Abstract
Context:
There is growing interest in the relationship between bone mineral density, bone strength, and fat depots. Marrow adipose tissue, a well-established component of the marrow environment, is metabolically distinct from peripheral fat depots, but its functional significance is unknown.
Objective:
In this review, we discuss animal and human data linking the marrow adipose tissue depot to parameters of bone density and integrity as well as the potential significance of marrow adipose tissue in metabolic diseases associated with bone loss, including type 1 diabetes mellitus and anorexia nervosa. Potential hormonal determinants of marrow adipose tissue are also discussed.
Conclusions:
We conclude that whereas most animal and human data demonstrate an inverse association between marrow adipose tissue and measures of bone density and strength, understanding the functional significance of marrow adipose tissue and its hormonal determinants will be critical to better understanding its role in skeletal integrity and the role of marrow adipose tissue in the pathophysiology of bone loss.
Marrow fat or marrow adipose tissue (MAT) is well established as a component of the bone marrow (BM) environment, but its function remains unknown. In clinical studies of healthy populations as well as in populations of individuals with metabolic disease, MAT has been shown to be inversely associated with measures of bone mineral density (BMD) and bone integrity and therefore may be an important regulator of bone turnover. With the recent advent of noninvasive methods of measuring MAT, including magnetic resonance spectroscopy, tools are now available to investigate the underlying physiology and function of this depot. This review will highlight what is known about MAT from clinical studies as well as from animal models (Table 1), focusing on the relationship of MAT to bone and other adipose tissue depots.
Table 1.
Marrow Adiposity and Skeletal Phenotype of Animal Models Discussed in This Review
| Mouse Model | Marrow Adiposity | Skeletal Phenotype: Bone Volume Fraction in the Distal Femur (Ref.) |
|---|---|---|
| C3H/HeJ | Increased | High cortical; normal trabecular [low vertebral] (60) |
| C57BL/6J | Reduced | Normal |
| Rosiglitazone treatment | Increased | Low in some strains of mice tested (60, 62) |
| 11 β OHase null (−/−) | None | Normal |
| Ebf1 null (−/−) | Increased | High early then subsequently low (32) |
| Glucocorticoid treatment | Increased or unchanged | Low (99) |
| Wnt10b overexpression in bone (osteocalcin promoter) | Reduced | High (100) |
| Small (Irs1 mutant) | None | Low (76) |
| Little (ghrh-deficient) | Increased | Low (80) |
| LID (targeted Igf-I liver-deficient) | Normal | Low (80) |
| Calorie restriction, C57BL/6 mice- young | Increased | Low (14, 15) |
| High-fat diet, C57BL/6 | Normal to increased | Normal to slightly low (15) |
| Ob/Ob (leptin deficient) | Increased | Low in femur; high in vertebrae (70) |
| C57BL/6 ovariectomy | Increased | Low (80) |
| Igfbp2 null −/− | Increased | Low (101) |
| Lactation at day 7, C57BL/6 | Reduced | Low (C.J.R., unpublished observation) |
| aP2-FoxC2 Tg, overexpression of FoxC2 in adipose cells | Increased | Increased (Beata Lecka-Czernik, PhD, unpublished observation) |
| Insulin deficiency, diabetes mellitus C57BL/6 | Increased | Decreased (57) |
| Insulin resistance, C57BL/6 | Normal to increased | Normal to slightly low (15) |
| Lrp5, High bone mass gene Tg (overexpression) | Reduced | Increased (102) |
| Lrp5-deficient mice | Increased | Decreased (103) |
| BADGE treatment (PPARγ antagonist) | Decreased | Unchanged or increased (104) |
| Radiation (therapeutic or ablative) | Increased | Reduced (105) |
| A-Zip | Reduce | High |
In early childhood, BM exists in a predominantly red or hematopoietic/osteogenic state (1). Exponential accumulation of MAT begins at birth (1), occurring more rapidly in distal than in proximal bones (2), such that by age 25, human BM is approximately 70% MAT. Detailed longitudinal analyses of patients from birth to age 90 years demonstrate that even after stabilization of the mature MAT phenotype, continued gradual accumulation of MAT continues throughout life (3). These observations helped lay the foundation for research into the relationship between MAT and measures of bone integrity, which is an area of active investigation (4).
An integrated approach, including animal models and in vitro work, has provided the framework for our present conceptualization of this adipose depot. However, future investigation using animal models and human studies will be necessary to gain a better understanding of the physiological role and clinical significance of MAT.
MAT Is Distinct From White Adipose Tissue (WAT)
As occurs in peripheral WAT, the lipid content of MAT is almost entirely composed of triglycerides (5). The fatty acid components of the triglyceride may be saturated, monounsaturated, or polyunsaturated, reflecting their number of carbon-carbon double bonds. In times of metabolic need, lipases break down the triglyceride to release free fatty acids into the bloodstream for utilization as an energy source. In BM, multiple studies have shown that free fatty acids have the capacity to regulate osteoblasts, osteoclasts, and blood populations (6–8). This has led to the hypothesis that fatty acids released by MAT may contribute locally to bone metabolism and hematopoiesis. This has also generated an interest in comparing the fatty acid composition of MAT to peripheral WAT depots, which are themselves quite heterogeneous in that WAT depots in various parts of the body—such as those in sc adipose tissue—have different metabolic responses as compared to other WAT depots, such as visceral adipose tissue. It has also been hypothesized that there may be reciprocal regulation, such that bone cells modulate the content of MAT.
In humans, the fatty acid composition of MAT appears to be different from that of sc adipose tissue (9), although older studies did not find such differences (10, 11) (Table 2). Both MAT and sc fat obtained during orthopedic surgery from a large group of predominantly female Chinese patients (mean age, 69.7 ± 10.5 y) were evaluated (9); monounsaturated fat content in MAT was found to be significantly lower and saturated fat content significantly higher than that of the sc fat depot (9). In the rabbit, which has a high proportion of fatty marrow, MAT has been shown to be distinct from WAT because the BM adipocytes are 4 to 6 times smaller in volume than that of perinephric WAT adipocytes (12, 13). One might expect such relative hypotrophy of BM adipocytes to result from decreased triacylglycerol synthesis and/or increased lipolysis; however, evidence suggests otherwise. For example, 14C-palmitate incorporation studies, which allow measurement of triacylglycerol synthesis by adipose tissue, have shown that incorporation of 14C-palmitate is 5 times greater in MAT than in perinephric WAT in rabbits, and uptake and incorporation of fatty acid is similar per adipocyte (12).
Table 2.
Differences in WAT as Compared to MAT
| General Characteristics | Subcutaneous WAT | Visceral WAT | MAT | First Author, y (Ref.) |
|---|---|---|---|---|
| Adipocyte cell size | + | 2++ | + | Trubowitz, 1977 (12); Bathija, 1979 (13); O.A.M., unpublished observations |
| Fatty acid contenta | Griffith, 2009 (9); Boyd, 1956 (10); Zach, 1974 (11) | |||
| Polyunsaturated | 9–21% | 20% | 9–20% | |
| Monounsaturated | 46–63% | 45% | 48–58% | |
| Saturated | 23–36% | 35% | 29–33% | |
| Fatty acid esterification capacity | + | + | 2++ | Trubowitz, 1977 (12) |
| Precursor cell | Lin-CD29+CD34+Sca1 +CD24+ | Lin-CD29+CD34+Sca1 +CD24+ | Unknown, not CD24+ | Rodeheffer, 2008 (20); M.C.H., unpublished observations |
These numbers represent results in humans. These differences do not hold true in all species.
States of nutritional deprivation also demonstrate significant differences between MAT and WAT; fasting of rabbits for up to 3 weeks does not diminish the fatty acid esterification capacity of MAT, whereas that of perinephric WAT decreases by as much as 60% (13). In a 10-day calorie-restriction study in this species, electron microscopic analysis demonstrated marked breakdown of the central lipid vacuole in white adipocytes, whereas marrow adipocytes of the distal tibia failed to mobilize lipid (14). A similar finding has been demonstrated with calorie restriction in young mice (ie, up to 16 wk of age) inducing BM adipocyte formation, accompanied by a decrease in the acquisition of bone mass (15) (Table 1). In contrast, older mice on a 30% calorie restriction diet show enhanced bone mass but no evidence of increased marrow adiposity. In human models of chronic calorie restriction, such as anorexia nervosa, there is also an increase in MAT as compared to healthy weight controls, and despite very low stores of WAT (16–18) (Table 3). These findings suggest that MAT and WAT respond to nutritional cues in distinct ways. The mechanism of how calorie restriction triggers the development of marrow adipocytes still remains to be elucidated. One possibility is that there is a systemic signal, released either centrally or locally, that triggers a hormonal response to induce marrow adipogenesis. One such candidate is the orexigenic hormone ghrelin, which has been shown to induce marrow adipogenesis in experimental animals (19).
Table 3.
Changes in the Amount of WAT vs MAT in Various Metabolic Disease States
| WAT | MAT | First Author, y (Ref.) | |
|---|---|---|---|
| Calorie restriction/anemia | Decrease | Increase | Tavassoli, 1974 (14); Devlin, 2010 (15); Bredella, 2009 (16); Ecklund, 2010 (17); Fazeli, 2012 (18) |
| Type 1 diabetes mellitus | Decrease | Increase | Botolin, 2005 (56); Botolin, 2007 (57) |
| Obesity | Increase | Variable | Bredella, 2011 (53) |
| Estrogen deficiency | Variable | Increase | Riggs, 2002 (48) |
| Type 2 diabetes mellitus | Variable | Variable | Baum, 2012 (59) |
| GH deficiency | Increase | Increase | Charlton, 1988 (78); Menagh, 2010 (80) |
Progenitor populations
It is generally accepted that MAT arises in BM from mesenchymal stem cells that are more closely related to osteoblasts than other cells of mesenchymal origin (chondrocytes, myocytes, and stromal cells). However, it remains unclear whether BM adipocytes are related to either white or brown adipocytes or whether they form another distinct class of adipocytes. The adipocyte progenitor in non-BM WAT depots is likely distinct from the cell that gives rise to BM adipocytes. Using flow cytometry, we analyzed BM from C57BL/6 mice to determine whether the adipocyte progenitor identified in WAT (Lin− CD29+CD34+Sca1+CD24+) (20) was present. In multiple experiments, the CD24+ adipocyte progenitor could not be found in normal C57BL/6 BM cells (M.S.R., M.C.H., and Jackie A. Fretz, PhD, unpublished observations).
Recently, gene profiling was used to compare adult C57BL/6 BM-derived adipocytes to epididymal adipocytes (21). Distinct gene patterns were seen between the two groups, with BM adipocytes having low expression of adipocyte-specific genes (eg, PPARγ, FABP4, perilipin) and high expression of genes associated with early adipocyte differentiation (C/EBPβ, RGS2). Interestingly, genes that regulate bone cell function (SFRP4, TNFα, TGFβ) were also markedly up-regulated in BM adipocytes.
MAT replaces “red” hematopoietic/osteogenic marrow within the long bones of humans at the time of peak bone acquisition and appears late in life at other skeletal sites (22, 23). A recent study suggests that MAT may express genes characteristic of brown adipose tissue (BAT) (24). In this study, RNA isolated from the tibias of C57BL/6 mice at 5 months of age were examined for BAT and WAT gene markers, and when compared to WAT, MAT expressed increased transcripts for Dio2, PGC1α, Prdm16, and FoxC2, all of which are established markers of BAT. The interpretation of these data is complicated, as discussed by the study investigators, because some of these genes are expressed in cells other than adipocytes, and whole tibias were used rather than isolated adipocytes (24). However, expression of WAT markers, including leptin and adiponectin, was reduced (24). Similarly, when comparing the marrow of C3H/HeJ mice, an inbred strain with high marrow fat, to that of C57BL/6J mice, markers of brown adipogenesis including uncoupling protein 1 (UCP1) and PGC1α, were markedly increased at 6 months of age in the male C3H/HeJ mice (C.J.R., unpublished observation). Interestingly, in older mice (26 mo old), the number of marrow adipocytes was increased compared to the younger mice, and the BAT genes, Prdm16, FoxC2, and Dio2, showed significantly lower expression than in the 5-month-old mice (24). These data support the concept that adipocytes in BM and non-BM depots are different, change with age, and may have distinct functions.
Potential function of MAT
The function of marrow adipocytes is poorly understood, with little specific data indicating roles in BM-dependent activities such as osteogenesis or hematopoiesis. In adult mice, adipogenesis in the spine increases in a gradient from proximal to distal vertebrae (25). As expected, the number of CD45+ hematopoietic cells in the BM of tail vertebrae is reduced by 25% compared to the BM from thoracic vertebrae. Importantly, the percentage of hematopoietic stem cells cKit+ Lin− Sca1+ Flk2−, multipotent progenitors, common myeloid progenitors, granulocyte-macrophage progenitors, and megakaryocyte-erythroid progenitors are all reduced 2- to 3-fold in BM from adipocyte-enriched tail vertebrae compared to BM from thoracic vertebrae (25). These data suggest that increased adipogenesis is an inhibitor of BM hematopoiesis. In contrast, C3H/HeJ mice, which have very high marrow adiposity, have intact hematopoiesis and high cortical bone density (M.C.H. and C.J.R., unpublished observations). In another example, although Ebf1 knockout mice are B-cell deficient, they have high BM fat, whereas the rest of their hematopoietic system is intact (26). These contradictory findings indicate that the interactions between marrow adipocytes and hematopoiesis are complex, and more work is required to understand these interactions.
With regard to bone, Meunier et al (27) and others have noted a strong association between reduced osteoblast function, as determined by histomorphometric measures and adipocytic infiltration of the marrow (27–29). A-Zip mice, which lack WAT, are also deficient in MAT and have high bone density (28). Conversely, C3H/HeJ mice have high trabecular bone mass and increased MAT.
Taken together, these data strongly suggest that MAT is not just “filler” or a default for other “more important” BM components (30). These cells may derive from a progenitor that is distinct from the one that gives rise to either WAT or BAT in depots outside the BM. In vitro and in vivo, functional adipocyte progenitors can be found in WAT depots consistent with the idea that adipocyte progenitors from the BM are not required to seed or replace adipocytes outside of the BM (31). Whether marrow adipocytes share a common progenitor with osteoblasts remains to be shown in vivo. Precursor cells in non-BM WAT depots, after enrichment and stimulation, can give rise to osteoblast-like cells capable of producing mineral. The fact that there are no bones in sc tissue suggests that under normal conditions these cells lack the required support cells and growth factors to produce osteoblasts, and/or are actively suppressed. In many situations, increased marrow adiposity is coincident with decreased bone mass and reduced hematopoiesis, although clear exceptions to this observation have been reported (32, 33). These data raise a series of critical questions, especially regarding the role BM adipocytes play in regulating BM cell differentiation and function.
Measuring MAT in Humans
With the use of magnetic resonance spectroscopy, noninvasive, accurate quantification of MAT has become possible in the form of a lipid-to-water ratio (34, 35). Some investigators have used T1-weighted magnetic resonance imaging for the measurement of MAT (36, 37), arguing that this method allows them to measure MAT in a larger volume of BM, whereas others have used computed tomography (CT) to quantify MAT (38). For the most part, all of these methods of quantification have led to similar findings in healthy individuals.
MAT in Healthy Populations
Studies of healthy populations demonstrate that MAT increases with age in both males and females (35, 36, 39, 40) (Figure 1), and males have greater amounts of MAT compared to females (35). The association between WAT depots and BMD is dependent upon the location of the adipose tissue. Visceral adipose tissue has been inversely associated with BMD in adolescents and adults (41–44), whereas sc adipose tissue depots may be positively associated with BMD (44). With respect to MAT, an inverse relationship between MAT and BMD is demonstrated in groups of healthy Caucasian women (36) and more recently in a population of middle-aged (ages 38–52 y) healthy Caucasian and African American men and women (37). This relationship holds for both young individuals (38) and older individuals, as Wren et al (45) have shown that femoral cortical bone area is inversely associated with MAT in both young subjects (<25 y old) and older men and women (>55 y old). Yet the relationship between BMD and MAT is complex and cannot be thought of solely as an inverse relationship. For example, during periods of development such as puberty, both MAT and bone density increase, suggesting that this differentiation process does not always occur in an inverse manner. Similarly, although males have more MAT than females, they also have greater bone mass. The in vitro data of Post et al (46), which demonstrated the presence of independent preosteoblastic and preadipocytic cell populations, provide further evidence of a complex relationship between bone mass and MAT.
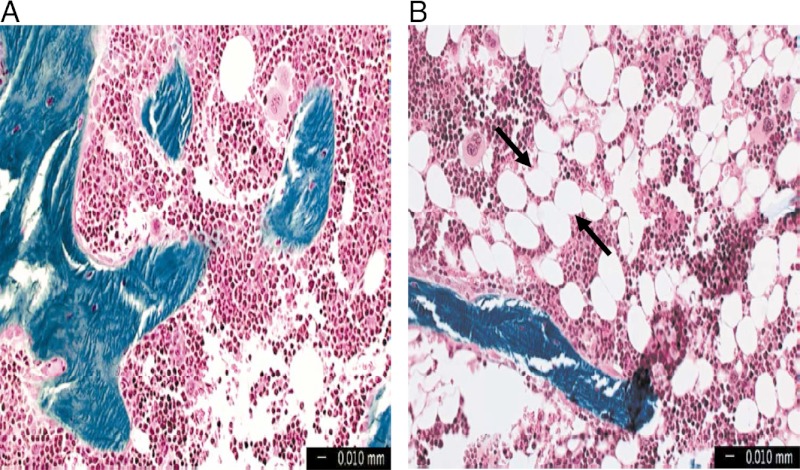
Figure 1.
Human marrow from the iliac crest of an 18 year old (A) and an 80 year old (B), demonstrating the age-related increase in marrow adipocyte “ghosts” (arrows) in human marrow.
MAT and aging
In rodents and humans, aging is associated with a significant increase in marrow adiposity. In an in vitro model, 17-β estradiol and DHT decrease the expression of PPARγ and the percentage of fat cells in human mesenchymal stem cells induced to differentiate into adipocytes after treatment with a PPARγ agonist (47), and therefore decreased levels of these hormones may contribute to the increase in marrow adiposity with age. C57BL/6 is the most frequent mouse strain studied, and in both males and females, mice older than 20 months accumulate marrow adipocytes in the distal femur and proximal tibia but not in the lumbar vertebrae. As noted, this is associated with the age-related decline in trabecular bone mass but not with cortical bone density. Ovariectomy reliably enhances adipocyte infiltration in the BM of all strains that have been studied, and MAT is greater in older retired breeders than younger mice (48). Finally, deletion of estrogen receptor-α, but not estrogen receptor-β, in adipocytes using an adiponectin promoter is also associated with a marked increase in marrow adiposity (Deborah Clegg, PhD, personal communication). Thus, the absence of estrogen receptor-α activation (either through deficient ligand or receptor) accelerates the development of marrow adipocytes; in contrast, estrogen supplementation can prevent marrow adiposity in ovariectomized animals. These findings recapitulate changes within the BM of postmenopausal women who are not replaced with estrogen (49).
MAT and Bone Strength
Early studies examining the adipose tissue content of bone using iliac crest bone biopsies in healthy individuals and those with osteoporosis demonstrate that osteoporotic individuals have increased MAT volume and decreased trabecular bone volume as compared to age-matched controls, suggesting an association between increased fracture risk and increased MAT (39). Schellinger et al (40) also found an inverse relationship between MAT and bone integrity using magnetic resonance spectroscopy; in subjects with weakened bone, as evidenced by the observation of Schmorl's nodes (intraosseous disk herniation), endplate depression, vertebral wedging, and vertebral compression fractures, the fraction of MAT was significantly higher as compared to controls. Wehrli et al (50) also demonstrated that MAT in the lumbar vertebrae is an independent predictor of fracture. Therefore, MAT is an important indicator of bone integrity, although the mechanism is not known.
Whether interventions such as increasing physical activity, which has been shown to increase BMD (51), can change levels of MAT has been studied in 20 young children (with a mean age of approximately 5 y) (52). Casazza et al (52) randomized children to a school-based physical activity intervention consisting of 20 minutes of moderate physical activity 3 times per week, or to usual physical activity, and found that those randomized to the physical activity group had a significant decrease in femoral MAT.
MAT and Metabolic Disease
MAT in obesity
Because MAT is a lipid-storage depot, one might expect increased levels of MAT in obese individuals as compared to normal weight individuals, but in a study of obese women only those with high levels of visceral fat also had increased MAT (53) (Table 3). In this group of 47 premenopausal women with a mean body mass index (BMI) of 30 ± 7 kg/m2, there was an inverse relationship between MAT and BMD (53), similar to that seen in healthy populations.
MAT and BMD in type 1 diabetes mellitus
Clinical evidence demonstrates that type 1 diabetes mellitus is a significant risk factor for low bone mass and an increased incidence of fractures, although the underlying mechanisms are not fully understood (54, 55). To understand the pathogenesis of low bone mass in diabetic patients, animal models of insulin deficiency have been generated, and marrow adiposity is a frequent accompaniment. The two most common mouse strains studied are the spontaneous diabetic NOD mouse and C57BL/6 mice in which diabetes is induced by streptozotocin (56, 57). In both of these models of type 1 diabetes mellitus, expression of proadipocytic genes such as PPARγ and aP2 (FABP4) was increased in long bones, and this correlated with decreased expression of osteocalcin (56, 57) (Table 3). Importantly, treatment with bisphenol A diglycidyl ether (BADGE), a PPARγ antagonist, decreased the marrow adiposity observed in streptozotocin-induced diabetic models, although skeletal loss was not reversed by the BADGE treatment. A recent study in individuals with type 1 diabetes mellitus found that levels of MAT were not significantly different in these subjects as compared to nondiabetic controls, and that there were no correlations between MAT and severity of disease, as assessed by hemoglobin A1C (58). Thus, it is still unclear whether marrow adipocytic infiltration in diabetic patients plays a central role in the development of bone loss, and further clinical studies are necessary to better understand the relationship of MAT and BMD in type 1 diabetic patients.
MAT and BMD in type 2 diabetes mellitus (T2DM)
In postmenopausal overweight women with T2DM, the inverse association between MAT and BMD is also seen (59). In one study, women with T2DM were found to have similar levels of MAT compared to a group of women of comparable BMI, age, and volumetric BMD as assessed by quantitative CT. Although, hemoglobin A1C levels were positively associated with vertebral MAT in the diabetic subjects; diabetic women with hemoglobin A1C levels > 7% had significantly higher levels of MAT as compared to those with levels ≤ 7% (Table 3). Therefore, in postmenopausal women with T2DM, MAT may influence or be influenced by glycemic control.
MAT and PPARγ activation
Activation of PPARγ is generally but not always associated with increased marrow adiposity as well as bone loss in rodent models, although the effect of PPARγ agonists on increased marrow adiposity is strain and drug specific (60). Treatment with rosiglitazone provides the best example of drug-induced marrow adiposity in rodents, although its effect in humans is still debated (60). Troglitazone has been shown to increase marrow adiposity in apolipoprotein E knockout mice, which are characterized by lipid abnormalities, but it does not appear to change bone mass (61). Netoglitazone, a relatively weak thiazolidinedione, was found to decrease whole body bone mineral content but did not affect trabecular bone volume or whole body areal BMD in C57BL/6 mice, but this thiazolidinedione also increased marrow adiposity (62). Importantly, aging is a key factor determining susceptibility to thiazolidinedione-induced bone loss because capacity for PPARγ activation increases with aging in the BM. Consistent with these findings, rosiglitazone-induced bone loss is much more pronounced in older female C57BL/6 mice and is associated with significant infiltration of marrow with large adipocytes. Histomorphometric indices in these mice reveal a marked suppression in bone formation and increases in bone resorption consistent with a profound imbalance in bone remodeling (24). Not surprisingly, this effect is strain-specific, such that some mice show only increased marrow adipogenesis but no bone loss (eg,C3H/HeJ), whereas others display no skeletal or marrow effects from rosiglitazone (60). However, the relevance of these findings in mice to humans is unclear because MAT is not increased in postmenopausal women treated with rosiglitazone (63), whereas another study that randomized individuals with T2DM to pioglitazone or placebo for 6 months demonstrated increases in MAT in the pioglitazone group (64).
MAT and leptin deficiency
Leptin is a protein secreted by adipocytes that regulates fertility, appetite, and energy metabolism. In addition, emerging evidence indicates the critical role of leptin in skeletal metabolism by relaying a sympathetic neural signal after binding in the hypothalamus (28, 65, 66). The ob/ob (obese) mouse, first described in 1950 by Ingalls et al (67), carries a spontaneous nonsense mutation at codon 105 of the leptin gene that results in a complete loss of leptin protein (67, 68). As the name of this mouse suggests, they are extremely obese: exhibiting profound hyperphagia, glucose intolerance, and hyperinsulinemia (68, 69). In the femur, ob/ob mice have increased marrow adipocytes, and peripheral administration of leptin corrects the marrow adiposity phenotype (70). Although the skeletal phenotype of ob/ob mice remains somewhat controversial, some papers indicate that there may be site- and compartment-specific determinants such that cortical and trabecular compartments of the femur exhibit lower bone mass (70–75). However, the vertebral compartment of these mice shows very high bone mass, despite the absence of gonadal steroids, and this is associated with decreased marrow adiposity (28, 66, 70), thus establishing the inverse relationship between marrow adiposity and bone mass in a compartment-specific manner.
MAT and insulin receptor substrate (IRS)-1-deficient models
Insulin is critical for adipogenesis and fat storage. The insulin receptor knockout mouse, which has global absence of the insulin receptor but in which expression of the receptor has been restored in the pancreas, liver, and brain, but not muscle or fat, has extremely low numbers of marrow adipocytes and normal trabecular BMD (56). Similarly, in a spontaneous mutant, small, which has a homozygous recessive mutation in the intact IRS-1 molecule, there is a virtual absence of marrow fat, which mirrors the absence of peripheral WAT depots, despite very low bone mass during neonatal and adult life (76). These mice have higher insulin levels than controls and are somewhat insulin resistant. As noted previously, bone loss, diabetes, and marrow adiposity have been described in several other mouse models, all of which show reduced osteocalcin expression (57, 77). Furthermore, as was noted in ob/ob mice, the increase in marrow adipocytes appears limited to the calvaria and long bones and not the vertebrae (56). Hence, there is site and context specificity to the relationship of marrow adiposity to insulin sensitivity and peripheral fat depots.
MAT and metabolic risk
GH deficiency is associated with known cardiometabolic risk factors including increased visceral fat, dyslipidemia, and insulin resistance. GH is produced and secreted from somatotrophs in the anterior pituitary and regulates linear growth, body composition, and lipid and glucose metabolism in both an IGF-I-dependent and -independent manner. GH is well-known to induce lipolysis in adipose tissue, and patients with GH deficiency have been shown to acquire more fat than normal controls, leading to the development of obesity-related metabolic complications. In bone, GH has both direct effects on long bone growth and indirect effects via IGF-I. Rats harboring the spontaneous dw/dw mutation are dwarfs, as a result of GH deficiency, and have a profound increase in both adipocyte number and adipocyte size in the marrow of long bones compared to wild-type controls (78) (Table 3). Treatment with GH results in a decrease in adipocyte number, whereas treatment with IGF-I results in a decrease in adipocyte volume (79). Recently, Turner and colleagues (80) demonstrated that acute pituitary insufficiency due to hypophysectomy resulted in significant marrow adiposity that can be reversed by GH, but not by IGF-I, estrogen, or cortisol. Interestingly, LID mice, in which hepatic IGF-I is deleted, have increased very high GH levels and low IGF-I but do not have marrow adiposity. On the other hand, little mice with a spontaneous mutation of the GHRH receptor have significant marrow fat (80). Thus, abnormalities in the GH–IGF-I axis are associated with marrow adipogenesis.
Whether MAT is associated with metabolic risk factors has been investigated in 131 young, healthy males and females between 16 and 25 years of age (81). Femoral MAT, as measured by CT, was not associated with any measure of metabolic risk, including waist-to-hip ratio, blood pressure, carotid intima-media thickness, insulin resistance, or lipid profile (81), suggesting that in young, healthy individuals MAT is metabolically distinct from visceral adipose tissue. Therefore, an association appears to exist between a higher metabolic risk profile and higher levels of MAT in obese women and those with T2DM; however, in young, healthy subjects, MAT does not seem to be associated with markers of metabolic risk.
MAT in anorexia nervosa
Anorexia nervosa is a primary psychiatric disorder characterized by extreme, self-induced starvation. Individuals affected by anorexia nervosa are predominantly female and have low levels of sc and visceral fat. Thus, one might expect their levels of MAT to also be decreased. On the contrary, compared to healthy controls, females with anorexia nervosa have higher levels of MAT (Table 3). Although an earlier study found that women with anorexia nervosa have less fat in the femur as compared to controls, the women with anorexia nervosa in this study were all inpatients and therefore likely significantly more ill than the patients with anorexia nervosa in the other studies (82). As the authors suggest, this decrease in MAT most likely represents serous atrophy, a gelatinous transformation, or degeneration of BM that has been described in women with anorexia nervosa who are very low in weight (82–84). A study of BM aspirates and biopsy specimens classified the BM of 40 women and 4 men with anorexia nervosa and found that only 11% had normal BM (84). Thirty-nine percent of the subjects had hypoplastic BM (defined as an increase in the proportion of adipocytes but with the presence of foci of erythroblasts, granulocytes, and megakaryocytes) or aplastic BM, whereas 50% had partial or complete gelatinous degeneration of the BM (defined as the absence of normal hematopoietic cells and the presence of degenerated adipocytes and hyaluronic acid matrix) (84). Nearly half of the subjects studied had an increase in the proportion of adipocytes (84). These cytological and histological findings also correlated with the degree of weight loss, and therefore those patients who lost more weight and were more ill were more likely to have gelatinous degeneration of the marrow (84). A subset of patients underwent biopsies after recovery from anorexia nervosa, and biopsy specimens demonstrated normalization of the marrow (84).
Normalization of MAT after recovery from anorexia nervosa has also been demonstrated in a cross-sectional study comparing women who have recovered from anorexia nervosa (defined as women with a history of anorexia nervosa with a weight greater than 85% of ideal body weight and regular menstrual cycles for at least 3 mo), women with active anorexia nervosa, and healthy controls. Women who had recovered from anorexia nervosa had significantly lower levels of MAT at the L4 vertebra as compared to women with anorexia nervosa (18). In fact, the levels of MAT at the L4 vertebra in women who had recovered from anorexia nervosa were comparable to levels in healthy controls (18).
MAT and BMD in anorexia nervosa
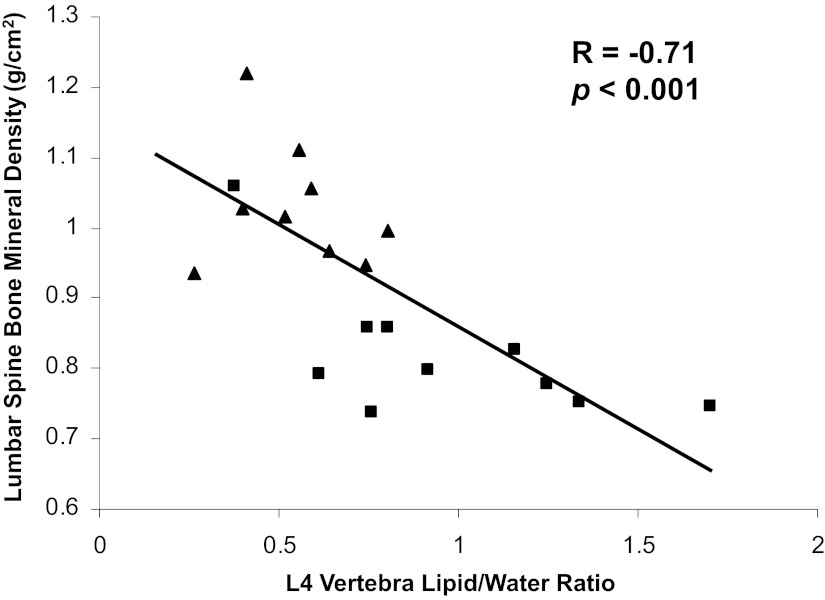
In women with anorexia nervosa, the inverse association between MAT and BMD remains intact (16) (Figure 2). In a study comparing MAT in 10 women with anorexia nervosa with 10 healthy controls of similar age, there was an inverse relationship between vertebral and femoral MAT and BMD of the lumbar spine and hip, and these relationships remained significant even after controlling for amenorrhea (16).
Figure 2.
Marrow fat of the L4 vertebra is inversely associated with lumbar spine BMD (R = −0.71; P < .001) in a group of women with anorexia nervosa (squares) and healthy controls (triangles).
MAT and body composition in anorexia nervosa
In anorexia nervosa, there is an inverse association between MAT of the femoral metaphysis and diaphysis and sc adipose tissue of the thigh, sc adipose tissue of the abdomen, and total abdominal adipose tissue (16). In women with anorexia nervosa, MAT (of the L4 vertebra, femoral metaphysis and diaphysis) is also inversely associated with BMI. Yet there is no relationship between disease duration and MAT in these patients (16, 17), suggesting that increased levels of MAT are a function of absolute weight as opposed to the chronicity of the disease, which is also suggested by the cytological and histological findings cited above (84).
Potential Determinants of MAT
Preadipocyte factor-1 (Pref-1)
Pref-1, a member of the epidermal growth factor-like family of proteins, is a known regulator of adipocyte and osteoblast differentiation and has been investigated as a potential determinant of MAT. Circulating Pref-1 concentrations are elevated in women with anorexia nervosa as compared to healthy controls (85). In women who have recovered from anorexia nervosa, Pref-1 levels are significantly lower as compared to women with active anorexia nervosa and are comparable to levels in normal-weight controls (18). Pref-1 has also been shown to be positively associated with MAT of the L4 vertebra in women with anorexia nervosa, whereas in healthy controls, Pref-1 is inversely associated with MAT of the L4 vertebra and therefore may have differential effects in different states of nutritional sufficiency (18).
Cortisol
Women with anorexia nervosa have higher levels of cortisol as compared to healthy controls (86, 87). Cortisol levels have also been shown to be a predictor of low BMD in anorexia nervosa (87). Because corticosteroids lead to adipocyte differentiation of human marrow preadipocytes in vitro (88), it is possible that the elevated cortisol levels in anorexia nervosa play a role in the elevated levels of MAT in this disorder as well. Further clinical studies will need to be performed to evaluate this potential determinant of MAT.
Early B-cell factor 1 (Ebf1)
The transcription factor Ebf1, originally identified as necessary for B lymphocyte maturation, also regulates the development of other cell types (26, 89, 90), including osteoblasts and adipocytes (91, 92). In vitro studies have shown that decreasing Ebf1 in mesenchymal progenitors decreases adipocyte formation, whereas overexpression of Ebf1 stimulates adipogenesis (92–94). An initial characterization of the bone phenotype of Ebf1−/− mice demonstrates that they not only have a large increase in the number of osteoblasts but also have more marrow adipocytes (91). This is noteworthy because under many conditions where MAT increases, osteoblast number and BMD decrease (95–97). A striking loss of both sc and visceral WAT was noted, as was an absence of subdermal adipocytes (32, 98). Therefore, with respect to adipocyte distribution, the loss of Ebf1 results in a phenotype similar to that of anorexia nervosa.
Conclusions
Both animal and human data demonstrate that MAT is context-specific and in many situations is inversely associated with measures of bone integrity. However, in other models MAT is positively associated with trabecular bone mass, raising a series of important questions about its functional significance and implications for the structural integrity of the skeleton. Longitudinal studies using different human models of disease including individuals with anorexia nervosa and obesity will be critical to understanding the relationship between MAT and other fat depots, including sc and visceral fat stores, as well as the hormonal determinants of MAT. Performing these studies will be critical to gaining insight into the role of MAT and its relationship to bone remodeling.
Acknowledgments
Writing of this review and experimental work were enabled by support from the National Institute of Diabetes, Digestive and Kidney Diseases/National Institutes of Health (Grants R24DK092759 and K23 DK094820 to P.K.F.), the Yale Core Center for Musculoskeletal Disorders (Grant P30AR046032), and the Department of Orthopedics and Rehabilitation, Yale University School of Medicine, New Haven, Connecticut (to M.C.H.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BADGE
- bisphenol A diglycidyl ether
- BAT
- brown adipose tissue
- BM
- bone marrow
- BMD
- bone mineral density
- BMI
- body mass index
- CT
- computed tomography
- Ebf1
- early B-cell factor 1
- IRS
- insulin receptor substrate
- MAT
- marrow adipose tissue
- Pref-1
- preadipocyte factor-1
- T2DM
- type 2 diabetes mellitus
- WAT
- white adipose tissue.
References
- 1. Custer RP. Studies on the structure and function of bone marrow. Part I. J Lab Clin Med. 1932;17:951–960 [Google Scholar]
- 2. Kricun ME. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985;14:10–19 [DOI] [PubMed] [Google Scholar]
- 3. Custer RP, Ahlfeldt FE. Studies on the structure and function of bone marrow. Part II. J Lab Clin Med. 1932;17:960–962 [Google Scholar]
- 4. Burkhardt R, Kettner G, Bohm W, et al. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone. 1987;8:157–164 [DOI] [PubMed] [Google Scholar]
- 5. Tavassoli M, Houchin DN, Jacobs P. Fatty acid composition of adipose cells in red and yellow marrow: a possible determinant of haematopoietic potential. Scand J Haematol. 1977;18:47–53 [DOI] [PubMed] [Google Scholar]
- 6. Cornish J, MacGibbon A, Lin JM, et al. Modulation of osteoclastogenesis by fatty acids. Endocrinology. 2008;149:5688–5695 [DOI] [PubMed] [Google Scholar]
- 7. Maurin AC, Chavassieux PM, Meunier PJ. Expression of PPARγ and β/δ in human primary osteoblastic cells: influence of polyunsaturated fatty acids. Calcif Tissue Int. 2005;76:385–392 [DOI] [PubMed] [Google Scholar]
- 8. Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. 2005;174:5390–5397 [DOI] [PubMed] [Google Scholar]
- 9. Griffith JF, Yeung DK, Ahuja AT, et al. A study of bone marrow and subcutaneous fatty acid composition in subjects of varying bone mineral density. Bone. 2009;44:1092–1096 [DOI] [PubMed] [Google Scholar]
- 10. Boyd HM, Peltier LF, Scott JR, Wheeler DH. Fat embolism. II. The chemical composition of fat obtained from human long bones and subcutaneous tissue. Surgery. 1956;40:661–664 [PubMed] [Google Scholar]
- 11. Zach E, Shafrir E. Composition of bone marrow adipose tissue in relation to body fat depots in various species. Isr J Med Sci. 1974;10:1541–1550 [PubMed] [Google Scholar]
- 12. Trubowitz S, Bathija A. Cell size and plamitate-1–14c turnover of rabbit marrow fat. Blood. 1977;49:599–605 [PubMed] [Google Scholar]
- 13. Bathija A, Davis S, Trubowitz S. Bone marrow adipose tissue: response to acute starvation. Am J Hematol. 1979;6:191–198 [DOI] [PubMed] [Google Scholar]
- 14. Tavassoli M. Differential response of bone marrow and extramedullary adipose cells to starvation. Experientia. 1974;30:424–425 [DOI] [PubMed] [Google Scholar]
- 15. Devlin MJ, Cloutier AM, Thomas NA, et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25:2078–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bredella MA, Fazeli PK, Miller KK, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:2129–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ecklund K, Vajapeyam S, Feldman HA, et al. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res. 2010;25:298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fazeli PK, Bredella MA, Freedman L, et al. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. J Bone Miner Res. 2012;27:1864–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thompson NM, Gill DA, Davies R, et al. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology. 2004;145:234–242 [DOI] [PubMed] [Google Scholar]
- 20. Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249 [DOI] [PubMed] [Google Scholar]
- 21. Liu LF, Shen WJ, Ueno M, Patel S, Kraemer FB. Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics. 2011;12:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moore SG, Dawson KL. Red and yellow marrow in the femur: age-related changes in appearance at MR imaging. Radiology. 1990;175:219–223 [DOI] [PubMed] [Google Scholar]
- 23. Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell. 2004;3:379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50:546–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin H, Grosschedl R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 1995;376:263–267 [DOI] [PubMed] [Google Scholar]
- 27. Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–154 [DOI] [PubMed] [Google Scholar]
- 28. Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207 [DOI] [PubMed] [Google Scholar]
- 29. Schellinger D, Lin CS, Lim J, Hatipoglu HG, Pezzullo JC, Singer AJ. Bone marrow fat and bone mineral density on proton MR spectroscopy and dual-energy x-ray absorptiometry: their ratio as a new indicator of bone weakening. AJR Am J Roentgenol. 2004;183:1761–1765 [DOI] [PubMed] [Google Scholar]
- 30. Gimble JM, Robinson CE, Wu X, Kelly KA. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996;19:421–428 [DOI] [PubMed] [Google Scholar]
- 31. Koh YJ, Kang S, Lee HJ, et al. Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. J Clin Invest. 2007;117:3684–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fretz JA, Nelson T, Xi Y, Adams DJ, Rosen CJ, Horowitz MC. Altered metabolism and lipodystrophy in the early B-cell factor 1-deficient mouse. Endocrinology. 2010;151:1611–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kveiborg M, Sabatakos G, Chiusaroli R, et al. δ-FosB induces osteosclerosis and decreases adipogenesis by two independent cell-autonomous mechanisms. Mol Cell Biol. 2004;24:2820–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schick F, Einsele H, Bongers H, et al. Leukemic red bone marrow changes assessed by magnetic resonance imaging and localized 1H spectroscopy. Ann Hematol. 1993;66:3–13 [DOI] [PubMed] [Google Scholar]
- 35. Schellinger D, Lin CS, Fertikh D, et al. Normal lumbar vertebrae: anatomic, age, and sex variance in subjects at proton MR spectroscopy—initial experience. Radiology. 2000;215:910–916 [DOI] [PubMed] [Google Scholar]
- 36. Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18:641–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen W, Scherzer R, Gantz M, et al. Relationship between MRI-measured bone marrow adipose tissue and hip and spine bone mineral density in African-American and Caucasian participants: the CARDIA study. J Clin Endocrinol Metab. 2012;97:1337–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Di Iorgi N, Rosol M, Mittelman SD, Gilsanz V. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metab. 2008;93:2281–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171 [DOI] [PubMed] [Google Scholar]
- 40. Schellinger D, Lin CS, Hatipoglu HG, Fertikh D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol. 2001;22:1620–1627 [PMC free article] [PubMed] [Google Scholar]
- 41. Russell M, Mendes N, Miller KK, et al. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab. 2010;95:1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bredella MA, Torriani M, Ghomi RH, et al. Determinants of bone mineral density in obese premenopausal women. Bone. 2011;48:748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Katzmarzyk PT, Barreira TV, Harrington DM, Staiano AE, Heymsfield SB, Gimble JM. Relationship between abdominal fat and bone mineral density in white and African American adults. Bone. 2012;50:576–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Campos RM, Lazaretti-Castro M, Mello MT, et al. Influence of visceral and subcutaneous fat in bone mineral density of obese adolescents. Arq Bras Endocrinol Metabol. 2012;56:12–18 [DOI] [PubMed] [Google Scholar]
- 45. Wren TA, Chung SA, Dorey FJ, Bluml S, Adams GB, Gilsanz V. Bone marrow fat is inversely related to cortical bone in young and old subjects. J Clin Endocrinol Metab. 2011;96:782–786 [DOI] [PubMed] [Google Scholar]
- 46. Post S, Abdallah BM, Bentzon JF, Kassem M. Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone. 2008;43:32–39 [DOI] [PubMed] [Google Scholar]
- 47. Benvenuti S, Cellai I, Luciani P, et al. Androgens and estrogens prevent rosiglitazone-induced adipogenesis in human mesenchymal stem cells. J Endocrinol Invest. 2012;35:365–371 [DOI] [PubMed] [Google Scholar]
- 48. Riggs BL, Khosla S, Melton LJ., III Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302 [DOI] [PubMed] [Google Scholar]
- 49. Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int. 2008;19:1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wehrli FW, Hopkins JA, Hwang SN, Song HK, Snyder PJ, Haddad JG. Cross-sectional study of osteopenia with quantitative MR imaging and bone densitometry. Radiology. 2000;217:527–538 [DOI] [PubMed] [Google Scholar]
- 51. Meyer U, Romann M, Zahner L, et al. Effect of a general school-based physical activity intervention on bone mineral content and density: a cluster-randomized controlled trial. Bone. 2011;48:792–797 [DOI] [PubMed] [Google Scholar]
- 52. Casazza K, Hanks LJ, Hidalgo B, Hu HH, Affuso O. Short-term physical activity intervention decreases femoral bone marrow adipose tissue in young children: a pilot study. Bone. 2012;50:23–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bredella MA, Torriani M, Ghomi RH, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring). 2011;19:49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int. 2007;18:427–444 [DOI] [PubMed] [Google Scholar]
- 55. McCabe LR. Understanding the pathology and mechanisms of type I diabetic bone loss. J Cell Biochem. 2007;102:1343–1357 [DOI] [PubMed] [Google Scholar]
- 56. Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-γ2 expression in type I diabetic mice. Endocrinology. 2005;146:3622–3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology. 2007;148:198–205 [DOI] [PubMed] [Google Scholar]
- 58. Slade JM, Coe LM, Meyer RA, McCabe LR. Human bone marrow adiposity is linked with serum lipid levels not T1-diabetes. J Diabetes Complications. 2012;26:1–9 [DOI] [PubMed] [Google Scholar]
- 59. Baum T, Yap SP, Karampinos DC, et al. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J Magn Reson Imaging. 2012;35:117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ackert-Bicknell CL, Shockley KR, Horton LG, Lecka-Czernik B, Churchill GA, Rosen CJ. Strain-specific effects of rosiglitazone on bone mass, body composition, and serum insulin-like growth factor-I. Endocrinology. 2009;150:1330–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tornvig L, Mosekilde LI, Justesen J, Falk E, Kassem M. Troglitazone treatment increases bone marrow adipose tissue volume but does not affect trabecular bone volume in mice. Calcif Tissue Int. 2001;69:46–50 [DOI] [PubMed] [Google Scholar]
- 62. Lazarenko OP, Rzonca SO, Suva LJ, Lecka-Czernik B. Netoglitazone is a PPAR-γ ligand with selective effects on bone and fat. Bone. 2006;38:74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Harslof T, Wamberg L, Moller L, et al. Rosiglitazone decreases bone mass and bone marrow fat. J Clin Endocrinol Metab. 2011;96:1541–1548 [DOI] [PubMed] [Google Scholar]
- 64. Grey A, Beckley V, Doyle A, et al. Pioglitazone increases bone marrow fat in type 2 diabetes: results from a randomized controlled trial. Eur J Endocrinol. 2012;166:1087–1091 [DOI] [PubMed] [Google Scholar]
- 65. Elefteriou F, Ahn JD, Takeda S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520 [DOI] [PubMed] [Google Scholar]
- 66. Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317 [DOI] [PubMed] [Google Scholar]
- 67. Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317–318 [DOI] [PubMed] [Google Scholar]
- 68. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432 [DOI] [PubMed] [Google Scholar]
- 69. Charlton HM. Mouse mutants as models in endocrine research. Q J Exp Physiol. 1984;69:655–676 [DOI] [PubMed] [Google Scholar]
- 70. Hamrick MW. Leptin, bone mass, and the thrifty phenotype. J Bone Miner Res. 2004;19:1607–1611 [DOI] [PubMed] [Google Scholar]
- 71. Bartell SM, Rayalam S, Ambati S, et al. Central (ICV) leptin injection increases bone formation, bone mineral density, muscle mass, serum IGF-1, and the expression of osteogenic genes in leptin-deficient ob/ob mice. J Bone Miner Res. 2011;26:1710–1720 [DOI] [PubMed] [Google Scholar]
- 72. Gryglewski RJ. Prostacyclin among prostanoids. Pharmacol Rep. 2008;60:3–11 [PubMed] [Google Scholar]
- 73. Iwaniec UT, Boghossian S, Trevisiol CH, Wronski TJ, Turner RT, Kalra SP. Hypothalamic leptin gene therapy prevents weight gain without long-term detrimental effects on bone in growing and skeletally mature female rats. J Bone Miner Res. 2011;26:1506–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Williams GA, Callon KE, Watson M, et al. Skeletal phenotype of the leptin receptor-deficient db/db mouse. J Bone Miner Res. 2011;26:1698–1709 [DOI] [PubMed] [Google Scholar]
- 75. Scheller EL, Song J, Dishowitz MI, Soki FN, Hankenson KD, Krebsbach PH. Leptin functions peripherally to regulate differentiation of mesenchymal progenitor cells. Stem Cells. 2010;28:1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. DeMambro VE, Kawai M, Clemens TL, et al. A novel spontaneous mutation of Irs1 in mice results in hyperinsulinemia, reduced growth, low bone mass and impaired adipogenesis. J Endocrinol. 2010;204:241–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Martin LM, McCabe LR. Type I diabetic bone phenotype is location but not gender dependent. Histochem Cell Biol. 2007;128:125–133 [DOI] [PubMed] [Google Scholar]
- 78. Charlton HM, Clark RG, Robinson IC, et al. Growth hormone-deficient dwarfism in the rat: a new mutation. J Endocrinol. 1988;119:51–58 [DOI] [PubMed] [Google Scholar]
- 79. Gevers EF, Loveridge N, Robinson IC. Bone marrow adipocytes: a neglected target tissue for growth hormone. Endocrinology. 2002;143:4065–4073 [DOI] [PubMed] [Google Scholar]
- 80. Menagh PJ, Turner RT, Jump DB, et al. Growth hormone regulates the balance between bone formation and bone marrow adiposity. J Bone Miner Res. 2010;25:757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Di Iorgi N, Mittelman SD, Gilsanz V. Differential effect of marrow adiposity and visceral and subcutaneous fat on cardiovascular risk in young, healthy adults. Int J Obes (Lond). 2008;32:1854–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Geiser F, Murtz P, Lutterbey G, et al. Magnetic resonance spectroscopic and relaxometric determination of bone marrow changes in anorexia nervosa. Psychosom Med. 2001;63:631–637 [DOI] [PubMed] [Google Scholar]
- 83. Lambert M, Hubert C, Depresseux G, et al. Hematological changes in anorexia nervosa are correlated with total body fat mass depletion. Int J Eat Disord. 1997;21:329–334 [DOI] [PubMed] [Google Scholar]
- 84. Abella E, Feliu E, Granada I, et al. Bone marrow changes in anorexia nervosa are correlated with the amount of weight loss and not with other clinical findings. Am J Clin Pathol. 2002;118:582–588 [DOI] [PubMed] [Google Scholar]
- 85. Fazeli PK, Bredella MA, Misra M, et al. Preadipocyte factor-1 is associated with marrow adiposity and bone mineral density in women with anorexia nervosa. J Clin Endocrinol Metab. 2010;95:407–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lawson EA, Misra M, Meenaghan E, et al. Adrenal glucocorticoid and androgen precursor dissociation in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:1367–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lawson EA, Donoho D, Miller KK, et al. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab. 2009;94:4710–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Greenberger JS. Corticosteroid-dependent differentiation of human marrow preadipocytes in vitro. In Vitro. 1979;15:823–828 [DOI] [PubMed] [Google Scholar]
- 89. Pongubala JM, Northrup DL, Lancki DW, et al. Transcription factor EBF restricts alternative lineage options and promotes B cell fate commitment independently of Pax5. Nat Immunol. 2008;9:203–215 [DOI] [PubMed] [Google Scholar]
- 90. Liberg D, Sigvardsson M, Akerblad P. The EBF/Olf/Collier family of transcription factors: regulators of differentiation in cells originating from all three embryonal germ layers. Mol Cell Biol. 2002;22:8389–8397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hesslein DG, Fretz JA, Xi Y, et al. Ebf1-dependent control of the osteoblast and adipocyte lineages. Bone. 2009;44:537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Akerblad P, Lind U, Liberg D, Bamberg K, Sigvardsson M. Early B-cell factor (O/E-1) is a promoter of adipogenesis and involved in control of genes important for terminal adipocyte differentiation. Mol Cell Biol. 2002;22:8015–8025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jimenez MA, Akerblad P, Sigvardsson M, Rosen ED. Critical role for Ebf1 and Ebf2 in the adipogenic transcriptional cascade. Mol Cell Biol. 2007;27:743–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Akerblad P, Mansson R, Lagergren A, et al. Gene expression analysis suggests that EBF-1 and PPARγ2 induce adipogenesis of NIH-3T3 cells with similar efficiency and kinetics. Physiol Genomics. 2005;23:206–216 [DOI] [PubMed] [Google Scholar]
- 95. Gimble JM, Morgan C, Kelly K, et al. Bone morphogenetic proteins inhibit adipocyte differentiation by bone marrow stromal cells. J Cell Biochem. 1995;58:393–402 [DOI] [PubMed] [Google Scholar]
- 96. Vost A. Osteoporosis: a necropsy study of vertebrae and iliac crests. Am J Pathol. 1963;43:143–151 [PMC free article] [PubMed] [Google Scholar]
- 97. Hartsock RJ, Smith EB, Petty CS. Normal variations with aging of the amount of hematopoietic tissue in bone marrow from the anterior iliac crest. A study made from 177 cases of sudden death examined by necropsy. Am J Clin Pathol. 1965;43:326–331 [DOI] [PubMed] [Google Scholar]
- 98. Festa E, Fretz J, Berry R, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone. 2012;50:534–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bennett CN, Ouyang H, Ma YL, et al. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res. 2007;22:1924–1932 [DOI] [PubMed] [Google Scholar]
- 101. DeMambro VE, Clemmons DR, Horton LG, et al. Gender-specific changes in bone turnover and skeletal architecture in igfbp-2-null mice. Endocrinology. 2008;149:2051–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Babij P, Zhao W, Small C, et al. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003;18:960–974 [DOI] [PubMed] [Google Scholar]
- 103. Kato M, Patel MS, Levasseur R, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Botolin S, McCabe LR. Inhibition of PPARγ prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol. 2006;209:967–976 [DOI] [PubMed] [Google Scholar]
- 105. Hui SK, Sharkey L, Kidder LS, et al. The influence of therapeutic radiation on the patterns of bone marrow in ovary-intact and ovariectomized mice. PLoS One. 2012;7:e42668. [DOI] [PMC free article] [PubMed] [Google Scholar]