were included. Treatment modalities performed in addition to TSS were ascertained from chart review, including pituitary radiation, γ-knife radiosurgery, adrenalectomy, or medical therapy. Available biochemical data were collected, including preoperative and immediate and long-term postoperative measures of cortisol status, including serum cortisol and ACTH concentrations, response to dexamethasone suppression testing, 24-hour urine-free cortisol (UFC), and midnight salivary free cortisol concentration. Results of other pituitary hormonal function tests were also recorded. The most recent biochemical data available from chart review were used to determine remission status. If clinical records were incomplete or postoperative data were ambiguous, attempts were made to contact patients to obtain the most updated results. When patient contact information was not current, patients' referring physicians were contacted to obtain details about their current clinical status, including biochemical tests, need for replacement GCs, additional therapy performed for CD, and presence or absence of comorbidities. Follow-up of patients was considered to be part of routine medical care, so informed consent was not required, as per Institutional Review Board protocol. This study was approved by the Institutional Review Board of the Mount Sinai School of Medicine (New York, New York).
Criteria for diagnosis of CD
Preoperative biochemical evaluation was performed by the referring physician. Confirmation of Cushing's syndrome (CS) was defined by elevated 24-hour UFC above the upper limit of the range for the assay used or lack of serum cortisol suppression after 1 mg dexamethasone (>5 μg/dL) in the setting of clinical features consistent with CS. When available, elevated midnight salivary free cortisol was also used to confirm CS. When measured, patients had normal or elevated preoperative plasma ACTH concentrations (n = 327), which confirmed ACTH-dependent disease. In the remaining patients (n = 19) for whom preoperative plasma ACTH results were not available, the final diagnosis of ACTH-dependent CD was established by: 1) pathology confirming corticotroph adenoma in the setting of clinical remission from CD after TSS (n = 13), 2) positive results on bilateral inferior petrosal sinus sampling (BIPSS) with a central to peripheral gradient greater than 3:1 (n = 4), or both (n = 2).
Localization of ACTH-dependent CD to a pituitary source was confirmed in the entire cohort (n = 346) based on one or more of the following criteria: 1) MRI findings demonstrating a pituitary adenoma in the setting of postoperative hypocortisolemia (cortisol <5 mg/dL) consistent with remission, 2) MRI findings of a pituitary adenoma in the setting of pathology demonstrating a corticotroph adenoma when no postoperative cortisol levels were available, or 3) if no tumor was seen on MRI in patients with confirmed biochemical CS, a positive BIPSS (>3:1 central:peripheral gradient) was used to make the diagnosis. For 7 patients operated on before 1990, for whom chart data were incomplete, we used the following criteria to establish CD: preoperative biochemical testing definitive for CS (UFC 3 times above the upper limit of normal for the assay used) in addition to: 1) postoperative clinical evidence of remission (n = 1), 2) immediate postoperative ACTH or cortisol values that were lower than preoperative values (n = 3), 3) postoperative GC requirement (n = 2) or preoperative serum cortisol suppression (<5 μg/dL) after high-dose dexamethasone suppression testing consistent with pituitary CD (n = 1).
Postoperative classification
Remission status was established after postoperative follow-up with subjects or their treating physicians. Biochemical data to definitively confirm remission status were considered adequate for 257 of the 346 subjects. Patients were categorized into the following groups for statistical analysis: overall remission, which was further subclassified as immediate surgical remission and late remission, and persistent disease.
Immediate surgical remission was defined as the presence of a postoperative serum cortisol concentration of <5 μg/dL measured 1 to 2 d after surgery, or in some patients the nadir serum cortisol value measured within 3 months after TSS, or requirement of postoperative GC replacement to prevent symptoms of adrenal insufficiency. If 24-hour UFC measurements were available off GC replacement after surgery, UFC that was low or in the normal range also confirmed immediate surgical remission.
Late remission was defined as remission experienced by patients who did not meet criteria for immediate remission but later became eucortisolemic or hypocortisolemic, requiring replacement GC therapy after receiving pituitary radiation, γ-knife radiosurgery, or bilateral adrenalectomy (including patients who developed Nelson's syndrome).
Patients with persistent disease had either normal or elevated immediate postoperative serum cortisol levels (>5 μg/dL), never required postoperative GC replacement therapy, or had elevated 24-hour UFC during serial follow-up. Patients requiring long-term steroidogenesis inhibitors or other medical therapy to reduce cortisol levels were considered to have persistent disease.
Disease recurrence was defined as presentation with an elevated 24-hour UFC, inability to suppress serum cortisol (>5 μg/dL) after low-dose dexamethasone testing, or elevated serum cortisol concentration with clinical symptoms after initial remission had been established.
Mortality data
In addition to standard chart review, mortality data were obtained by searching the National Social Security Death Index (until December 31, 2011). Cause of death was determined by chart review, speaking to patient's family members, or contacting the patient's referring physician.
Statistical analysis
Univariate Cox proportional hazard regression models were used to identify predictors (age, sex, comorbidities at diagnosis, pre- and postoperative biochemical data, MRI findings, and pathology) of mortality, recurrence, and CV events. Multivariate Cox proportional hazard models were constructed in a stepwise fashion to include variables with either clinical or statistical significance on univariate analysis. Multivariate logistic regression analysis with models adjusted, as follows: model A, age at diagnosis, exposure time to excess GCs, preoperative plasma ACTH and serum cortisol concentrations; and model B, age at diagnosis, sex, diabetes and depression were conducted on the total cohort, as well as the overall and immediate remission subgroups to identify possible predictors of mortality. Model B was also used to determine predictors of CV events in the same subgroups of patients. To determine the interaction between remission and predictors of mortality, remission status was added as a covariate to each multivariate model. Statistical significance was defined as a P value of <.05 on a 2-tailed test. All analyses were done using STATA version 11.2 (STATACORP, College Station, Texas). Kaplan-Meier analysis was used to estimate survival curves for patients in remission who presented with depression at the time of diagnosis. Subjects were arbitrarily divided into tertiles based on their estimated time of exposure to excess GCs, and Kaplan-Meier analysis was used to compare their survival curves.
Results
Baseline characteristics
Baseline demographic details of the patient cohort are shown in Table 1. The mean age at diagnosis of CD was 39.9 years (range, 7–77). The mean duration of follow-up was 6.3 years (range, 1 mo to 30 y). There were 265 (77%) female and 81 (23%) male patients. Mean GC exposure, estimated based on the duration of symptoms before diagnosis until time at which patients achieved remission after surgery by any means, was 40.0 months (range, 2–240). At the time of diagnosis, 72% of the total cohort had HTN, 27% had diabetes mellitus, and 22% reported depression. At baseline, the mean BMI was 31.5 kg/m2 (range, 17–51.6) (for 105 patients for whom it was available).
Postoperative classification/treatment outcomes
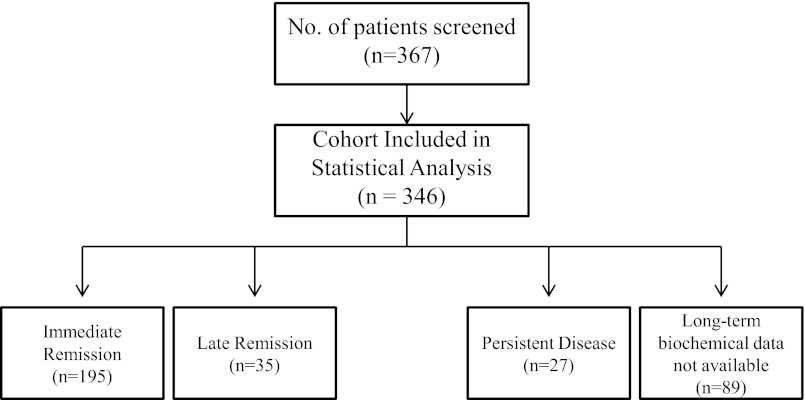
Figure 1 depicts treatment outcomes of the cohort based on follow-up biochemical data. Among the 257 patients for whom adequate postoperative biochemical data were available, 89.4% (230/257) achieved overall remission from CD; 75.9% (195/257) of the whole cohort (for whom remission status was determined) achieved immediate surgical remission, defined by low serum cortisol concentration (<5 μg/dL), requirement of postoperative GC replacement to prevent symptoms of adrenal insufficiency, or normal postoperative 24-hr UFC. Another 13.6% (35/257) achieved late remission, and 10.5% (27/257) had persistent disease.
Figure 1.
Study design and classification of patients based on treatment outcomes. A total of 367 patients with CD were screened from retrospective chart review of patients seen and operated on by a single surgeon. After excluding 21 patients from statistical analysis based on ectopic CS, silent ACTH tumors, and lack of definitive CD diagnosis because of inconclusive biochemical testing, 346 subjects were included in the statistical analysis. After surgery, patients were classified into 1 of 3 outcomes: immediate remission vs late remission (based on the need for subsequent postoperative therapy that resulted in endocrine cure of CD) vs persistent disease. Sufficient postoperative laboratory and clinical follow-up were not available for 89 patients in the cohort, and subsequently remission status could not be confirmed for these patients. At the time of most recent biochemical follow-up, patients were classified as overall remission (including immediate and late) vs persistent disease.
Table 2 depicts postoperative outcomes in the total cohort and each subgroup for which follow-up biochemical data were available. Among the 35 patients who achieved late remission, 23 required radiation treatment and 17 required postoperative adrenalectomy after unsuccessful TSS (n = 13) or disease recurrence (n = 22). Two patients for whom follow-up biochemical data were available underwent bilateral adrenalectomy and subsequently developed Nelson's syndrome. Because they were no longer hypercortisolemic, they were considered to be in late remission. Among the 27 patients with persistent disease, 16 had received radiation treatment. In the overall cohort, 21.1% (73/346) had recurrent disease (at any time point, either before initial presentation to the study's surgeon or after remission). The mean time to recurrence was 5.8 years (range, 1.2–28.8). Of the patients who achieved immediate remission, 10.8% (21/195) experienced recurrence, 57.1% (20/35) of the patients who achieved late remission experienced recurrence, and 44.4% (12/27) of the patients with persistent disease experienced recurrence at any point in time, including before presentation to our neurosurgeon.
Table 2.
Postoperative Outcomes in the Total Cohort and Each Treatment Subgroup
| Classification | Total Cohort (n = 346) | Immediate Remission (n = 195) | Late Remission (n = 35) | Persistent Disease (n = 27) |
|---|---|---|---|---|
| Average length of time (in mo) on glucocorticoids after surgery (range) | 11.06 (0.25–156) | 13.2 (0.5–120) | 5.2 (1–24) | 3.7 (0.25–12) |
| Radiation treatmenta | 52 | 0 | 23 | 16 |
| Adrenalectomy | 20 | 0 | 17 | 0 |
| Hypopituitarism | 83 | 42 | 12 | 5 |
| Postoperative serum cortisol, μg/dL | 6.8 (range, 0.2–50) | 3.7 (range, 0.2–29.6) | 14.8 (range 1–47.6) | 13.3 (range 1–42) |
| Positive ACTH pathology | 277 (80%) | 170 (87%) | 28 (80%) | 27 (100%) |
| No. of TSS required for endocrine cure (range) | 1.3 (1–3) | 1.2 (1–3) | 1.9 (1–3) | N/A |
| Recurrence | 73 (21%) | 21 (11%) | 20 (57%) | 12 (44%) |
| Death from all cause | 31 | 7 | 3 | 3 |
| Cardiovascular events | 30 (9%) | 18 (9%) | 3 (8.5%) | 3 (11%) |
Abbreviation: N/A, not applicable. Data shown as mean (range) or actual number, unless otherwise specified.
Remission status was not confirmed in 13 patients in the total cohort who received radiation treatment.
Predictors of mortality
Mortality data were available for all subjects, with a total of 31 deaths in 346 patients (9.0%) occurring by the end of the study period (December 2011). All 31 deaths were included in the total cohort analysis. The 15 deaths that occurred in subjects for whom insufficient follow-up biochemical data were available were not included in analysis of the subgroup of patients in remission. The mean age of death was 61.3 years (range, 31–89). Death occurred at a mean of 11.4 years (range, 1–23) after surgery. Nine (29%) were male and 22 (71%) female. Ten deaths occurred in the overall remission subgroup (4.4% of 228 patients), and 3 deaths occurred in both the persistent disease (11.1% of 27 patients) and late remission (8.5% of 35 patients) subgroups. Death was not associated with any subgroup (by χ-square and Fisher exact test). There were too few deaths in the persistent disease subgroup to determine a statistically significant difference in mortality between CD patients in remission and those with persistent disease. Cause of death was known in only 29% of cases, with CV disease being the most common etiology (44%).
Among the entire cohort, the following variables increased the risk of death: exposure time to excess GCs, estimated by duration of preoperative symptoms until postoperative remission was achieved by any means (hazard ratio [HR] 1.007, 95% confidence interval [CI] 1.000–1.014; P = .038, per month of GC exposure), older age at diagnosis (HR 1.057, 95% CI 1.025–1.091; P = .0001, per year), and preoperative plasma ACTH concentration (HR 1.070, 95% CI 1.018–1.129; P = .007, per 10 U increase in ACTH concentration). Among patients who achieved overall (immediate and late) and immediate remission, depression at presentation increased the risk of death (HR 6.77, 95% CI 1.70–26.99, P = .007 for immediate remission; HR 4.99, 95% CI 1.37–18.15, P = .015 for overall remission). In the overall remission and immediate remission cohorts, male sex increased the risk of death (HR 4.20, 95% CI 1.21–14.61; P = .024 and HR 4.31, 95% CI 1.24–15.02; P = .022, respectively) (Table 3).
Table 3.
Predictors of Mortality
| Variable | HR (95% CI) | P Value |
|---|---|---|
| Total cohorta | ||
| Age at diagnosis | 1.057 (1.025–1.091) | .0001 |
| Male sex | 1.871 (0.836–4.191) | .128 |
| Preoperative ACTH | 1.070 (1.018–1.129) | .007 |
| Preoperative cortisol | 0.999 (0.979–1.020) | .917 |
| Depression | 1.851 (0.765–4.484) | .173 |
| Exposure to excess GC | 1.007 (1.000–1.014) | .038 |
| Diabetes | 1.942 (0.943–4.001) | .072 |
| Immediate remissionb | ||
| Age at diagnosis | 1.103 (1.039–1.171) | .001 |
| Male genderc | 4.310 (1.237–15.024) | .022 |
| Preoperative ACTH | 1.063 (0.993–1.138) | .077 |
| Preoperative cortisol | 0.999 (0.974–1.025) | .949 |
| Depressionc | 6.770 (1.698–26.992) | .007 |
| Exposure to excess GC | 1.002 (0.991–1.013) | .694 |
| Diabetes | 0.932 (0.262–3.320) | .914 |
Multivariate logistic regression analysis performed in the overall and immediate remission cohorts. Models A and B refer to two multivariate models that were constructed from two distinct sets of variables. Both models were constructed in a stepwise fashion to include variables of statistical or clinical significance on univariate analysis. Model A includes age at diagnosis, preoperative plasma ACTH (per 10 U increase), preoperative serum cortisol and exposure to excess GCs (estimated based on symptom duration before diagnosis until remission was achieved by any means). Model B includes age at diagnosis, sex, depression, and diabetes. These models were used to study the total cohort and different patient subgroups, including immediate remission, as demonstrated in the table.
Model A: n = 271, number of events = 25; model B: n = 345, number of events = 31.
Model A: n = 207, number of events = 11; model B: n = 255, number of events = 13.
Also significant in the overall remission group, HR 4.20, 95% CI 1.21–14.61, P = .024 for male gender and HR 4.99, 95% CI 1.37–18.15, P = .015 for depression.
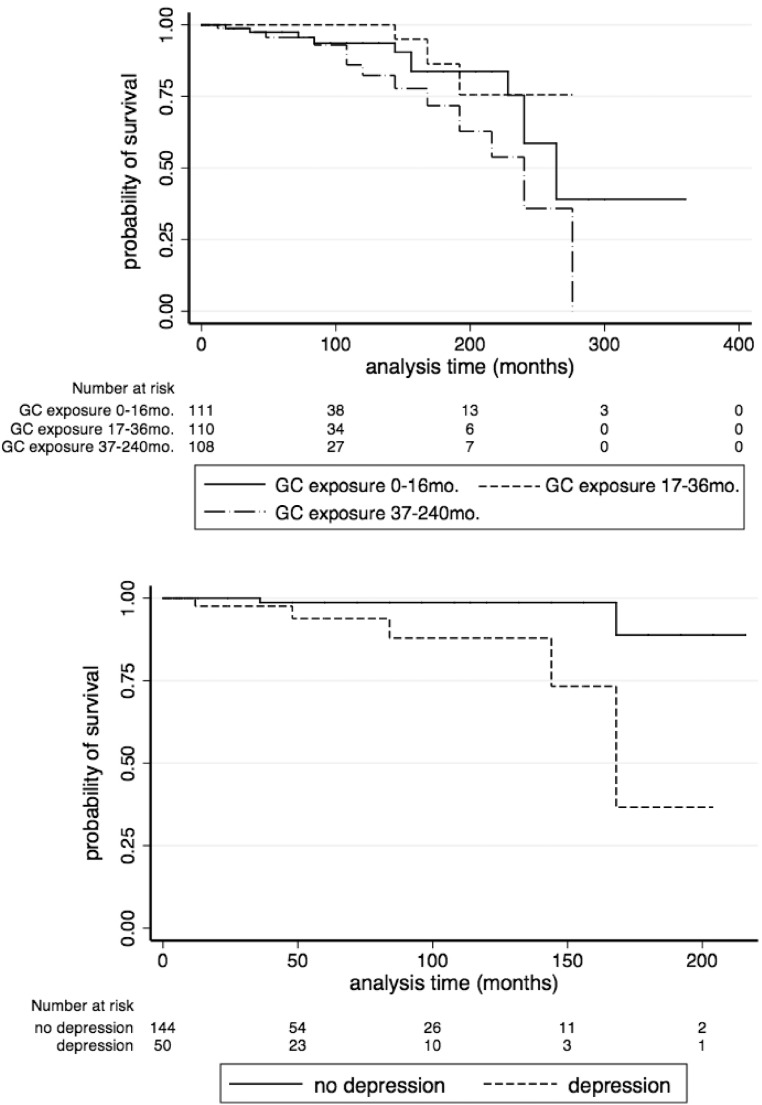
The influence of duration of excess GC exposure and depression on survival are depicted in the Kaplan-Meier plots shown in Figure 2.
Figure 2.
Kaplan-Meier survival curves. Top, Mortality and length of GC exposure in the total cohort. (Because there are no known clinically relevant cutoffs for risk associated with GC exposure, exposure length was arbitrarily divided into tertiles for Kaplan-Meier curve.) Bottom, Mortality and depression in subjects who achieved immediate remission.
Because of the small number of deaths in the persistent disease and late remission subgroups, multivariate analyses for predictors of mortality could not be performed for these groups.
Predictors of CV events
Among the total cohort, 30 CV events, including myocardial infarction, stroke, and thromboembolism (pulmonary embolism or deep vein thrombosis), were documented in patients' charts and analyzed by a logistic regression model that included age, sex, preoperative diagnoses of diabetes, and depression (Table 4). As demonstrated in Table 4, age at diagnosis and male sex increased the hazard of CV events in the entire cohort and in the total and immediate remission subgroups. Among the entire cohort only, preoperative depression (HR 2.32, 95% CI 1.06–5.07; P = .036) and diabetes (HR 2.64, 95% CI 1.27–5.50; P = .009) were found to predict CV events. HTN demonstrated a near significant trend as a predictor of CV events in the univariate analysis (HR 3.307, 95% CI 0.998–10.957; P = .051).
Table 4.
Predictors of Cardiovascular Events in the Total Cohort
| Variable | HR (95% CI) | P Value |
|---|---|---|
| Age at diagnosis | 1.056 (1.026–1.088) | 0.000 |
| Male sex | 2.969 (1.026–6.215) | 0.004 |
| Depression | 2.315 (1.057–5.068) | 0.036 |
| Diabetes | 2.645 (1.270–5.505) | 0.009 |
Multivariate regression model included age at diagnosis, sex, preoperative diabetes, and depression. Total number of subjects = 345, number of events = 30.
Predictors of recurrence
Biochemical recurrence of CD occurred in 73 (21.1%) subjects in the total cohort of 346 subjects during follow-up, which includes patients presenting initially to our surgeon with disease recurrence and patients who experienced recurrence after TSS. Neither demographic variables nor specific pre- or postoperative biochemical data predicted risk of recurrence. Immediate postoperative serum cortisol concentration was not associated with recurrence (P = .58).
Discussion
Our study of 346 patients with CD represents one of the largest CD cohorts operated on by a single surgeon, spanning 31 years of follow-up data, with an emphasis on identifying predictors of mortality, CV events, and recurrence. Underlying these objectives is the aim to recognize modifiable risk factors that can be targeted by treating physicians to prevent and manage adverse outcomes in patients treated for CD. Our data demonstrated that duration of exposure to excess GCs was associated with increased mortality in the whole cohort, and depression at presentation was associated with increased mortality among patients who achieved immediate and late remission. Older age at diagnosis, preoperative plasma ACTH concentration, and male sex were also associated with increased mortality.
Patients with CD have increased mortality compared with patients with nonfunctioning (8) and growth-hormone–secreting (12) pituitary adenomas, suggesting that hypercortisolemia itself increases mortality, independent of the presence of a pituitary tumor or history of TSS. Although several studies have investigated the long-term mortality outcomes in treated CD (7, 9, 10, 13, 14), few have identified predictors of mortality (8, 14).
We found that longer exposure to excess GCs, estimated by duration of symptoms before diagnosis until postoperative remission was achieved by any means, increased the risk of death in the whole cohort (P = .038). Other studies focusing on surgical outcomes have speculated that increased mortality is associated with duration of hypercortisolemia, in short- (2) and long-term (9) follow-up, particularly among those with persistent disease. When evaluating our subgroup of patients in remission, the duration of exposure to excess GCs was no longer a significant predictor of mortality. This may be because of insufficient sample size or a true lack of association between hypercortisolemia and mortality in patients who achieve remission. Nevertheless, the link between GC excess and mortality emphasizes the importance of early diagnosis and treatment of CD.
Another novel finding shown here is that the preoperative report of depression was associated with excess mortality by nearly 7-fold among those who achieved immediate remission from CD (P = .007) and nearly 5-fold in patients who experienced remission overall (including immediate and late remission, P = .015). Although mechanisms underlying this relationship are unclear, one can hypothesize that depression may be a marker for disease severity and a prognostic indicator for future outcomes. It has been well documented in other populations that major depression is associated with all-cause mortality (15–17). In a cross-sectional study of 162 patients with CD, major depression was associated with more severe CD (18). Depression and other neuropsychiatric diseases have been well described as adverse outcomes of chronic GC overexposure (18, 19), although the extent of the reversibility of the psychopathology requires further investigation. A study by Starkman et al (20) demonstrated an improvement in depressed mood among those in remission from CD, whereas other studies suggest persistent mood abnormalities even after remission is achieved (21–23). Chronic hypercortisolemia is associated with decreased hippocampal volume (24) and local neurohormonal alterations involving the inhibition of dopaminergic activity (25) that may lead to irreversible changes in neural function (26). However, whether or not GC overexposure leads to irreversible changes that confer a unique mortality risk even when GC levels normalize remains unclear. Prospective studies are needed to clarify the potential role of persistent psychiatric disease in the mortality risk in patients with CD remission.
Older age at diagnosis also predicted mortality among all patients in our study and remained significant throughout subgroup analyses of patients with immediate and late remission. There are limited data associating advanced age with morbidity and mortality outcomes in treated CD. In a study by Patil et al (27) that examined outcomes and mortality using a National Inpatient Sample database of an estimated 3525 patients with CD operated on in the United States from 1993 to 2002, advanced age was a significant risk factor for adverse outcomes and greater length of hospital stay. An examination of 253 patients with CS in New Zealand reported that age at diagnosis was one of the strongest predictors of mortality among those with pituitary CD (odds ratio 1.08) (14). These findings emphasize the importance of close follow-up in older patients, even in those in remission from CD, perhaps with more aggressive management of comorbidities that could contribute to increased mortality.
Male gender was found to predict mortality in the overall and immediate remission subgroup analysis. There are limited data associating male gender with an increased risk of death in CD, perhaps because the disease is more commonly observed in female patients, with long-term follow-up of male subjects potentially being inadequate. In the study by Patil et al (27), women were 0.3 times less likely to experience a postoperative adverse outcome than were men, with adverse outcome being defined as death or discharge to an institution other than home, independent of comorbidities. In contrast, a long-term follow-up study of CD demonstrated that female sex had a relative risk of 4.5 in predicting mortality (10). These conflicting data underscore the need for long-term follow-up of all patients treated for CD.
Preoperative plasma ACTH concentrations, but not preoperative serum or urine cortisol concentrations, were associated with increased risk of death in our cohort (P = .007). The significance of this finding is unclear and should be interpreted with caution because of the lack of standardization of ACTH values in this retrospective chart review. It is possible that high ACTH levels may be associated with more aggressive tumors or that ACTH itself may confer risk. A series of case reports demonstrates that remission from CD is associated with rebound immunity, and interestingly this was described only in ACTH-dependent but not ACTH-independent CS (28). Moreover, CD remission has been associated with a higher risk of postoperative venous thromboembolism compared with a control population of patients treated for nonfunctioning adenomas (29). In this study, the risk of postoperative venous thromboembolism was 3.4% among patients with ACTH-dependent CS, many of whom were eucortisolemic at the time, compared with 0% for patients with ACTH-independent CS, thus postulating a role for ACTH in predisposing to this prothrombotic tendency. In fact, previous data suggest that ACTH may have immunomodulatory effects (30) and, if so, could contribute to a proinflammatory state, although the mechanism remains to be determined. Finally, outcomes of patients with ACTH-dependent CD versus ACTH-independent CS have been shown to be worse (2, 13, 31–33), and whether this is a result of tumor type and location, treatment modality and remission rate, outcomes from pituitary endocrine deficits resulting from treatment of pituitary disease, or direct effects of ACTH itself remains unknown.
Overall, our study has several limitations that deserve mention. The retrospective nature of the study is a limitation that prevented us from obtaining complete biochemical data sets for all patients in the cohort. Consequently, we were unable to definitively confirm remission status in 89 of the 346 patients who underwent TSS. With regard to the estimation of GC exposure by symptom duration at presentation, the accuracy of these data are affected by not having been collected in a dedicated manner and by the nonspecific nature of some CD symptoms. However, this method has been used in other studies and therefore is a validated method for estimating GC exposure in CD (31, 34). Different ACTH assays were used to perform preoperative biochemical evaluation in our cohort. In this large retrospective study, it was not feasible to perform biochemical evaluations for all patients in one lab. Therefore, the possible association between plasma ACTH concentration and mortality needs to be confirmed with prospective studies. Additionally, there may have been referral or selection bias because this is a single-surgeon cohort. A final limitation is that the preoperative diagnosis of depression, which was found to be a strong predictor of mortality, was obtained by patient report via chart review and not consistently confirmed by Diagnostic and Statistical Manual of Mental Disorders criteria. Despite these limitations, this study represents one of the largest cohorts of patients with CD operated on by a single surgeon who has significant experience in transsphenoidal pituitary surgery using modern surgical techniques, with a long duration of follow-up.
To conclude, our study has identified several predictors of mortality in patients with treated CD, including duration of exposure to excess GCs, preoperative ACTH concentration, and older age at diagnosis. Depression and male gender predicted mortality among patients who achieved remission. These data illustrate the importance of early recognition and treatment of CD. Long-term follow-up, with management of persistent comorbidities by an experienced endocrinologist, is needed even after successful treatment of CD. Additional research is required to understand the mechanisms of persistent complications associated with the post-hypercortisolemic state caused by treated CD or prior exposure to exogenous GCs.
Acknowledgments
This work was supported by National Institutes of Health Grants K23 DK 082617 (to E.B.G.), and by Grant TL1RR029886 from the National Center for Research Resources.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BIPSS
- bilateral inferior petrosal sinus sampling
- BMI
- body mass index
- BP
- blood pressure
- CD
- Cushing's disease
- CI
- confidence interval
- CS
- Cushing's syndrome
- CV
- cardiovascular
- GC
- glucocorticoid
- HR
- hazard ratio
- HTN
- hypertension
- MRI
- magnetic resonance imaging
- TSS
- transsphenoidal surgery
- UFC
- urine free cortisol.
References
- 1. Etxabe J, Vazquez JA. Morbidity and mortality in Cushing's disease: an epidemiological approach. Clin Endocrinol (Oxf). 1994;40:479–484 [DOI] [PubMed] [Google Scholar]
- 2. Lindholm J, Juul S, Jørgensen JO, et al. Incidence and late prognosis of cushing's syndrome: a population-based study. J Clin Endocrinol Metab. 2001;86:117–123 [DOI] [PubMed] [Google Scholar]
- 3. Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). 1932. Obes Res. 1994;2:486–508 [DOI] [PubMed] [Google Scholar]
- 4. Plotz CM, Knowlton AI, Ragan C. The natural history of Cushing's syndrome. Am J Med. 1952;13:597–614 [DOI] [PubMed] [Google Scholar]
- 5. Pivonello R, Faggiano A, Lombardi G, Colao A. The metabolic syndrome and cardiovascular risk in Cushing's syndrome. Endocrinol Metab Clin North Am. 2005;34:327–339 [DOI] [PubMed] [Google Scholar]
- 6. Colao A, Pivonello R, Spiezia S, et al. Persistence of increased cardiovascular risk in patients with Cushing's disease after five years of successful cure. J Clin Endocrinol Metab. 1999;84:2664–2672 [DOI] [PubMed] [Google Scholar]
- 7. Clayton RN, Raskauskiene D, Reulen RC, Jones PW. Mortality and morbidity in Cushing's disease over 50 years in Stoke-on-Trent, UK: audit and meta-analysis of literature. J Clin Endocrinol Metab. 2011;96:632–642 [DOI] [PubMed] [Google Scholar]
- 8. Dekkers OM, Biermasz NR, Pereira AM, et al. Mortality in patients treated for Cushing's disease is increased, compared with patients treated for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab. 2007;92:976–981 [DOI] [PubMed] [Google Scholar]
- 9. Hammer GD, Tyrrell JB, Lamborn KR, et al. Transsphenoidal microsurgery for Cushing's disease: initial outcome and long-term results. J Clin Endocrinol Metab. 2004;89:6348–6357 [DOI] [PubMed] [Google Scholar]
- 10. Hassan-Smith ZK, Sherlock M, Reulen RC, et al. Outcome of Cushing's disease following transsphenoidal surgery in a single center over 20 years. J Clin Endocrinol Metab. 2012;97:1194–1201 [DOI] [PubMed] [Google Scholar]
- 11. Swearingen B, Biller BM, Barker FG, 2nd, et al. Long-term mortality after transsphenoidal surgery for Cushing disease. Ann Intern Med. 1999;130:821–824 [DOI] [PubMed] [Google Scholar]
- 12. Biermasz NR, Dekker FW, Pereira AM, et al. Determinants of survival in treated acromegaly in a single center: predictive value of serial insulin-like growth factor I measurements. J Clin Endocrinol Metab. 2004;89:2789–2796 [DOI] [PubMed] [Google Scholar]
- 13. Graversen D, Vestergaard P, Stochholm K, Gravholt CH, Jorgensen JO. Mortality in Cushing's syndrome: a systematic review and meta-analysis. Eur J Intern Med. 2012;23:278–282 [DOI] [PubMed] [Google Scholar]
- 14. Bolland MJ, Holdaway IM, Berkeley JE, et al. Mortality and morbidity in Cushing's syndrome in New Zealand. Clin Endocrinol (Oxf). 2011;75:436–442 [DOI] [PubMed] [Google Scholar]
- 15. Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2000;160:1761–1768 [DOI] [PubMed] [Google Scholar]
- 16. Whooley MA, Browner WS. Association between depressive symptoms and mortality in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1998;158:2129–2135 [DOI] [PubMed] [Google Scholar]
- 17. Wulsin LR, Vaillant GE, Wells VE. A systematic review of the mortality of depression. Psychosom Med. 1999;61:6–17 [DOI] [PubMed] [Google Scholar]
- 18. Sonino N, Fava GA, Raffi AR, Boscaro M, Fallo F. Clinical correlates of major depression in Cushing's disease. Psychopathology. 1998;31:302–306 [DOI] [PubMed] [Google Scholar]
- 19. Starkman MN, Schteingart DE. Neuropsychiatric manifestations of patients with Cushing's syndrome. Relationship to cortisol and adrenocorticotropic hormone levels. Arch Intern Med. 1981;141:215–219 [PubMed] [Google Scholar]
- 20. Starkman MN, Schteingart DE, Schork MA. Cushing's syndrome after treatment: changes in cortisol and ACTH levels, and amelioration of the depressive syndrome. Psychiatry Res. 1986;19:177–188 [DOI] [PubMed] [Google Scholar]
- 21. Dorn LD, Burgess ES, Friedman TC, Dubbert B, Gold PW, Chrousos GP. The longitudinal course of psychopathology in Cushing's syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82:912–919 [DOI] [PubMed] [Google Scholar]
- 22. van Aken MO, Pereira AM, Biermasz NR, et al. Quality of life in patients after long-term biochemical cure of Cushing's disease. J Clin Endocrinol Metab. 2005;90:3279–3286 [DOI] [PubMed] [Google Scholar]
- 23. Pereira AM, Tiemensma J, Romijn JA. Neuropsychiatric disorders in Cushing's disease. Neuroendocrinology. 2010;92:65–70 [DOI] [PubMed] [Google Scholar]
- 24. Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing's syndrome. Biol Psychiatry. 1992;32:756–765 [DOI] [PubMed] [Google Scholar]
- 25. Pacak K, Tjurmina O, Palkovits M, et al. Chronic hypercortisolemia inhibits dopamine synthesis and turnover in the nucleus accumbens: an in vivo microdialysis study. Neuroendocrinology. 2002;76:148–157 [DOI] [PubMed] [Google Scholar]
- 26. Heald A, Ghosh S, Bray S, et al. Long-term negative impact on quality of life in patients with successfully treated Cushing's disease. Clin Endocrinol (Oxf). 2004;61:458–465 [DOI] [PubMed] [Google Scholar]
- 27. Patil CG, Lad SP, Harsh GR, Laws ER, Jr, Boakye M. National trends, complications, and outcomes following transsphenoidal surgery for Cushing's disease from 1993 to 2002. Neurosurg Focus. 2007;23:E7. [DOI] [PubMed] [Google Scholar]
- 28. da Mota F, Murray C, Ezzat S. Overt immune dysfunction after Cushing's syndrome remission: a consecutive case series and review of the literature. J Clin Endocrinol Metab. 2011;96:E1670–1674 [DOI] [PubMed] [Google Scholar]
- 29. Stuijver DJ, van Zaane B, Feelders RA, et al. Incidence of venous thromboembolism in patients with Cushing's syndrome: a multicenter cohort study. J Clin Endocrinol Metab. 2011;96:3525–3532 [DOI] [PubMed] [Google Scholar]
- 30. Heijnen CJ, Zijlstra J, Kavelaars A, Croiset G, Ballieux RE. Modulation of the immune response by POMC-derived peptides. I. Influence on proliferation of human lymphocytes. Brain Behav Immun. 1987;1:284–291 [DOI] [PubMed] [Google Scholar]
- 31. Giordano R, Picu A, Marinazzo E, et al. Metabolic and cardiovascular outcomes in patients with Cushing's syndrome of different aetiologies during active disease and 1 year after remission. Clin Endocrinol (Oxf). 2011;75:354–360 [DOI] [PubMed] [Google Scholar]
- 32. Pikkarainen L, Sane T, Reunanen A. The survival and well-being of patients treated for Cushing's syndrome. J Intern Med. 1999;245:463–468 [DOI] [PubMed] [Google Scholar]
- 33. Steffensen C, Bak AM, Rubeck KZ, Jorgensen JO. Epidemiology of Cushing's syndrome. Neuroendocrinology. 2010;92:1–5 [DOI] [PubMed] [Google Scholar]
- 34. Barahona MJ, Sucunza N, Resmini E, et al. Persistent body fat mass and inflammatory marker increases after long-term cure of Cushing's syndrome. J Clin Endocrinol Metab. 2009;94:3365–3371 [DOI] [PubMed] [Google Scholar]