Abstract
Objective:
The purpose of this study was to determine 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] and 1,25-dihydroxyvitamin D2 [1,25(OH)2D2] levels in healthy adults consuming 1000 IU vitamin D2 or vitamin D3 per day for 11 weeks.
Subjects and Design:
Blood from 34 healthy male and female adults, aged 18 to 79 years, from a placebo-controlled, double-blind study who received a placebo, 1000 IU vitamin D3, or 1000 IU vitamin D2 daily for 11 weeks at end of winter was analyzed. Serum levels of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3, 1,25(OH)2D2, and 1,25(OH)2D3 were determined by liquid chromatography–tandem mass spectroscopy.
Results:
Of the adults, 82% were vitamin D insufficient (serum 25-hydroxyvitamin D [25(OH)D <30 ng/mL]) at the start of the study. Administration of vitamin D2 and vitamin D3 induced similar increases in total 25(OH)D as well as in 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3, respectively. Compared with placebo and adjusting for baseline levels, 1000 IU daily of vitamin D2 was associated with a mean increase of 7.4 pg/mL (95% confidence interval, 4.4–10.3) in 1,25(OH)2D2, which was accompanied by a mean decrease of 9.9 pg/mL (−15.8 to −4.0) in 1,25(OH)2D3. No such differences accompanied administration of 1000 IU daily of vitamin D3.
Conclusion:
Vitamin D2 and vitamin D3 were effective in raising and maintaining total serum concentrations of 25(OH)D. Ingestion of vitamin D2 also resulted in an increase in serum concentrations of 1,25(OH)2D2. This increase was accompanied by a comparable decrease in serum concentrations of 1,25(OH)2D3; therefore, the total 1,25-dihydroxyvitamin D [1,25(OH)2D] concentrations did not significantly change after 11 weeks compared with baseline levels. Ingestion of vitamin D3 did not alter serum concentrations of 1,25(OH)2D3 or total 1,25(OH)2D. Therefore, ingestion of 1000 IU vitamin D2 or vitamin D3 for 11 weeks was effective in raising total serum concentrations of 25(OH)D as well as sustaining serum concentrations of total 1,25(OH)2D.
Vitamin D plays an integral role in bone health, specifically, in calcium and phosphorus homeostasis (1). Vitamin D deficiency and insufficiency, defined as serum 25-hydroxyvitamin D [25(OH)D] levels <20 and 21 to 29 ng/mL, respectively, is a major health concern that extends beyond skeletal ailments (2). Because there is a vitamin D receptor in most every tissue in the body, people of any age or race are at risk of becoming vitamin D deficient (3–5). It is an indiscriminate affliction that has been associated with a wide range of health issues such as autoimmune diseases, diabetes, cardiovascular disease, and certain cancers (6–18). In a vitamin D–deficient state, the body only absorbs 10% to 15% of dietary calcium and approximately 60% of phosphorus. 1,25-Dihydroxyvitamin D [1,25(OH)2D], the active form of vitamin D, increases the efficiency of intestinal calcium and phosphorus absorption by up to 40% and 80%, respectively (19).
Humans obtain vitamin D from diet and sunlight (1, 20–22). The main source of vitamin D3 is from exposure to sunlight, accounting for more than 90% of the body's vitamin D requirement (23). Vitamin D3 is synthesized in the skin and naturally found in oily fish, whereas vitamin D2 is present in sundried and UV light–exposed mushrooms (24). Vitamin D2 is produced from the irradiation of yeast and vitamin D3 is produced from lanolin and both are used to fortify milk, other dairy products, orange juice, and cereals (1, 25). Both vitamin D3 and vitamin D2 are found in dietary supplements, but vitamin D2 is the only prescription form available in the United States (1).
Endogenous, dietary, and supplemental forms of vitamin D2 and vitamin D3 are metabolized in sequential hydroxylations in the liver and kidneys to 25(OH)D and 1,25(OH)2D, respectively (1, 26). Some studies have suggested that vitamin D2 was 30% to 50% less effective than vitamin D3 in maintaining serum 25(OH)D levels (27–29). However, children receiving 2000 IU vitamin D2 daily increased their serum 25(OH)D to the same level as children receiving a daily dose of 2000 IU vitamin D3 (30). In addition, healthy adults taking 1000 IU vitamin D2 or 1000 IU vitamin D3 in a capsule or in orange juice were able to raise their blood level of 25(OH)D to the same level by the end of the 11-week study (31, 32). Men and women who took 50,000 IU vitamin D2 twice a month for up to 6 years were able to maintain their serum 25(OH)D at >30 ng/mL without toxicity (33).
The purpose of this study was to determine 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] and 1,25-dihydroxyvitamin D3 [1,25(OH)2D2] levels in adults consuming 1000 IU vitamin D2 or vitamin D3 daily for 11 weeks using the state-of-the-art liquid chromatography-mass spectroscopy assay.
Subjects and Methods
Subjects and serum samples
To determine the effect of ingesting 1000 IU vitamin D2 or 1000 IU vitamin D3 on circulating blood levels of 1,25(OH)2D2 and 1,25(OH)2D3 and total 1,25(OH)2D we evaluated blood samples from a double-blind, placebo-controlled study that was approved by our institutional review board at Boston University Medical Center and published previously (32). Of the 105 subjects in the parent study, we were able to retrieve the 1 mL of the serum required for the 1,25(OH)2D2 and 1,25(OH)2D3 assays from 8 subjects (7 female and 1 male) who received the placebo, 9 subjects (8 female and 1 male) who received 1000 IU vitamin D3, and 17 subjects (10 female and 7 male) who received 1000 IU vitamin D2 daily for 11 weeks.
Analytical methods
Serum 1,25(OH)2D2 and 1,25(OH)2D3 concentrations were determined by liquid chromatography–tandem mass spectroscopy (LC-MS/MS) at Quest Diagnostic Laboratory (San Juan Capistrano, California). Before analysis, 1,25(OH)2D2 and 1,25(OH)2D3 were extracted from 1 mL of the patient serum samples through the use of a cospecific antibody targeted to the A ring of 1,25(OH)2D. To improve ionization and fragmentation in the mass spectrometer, analytes were derivatized using a Cookson-type reagent, 4′-phenyl-1,2,4-triazoline-3,5-dione. Stable isotope-labeled internal standards for both 1,25(OH)2D2 and 1,25(OH)2D3 were used throughout to improve accuracy and precision.
A multiplexed analytical HPLC system using a reverse-phase column and reverse-phase gradient was used to effect chromatographic separation. The flow of liquid solvent from the high-performance liquid chromatograph entered a heated nebulizer interface of a LC-MS/MS analyzer (Thermo Fisher Scientific, Waltham, Massachusetts).
Analytes were measured using the selected reaction monitoring mode whereby the mass of the intact analyte was selected in the first quadrupole, collisionally induced dissociation effected in the second quadrupole, and multiple fragment ions sequentially isolated by the third quadrupole.
The areas under the chromatographic peaks were determined, and calibration curves were constructed by plotting standard concentration vs peak area ratio of analyte/internal standard. Using the calibration curves, the concentrations of 1,25(OH)2D2 and 1,25(OH)2D3 were quantitated for patient samples.
The assay has an intraassay coefficient of variation (CV) of 9% and interassay CV of 12%. Serum PTH was determined using immunoassay/spectrophotometry (Quest Diagnostics Nichols Institute, San Juan Capistrano, California). The assay has an intraassay CV of 8% and interassay CV of 10%. Serum 25-hydroxyvitamin D2 [25(OH)D2] and 25-hydroxyvitamin D3 [25(OH)D3] concentrations were determined by LC-MS/MS as described previously (32).
Statistical analyses
Descriptive statistics (means, medians, and SDs) were computed for baseline and total change at end of the study for each treatment group. Comparisons of trends between groups using all time points were performed using generalized estimating equations, which acknowledge the repeated measurements on subjects in computing estimates and confidence intervals, with statistical control for baseline levels; ie, comparisons of 25(OH)D2 on treatment were performed with control for baseline 25(OH)D2 levels. Differences were considered statistically significant if null hypotheses of no difference could be rejected at the .05 level.
Results
Of the 34 subjects, 28 subjects (82%) had 25(OH)D levels <30 ng/mL at baseline. Descriptions of baseline levels of all vitamin D metabolite concentrations are given in Table 1. Baseline levels were roughly comparable, although subjects assigned to 1000 IU vitamin D2 had slightly greater 25(OH)D2 levels at study start, compared with those of other subjects, and those assigned to 1000 IU vitamin D3 had slightly greater 25(OH)D3 levels at study start. Most subjects began the study with 1,25(OH)2D2 levels that were undetectable, whereas the overall mean (SD) 1,25(OH)2D3 at study start was 32.0 (10.2) pg/mL.
Table 1.
Baseline of Vitamin D Metabolite Concentrations, by Treatment Groupa
| Placebo (n = 8) | D2 (n = 17)b | D3 (n = 9)c | |
|---|---|---|---|
| 25(OH)D2, ng/mL | 0.9 (2.1) | 3.8 (4.9) | 0.9 (2.7) |
| 25(OH)D3, ng/mL | 17.6 (7.8) | 15.5 (6.9) | 21.3 (12.9) |
| 1,25(OH)2D2, pg/mL | 0.0 (0.0) | 2.8 (6.7) | 0.0 (0.0) |
| 1,25(OH)2D3, pg/mL | 30.3 (8.1) | 30.5 (9.0) | 35.4 (13.0) |
Data are means (SD).
1000 IU vitamin D2/d.
1000 IU vitamin D3/d.
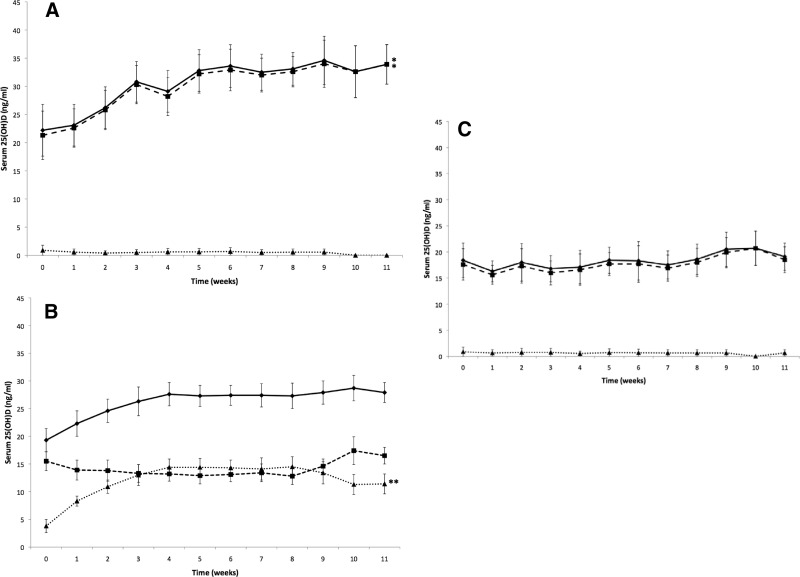
The trajectory of change in 25(OH)D levels is depicted in Figure 1, with change from the first to last visit described in Table 2. Subjects assigned to 1000 IU vitamin D2 or vitamin D3 exhibited the expected increases in 25(OH)D2 and 25(OH)D3, respectively. In contrast, assignment to 1000 IU vitamin D2 appeared to be associated with increases in 1,25(OH)2D2 and similar decreases in 1,25(OH)2D3 at study end, whereas those subjects assigned to 1000 IU vitamin D3 exhibited no appreciable change in either 1,25(OH)2D2 or 1,25(OH)2D3; this observation was consistent with trends over the course of the treatment period (Figure 2).
Figure 1.
A, Total 25(OH)D levels are demonstrated over time. Shown are mean (± SEM) serum total 25(OH)D (♦; n = 9), serum 25(OH)D3 (■; n = 9), and serum 25(OH)D2 (▴; n = 9) after oral administration of either 1000 IU vitamin D3 in orange juice or vitamin D3 in capsules. There were no statistically significant differences in serum 25(OH)D2 over time in the groups receiving either 1000 IU vitamin D3 in orange juice or vitamin D3 in capsules (2-tailed paired t test, P > .05). *P < .05 comparing serum total 25(OH)D and 25(OH)D3 over time in the groups receiving either 1000 IU vitamin D3 in orange juice or vitamin D3 in capsules. B, Total 25(OH)D levels are demonstrated over time. Shown are mean (± SEM) serum total 25(OH)D (♦; n = 17), serum 25(OH)D3 (■; n = 17), and serum 25(OH)D2 (▴; n = 17) after oral administration of either 1000 IU vitamin D2 in orange juice or vitamin D2 in capsules. There were no statistically significant differences in serum total 25(OH)D and 25(OH)D3 over time in the groups receiving either 1000 IU vitamin D2 in orange juice or vitamin D2 in capsule (2-tailed paired t test, P > .05). **P = .0005 comparing serum 25(OH)D2 over time in the groups receiving either 1000 IU vitamin D2 in orange juice or vitamin D2 in capsules. C, Total 25(OH)D levels are demonstrated over time. Shown are mean (± SEM) serum total 25(OH)D (♦; n = 8), serum 25(OH)D3 (■; n = 8), and serum 25(OH)D2 (▴; n = 8) after oral administration of either unfortified orange juice or placebo capsule. There were no statistically significant differences in serum total 25(OH)D, 25(OH)D3, and 25(OH)D2 over time in the groups receiving either unfortified orange juice or placebo capsule (2-tailed paired t test, P > .05).
Table 2.
Change From Baseline in Vitamin D Parameters, by Treatment Groupa
| Placebo (n = 8) | D2 (n = 17)b | D3 (n = 9)c | |
|---|---|---|---|
| 25(OH)D2, ng/mL | −1.0 (2.4) | 6.5 (5.5) | −0.9 (2.7) |
| P | .31 | <.001 | .35 |
| 25(OH)D3, ng/mL | 1.8 (4.0) | 1.3 (11.2) | 12.3 (7.5) |
| P | .28 | .65 | .001 |
| 1,25(OH)2D2, pg/mL | 0.0 (0.0) | 5.2 (11.4) | 0.0 (0.0) |
| P | >.99 | .09 | >.99 |
| 1,25(OH)2D3, pg/mL | 0.7 (7.2) | −6.8 (14.1) | −1.1 (13.8) |
| P | .81 | .07 | .82 |
Data are means (SD). Baseline represents the last visit minus baseline, other visits ignored. P values from 2-sided Student's t test of hypothesis of zero change.
1000 IU vitamin D2/d.
1000 IU vitamin D3/d.
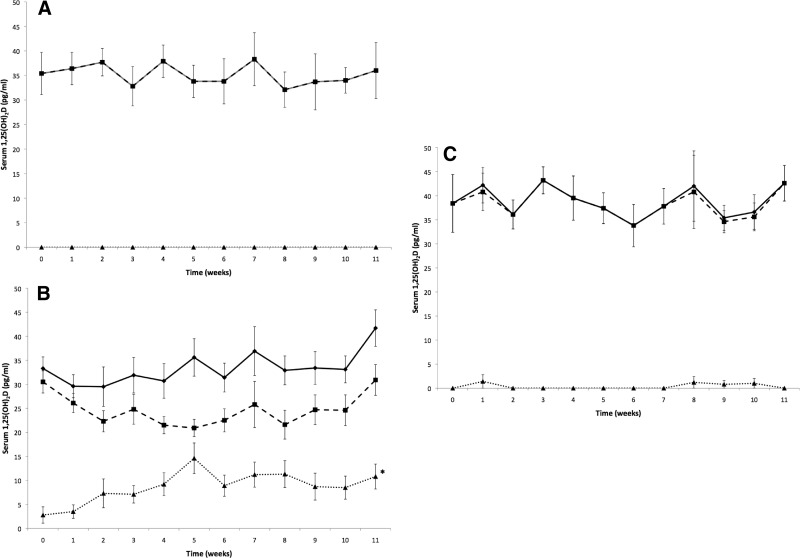
Figure 2.
A, Total 1,25(OH)2D levels are demonstrated over time. Shown are mean (± SEM) serum total 1,25(OH)2D (♦; n = 9), serum 1,25(OH)2D3 (■; n = 9), and serum 1,25(OH)2D2 (▴; n = 9) after oral administration of either 1000 IU vitamin D3 in orange juice or vitamin D3 in capsules. There were no statistically significant differences in serum 1,25(OH)2D, 1,25(OH)2D3, or 1,25(OH)2D2 over time in the groups receiving either 1000 IU vitamin D3 in orange juice or vitamin D3 in capsules (2-tailed paired t test, P > .05). B, Total 1,25(OH)2D levels are demonstrated over time. Shown are mean (± SEM) serum total 1,25(OH)2D (♦; n = 17), serum 1,25(OH)2D3 (■; n = 17), and serum 1,25(OH)2D2 (▴; n = 17) after oral administration of either 1000 IU vitamin D2 in orange juice or vitamin D2 in capsules. There were no statistically significant differences in serum total 1,25(OH)2D and 1,25(OH)2D3 over time in the groups receiving either 1000 IU vitamin D2 in orange juice or vitamin D2 in capsules (2-tailed paired t test, P > .05). *P < .05 comparing serum 1,25(OH)2D2 over time in the groups receiving 1000 IU vitamin D2 in orange juice or in capsules. C, Total 1,25(OH)2D levels demonstrated over time. Shown are mean (± SEM) serum total 1,25(OH)2D (♦; n = 8), serum 1,25(OH)2D3 (■; n = 8), and serum 1,25(OH)2D2 (▴; n = 8) after oral administration of either unfortified orange juice or placebo capsule. There were no statistically significant differences in serum total 1,25(OH)2D, 1,25(OH)2D3, and 1,25(OH)2D2 over time in the groups receiving either unfortified orange juice or placebo capsule (2-tailed paired t test, P > .05).
Results of longitudinal regression analysis were consistent with these exploratory findings (Table 3). Compared with placebo, assignment to 1000 IU vitamin D2 was associated with mean (95% confidence interval) on-treatment increases of 7.4 (4.4–10.3) pg/mL in 1,25(OH)2D2 accompanied by decreases of similar magnitude in 1,25(OH)2D3, whereas those assigned to 1000 IU vitamin D3 exhibited little appreciable change in either parameter. The mean total 25(OH)D increase was greater in those assigned to 1000 IU vitamin D3 than in those assigned to 1000 IU vitamin D2, but evidence in favor of a greater increase associated with 1000 IU vitamin D3 did not achieve statistical significance when all data were taken into consideration in the longitudinal model (P = .07).
Table 3.
Comparison of Change in Vitamin D Metabolite Concentrationsa
| 25(OH)D2, ng/mL | 25(OH)D3, ng/mL | 1,25(OH)2D2, pg/mL | 1,25(OH)2D3, pg/mL | |
|---|---|---|---|---|
| Placebo (n = 8) | Referent | Referent | Referent | Referent |
| D2 (n = 17)b | 8.7 (7.1 to 10.3) | −1.9 (−3.8 to 0.01) | 7.4 (4.4 to 10.3) | −9.9 (−15.8 to −4.0) |
| D3 (n = 9)c | −0.2 (−1.1 to 0.7) | 11.1 (7.4 to 14.8) | −0.3 (−0.7 to 0.1) | −0.6 (−6.9 to 5.7) |
Data are means (95% confidence interval) for on-treatment minus baseline. Estimates were obtained via generalized estimating equations, controlling for baseline vitamin D metabolite levels.
25(OH)D and 1,25(OH)2D.
1000 IU vitamin D2/d.
1000 IU vitamin D3/d.
Discussion
Although there continues to be debate as to whether vitamin D2 is as effective as vitamin D3 in maintaining blood levels of 25(OH)D, less is known about how 25(OH)D2 is metabolized to 1,25(OH)2D2 and what happens to 1,25(OH)2D3 and total 1,25(OH)2D concentrations. It had been reported previously that the concentrations of 1,25(OH)2D2 and 1,25(OH)2D3 were proportional to distribution of 25(OH)D2 and 25(OH)D3 (34). Hartwell et al (35) observed that premenopausal women who received 4000 IU vitamin D2 for 8 weeks demonstrated significant increases in serum 1,25(OH)2D2 at 4 weeks that continued to increase at the end of the 8-week study with comparable declines in the serum concentrations of 1,25(OH)2D3. They further observed over this 8-week period that the total serum concentrations of 1,25(OH)2D did not significantly change. It remained to be determined whether and when the 1,25(OH)2D2 would reach a plateau and what the consequences would be on the serum concentrations of 1,25(OH)2D3 because Hartwell et al (35) only measured blood levels at 4 and 8 weeks. Furthermore, because they only performed their study in premenopausal women, it is not known whether men and postmenopausal women would also metabolize vitamin D2 in a manner similar to that of premenopausal women.
In our previous studies, we observed that healthy male and female adults with a wide age range (18–79 years) who ingested 1000 IU vitamin D2 or vitamin D3 in a capsule or in orange juice were able to raise their blood levels of 25(OH)D2 and 25(OH)D3 by ∼10 ng/mL (31, 32). Furthermore, the groups who received vitamin D2 demonstrated no significant change in the circulating concentrations of 25(OH)D3, and the total blood levels of 25(OH)D were the same whether the adults ingested 1000 IU vitamin D2 or vitamin D3. These blood samples were archived and stored at −80°C. Because the 1,25(OH)2D assay required 1 mL of serum, we recovered 34 samples meeting this requirement; 28.1% were from male participants. The concentrations of 25(OH)D2 and 25(OH)D3 gradually increased in a similar fashion over a period of 6 weeks to a plateau level that was sustained for the duration of the trial period. In this analysis, we confirmed that there was no significant difference using our longitudinal model in the increase in serum 25(OH)D2 for the group who received vitamin D2 compared with the increase in 25(OH)D3 in the group who received vitamin D3. This was reflected by the observation that the total serum 25(OH)D concentrations were no different for the groups ingesting 1000 IU vitamin D2 or 1000 IU vitamin D3 for 11 weeks in this study and the parent studies (31, 32). However, when only the change from baseline in serum 25(OH)D2 and 25(OH)D3 concentrations in this smaller subset of samples from the parent study were compared with the levels at 11 weeks, there was a small, significant (P < .04) difference with a 2-sample t test. No significant change was observed in the group who received placebo (Figure 1).
Serum concentrations of 1,25(OH)2D3 and total 1,25(OH)2D did not change throughout the 11 weeks in the adults who ingested 1000 IU vitamin D3 daily nor in the group who had no additional vitamin D, ie, the placebo group. However, for the group who received 1000 IU vitamin D2, there was a gradual increase in the serum concentrations of 1,25(OH)2D2, which reached a peak concentration at 6 weeks that was sustained for the ensuing 5 weeks and mirrored the increase in the serum 25(OH)D2 concentrations (Figure 2). Although there appeared to be a slight decline in the serum concentrations of 1,25(OH)2D3, it was not statistically significant, and, furthermore, at the end of 11 weeks, the blood levels were the same as they were at baseline. There was a trend for an increase in total 1,25(OH)2D, but the total at baseline (33 ± 2.4 pg/mL) was not statistically significantly different from that at 11 weeks (41.7 ± 3.8 pg/mL).
These results add to the initial observation (35) that vitamin D2 was as effective as vitamin D3, not only in raising blood levels of total 25(OH)D (31, 32) but also in sustaining blood levels of total 1,25(OH)2D because the total 1,25(OH)2D was the same after 11 weeks in both of the groups who received 1000 IU vitamin D2 or 1000 IU vitamin D3. Results from this study suggest that in both young and older men and women 25(OH)D2 was recognized by the kidneys and efficiently converted to 1,25(OH)2D2. The increase in the blood level was approximately 10 pg/mL for 1000 IU vitamin D2, which was 1000 times less than the 10 ng/mL increase observed for 25(OH)D2. What remains unknown is whether 25(OH)D2 is recognized differently by the kidneys and, instead of substituting for 25(OH)D3, it acts as an additional substrate possibly increasing the total blood levels of 1,25(OH)2D as suggested in Figure 2. Although this trial was not powered to detect such an increase, it is intriguing to speculate that ingesting vitamin D2 could potentially increase total circulating concentrations of 1,25(OH)2D.
In conclusion, our results demonstrate that vitamin D2 is metabolized in men and women in a fashion similar to that of vitamin D3 to both its 25-hydroxy and 1,25-dihydroxy metabolites.
Acknowledgments
This work was supported in part by the UV Foundation, the Mushroom Council, and National Institutes of Health Clinical Translational Science Institute Grant UL-1-RR-25711.
Disclosure Summary: N.C. and R.E.R. are employed by Quest Diagnostics and have equity interests in Quest Diagnostics. M.F.H. is an Academic Associate for Quest Diagnostics. The other authors have nothing to disclose.
Footnotes
- CV
- coefficient of variation
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- 1,25(OH)2D2
- 1,25-dihydroxyvitamin D2
- 1,25(OH)2D3
- 1,25-dihydroxyvitamin D3
- 25(OH)D
- 25-hydroxyvitamin D
- 25(OH)D2
- 25-hydroxyvitamin D2
- 25(OH)D3
- 25-hydroxyvitamin D3
- LC-MS/MS
- liquid chromatography–tandem mass spectroscopy.
References
- 1. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281 [DOI] [PubMed] [Google Scholar]
- 2. Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes Am J Clin Nutr. 2006;84:18–28 [DOI] [PubMed] [Google Scholar]
- 3. Hewison M, Burke F, Evans KN, et al. Extra-renal 25-hydroxyvitamin D3-1-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–321 [DOI] [PubMed] [Google Scholar]
- 4. Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin D3-1α-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–894 [DOI] [PubMed] [Google Scholar]
- 5. Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30 [DOI] [PubMed] [Google Scholar]
- 6. Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–371 [DOI] [PubMed] [Google Scholar]
- 7. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancer and cardiovascular disease. Am J Clin Nutr. 2004;80(suppl):1678S–1688S [DOI] [PubMed] [Google Scholar]
- 8. Harries AD, Brown R, Heatley RV, Williams LA, Woodhead S, Rhodes J. Vitamin D status in Crohn's disease: association with nutrition and disease activity. Gut. 1985;26:1197–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503 [DOI] [PubMed] [Google Scholar]
- 10. Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight year prospective study. Lancet. 1989;2:1176–1178 [DOI] [PubMed] [Google Scholar]
- 11. Martinez ME, Giovannuccci EL, Colditz GA, et al. Calcium, vitamin D and the occurrence of colorectal cancer among women. J Natl Cancer Inst. 1996;88:1375–1382 [DOI] [PubMed] [Google Scholar]
- 12. Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control. 2000;11:847–852 [DOI] [PubMed] [Google Scholar]
- 13. Cheng S, Massaro JM, Fox CS, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010. 59:242–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165 [DOI] [PubMed] [Google Scholar]
- 15. Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069 [DOI] [PubMed] [Google Scholar]
- 16. Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D–mediated human antimicrobial response. Science. 2006;311:1770–1773 [DOI] [PubMed] [Google Scholar]
- 17. Bischoff-Ferrari HA, Zhang Y, Kiel DP, Felson DT. Positive association between serum 25-hydroxyvitamin D level and bone density in osteoarthritis. Arthritis Rheum. 2005;53:821–826 [DOI] [PubMed] [Google Scholar]
- 18. Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–146 [DOI] [PubMed] [Google Scholar]
- 20. Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–378 [DOI] [PubMed] [Google Scholar]
- 21. Poskitt EM, Cole TJ, Lawson DE. Diet, sunlight and 25-hydroxyvitamin D in healthy children and adults. Br Med J. 1979;1:221–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moore C, Murphy MM, Keast DR, Holick MF. Vitamin D Intake in the United States. J Am Diet Assoc. 2004;104:980–983 [DOI] [PubMed] [Google Scholar]
- 23. Holick MF. Vitamin D for health and in chronic kidney disease. Semin Dial. 2005;18:266–275 [DOI] [PubMed] [Google Scholar]
- 24. Lu Z, Chen TC, Zhang A, et al. An evaluation of the vitamin D3 content in fish: is the vitamin D content adequate to satisfy the dietary requirement for vitamin D? J Steroid Biochem Mol Biol. 2007;103:642–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holick MF, Shao Q, Liu WW, Chen TC. The vitamin D content of fortified milk and infant formula. N Engl J Med. 1992;326:1178–1181 [DOI] [PubMed] [Google Scholar]
- 26. Jones G, Strugnell S, DeLuca H. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231 [DOI] [PubMed] [Google Scholar]
- 27. Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68:854–858 [DOI] [PubMed] [Google Scholar]
- 28. Armas LA, Hollis B, Heaney RP. Vitamin D2 is much less effective than vitamin D3 ion humans. J Clin Endocrinol Metab. 2004;89:5387–5391 [DOI] [PubMed] [Google Scholar]
- 29. Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D3 is more potent than vitamin D2 in humans. J Clin Endocrinol Metab. 2011;96:E447–E452 [DOI] [PubMed] [Google Scholar]
- 30. Gordon CM, Williams AL, Feldman HA, et al. Treatment of Hypovitaminosis in infants and toddlers. J Clin Endocrinol Metab. 2008;93:2716–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Biancuzzo RM, Cai MH, Winter MR, et al. Fortification of orange juice with vitamin D2 or vitamin D3 is as effective as an oral supplement in maintaining vitamin D status in adults. Am J Clin Nutr. 2010;91:1621–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pietras SM, Obayan BK, Cai MH, Holick MF. Research letter: vitamin D2 treatment for vitamin D deficiency and insufficiency for up to 6 years. Arch Intern Med. 2009;169:1806–1808 [DOI] [PubMed] [Google Scholar]
- 34. Clemens TL, Zhou XY, Myles M, Endres D, Lindsay R. Serum vitamin D2 and vitamin D3 metabolite concentrations and absorption of vitamin D2 in elderly subjects. J Clin Endocrinol Metab. 1986;63:656–660 [DOI] [PubMed] [Google Scholar]
- 35. Hartwell D, Hassager C, Christiansen C. Effect of vitamin D2 and vitamin D3 on the serum concentrations of 1,25(OH)2D3 in normal subjects. Acta Endocrinol (Copenh). 1987;115:378–384 [DOI] [PubMed] [Google Scholar]