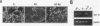Abstract
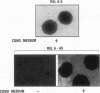
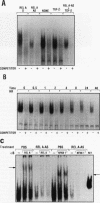
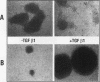
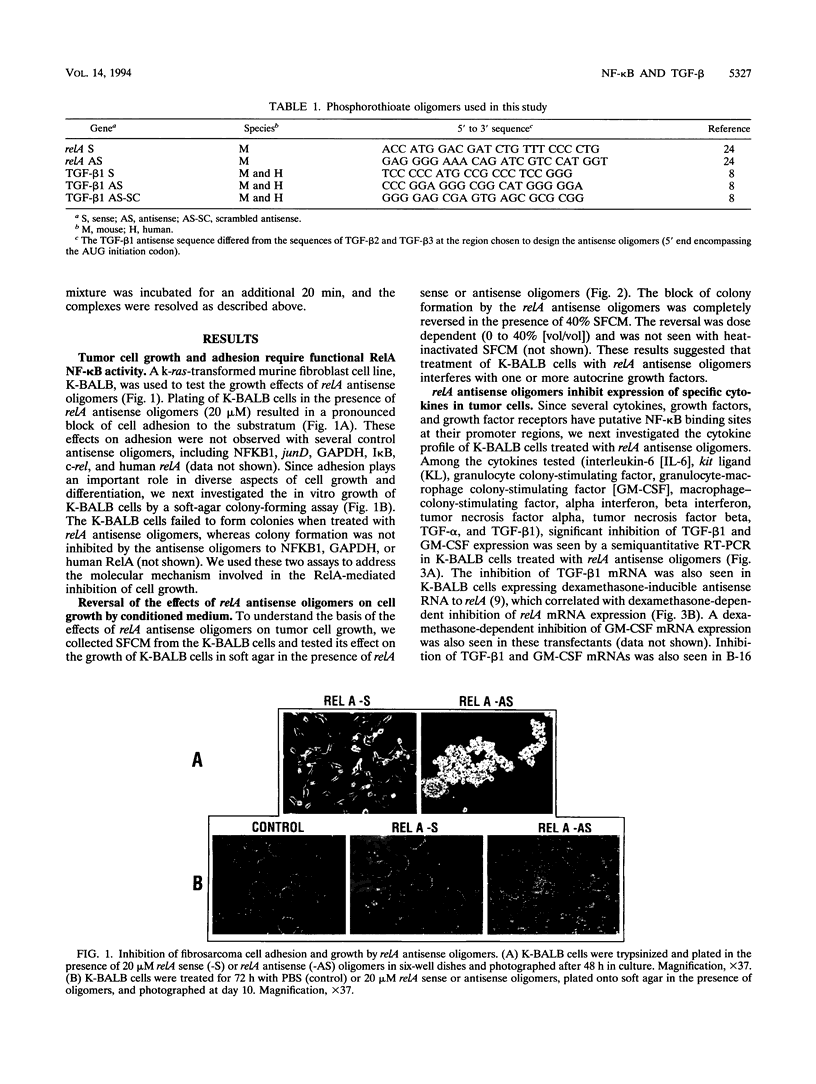
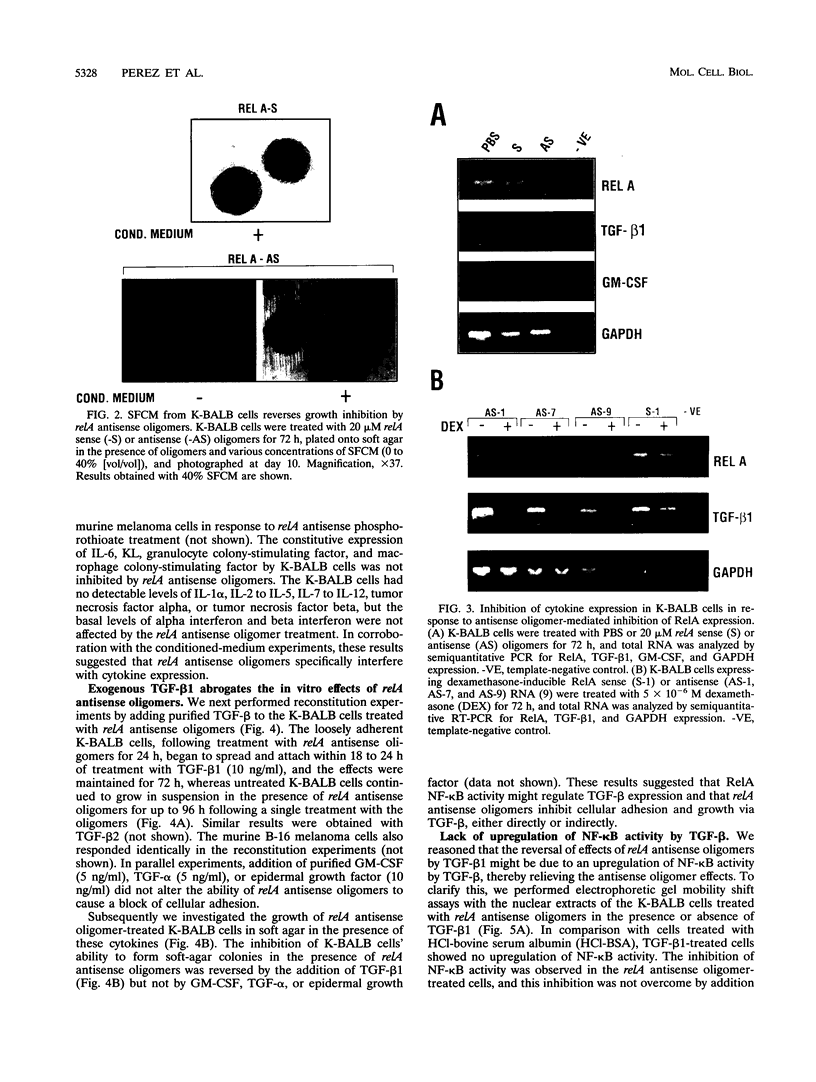
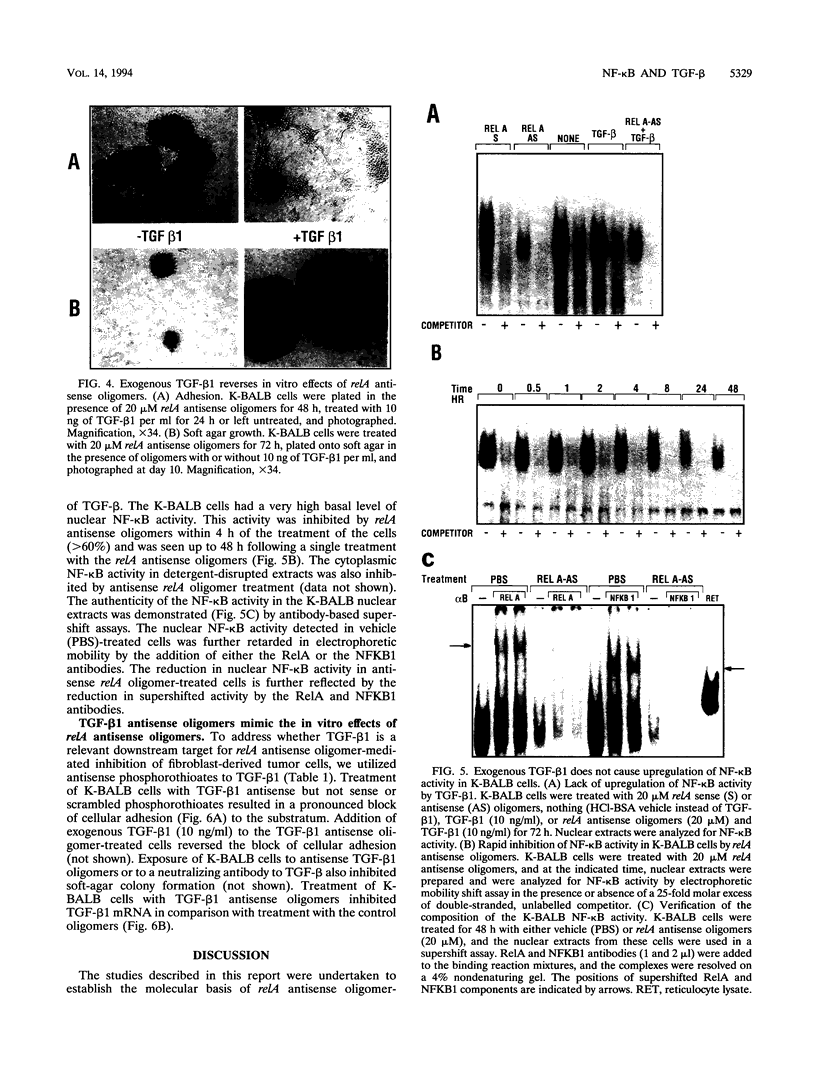
The NF-kappa B transcription factor is a pleiotropic activator that participates in the induction of a wide variety of cellular genes. Antisense oligomer inhibition of the RelA subunit of NF-kappa B results in a block of cellular adhesion and inhibition of tumor cell growth. Investigation of the molecular basis for these effects showed that in vitro inhibition of the growth of transformed fibroblasts by relA antisense oligonucleotides can be reversed by the parental-cell-conditioned medium. Cytokine profile analysis of these cells treated with relA antisense oligonucleotides revealed inhibition of transforming growth factor beta 1 (TGF-beta 1 to the transformed fibroblasts reversed the inhibitory effects of relA antisense oligomers on soft agar colony formation and cell adhesion to the substratum. Direct inhibition of TGF-beta 1 expression by antisense phosphorothioates to TGF-beta 1 mimicked the in vitro effects of blocking cell adhesion that are elicited by antisense relA oligomers. These results may explain the in vitro effects of relA antisense oligomers on fibrosarcoma cell growth and adhesion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anzano M. A., Roberts A. B., De Larco J. E., Wakefield L. M., Assoian R. K., Roche N. S., Smith J. M., Lazarus J. E., Sporn M. B. Increased secretion of type beta transforming growth factor accompanies viral transformation of cells. Mol Cell Biol. 1985 Jan;5(1):242–247. doi: 10.1128/mcb.5.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Ballard D. W., Dixon E. P., Peffer N. J., Bogerd H., Doerre S., Stein B., Greene W. C. The 65-kDa subunit of human NF-kappa B functions as a potent transcriptional activator and a target for v-Rel-mediated repression. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1875–1879. doi: 10.1073/pnas.89.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eck S. L., Perkins N. D., Carr D. P., Nabel G. J. Inhibition of phorbol ester-induced cellular adhesion by competitive binding of NF-kappa B in vivo. Mol Cell Biol. 1993 Oct;13(10):6530–6536. doi: 10.1128/mcb.13.10.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser A. G., Kim S. J., Roberts A. B., Sporn M. B. Characterization of the mouse transforming growth factor-beta 1 promoter and activation by the Ha-ras oncogene. Mol Cell Biol. 1991 Jan;11(1):84–92. doi: 10.1128/mcb.11.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore T. D. NF-kappa B, KBF1, dorsal, and related matters. Cell. 1990 Sep 7;62(5):841–843. doi: 10.1016/0092-8674(90)90257-f. [DOI] [PubMed] [Google Scholar]
- Grilli M., Chiu J. J., Lenardo M. J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Hatzfeld J., Li M. L., Brown E. L., Sookdeo H., Levesque J. P., O'Toole T., Gurney C., Clark S. C., Hatzfeld A. Release of early human hematopoietic progenitors from quiescence by antisense transforming growth factor beta 1 or Rb oligonucleotides. J Exp Med. 1991 Oct 1;174(4):925–929. doi: 10.1084/jem.174.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins K. A., Perez J. R., Coleman T. A., Dorshkind K., McComas W. A., Sarmiento U. M., Rosen C. A., Narayanan R. Antisense inhibition of the p65 subunit of NF-kappa B blocks tumorigenicity and causes tumor regression. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9901–9905. doi: 10.1073/pnas.90.21.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A., Heino J., Massagué J. Regulation of cell adhesion receptors by transforming growth factor-beta. Regulation of vitronectin receptor and LFA-1. J Biol Chem. 1989 Jan 5;264(1):389–392. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Cell adhesion protein receptors as targets for transforming growth factor-beta action. Cell. 1987 Oct 23;51(2):189–197. doi: 10.1016/0092-8674(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Kaszubska W., Hooft van Huijsduijnen R., Ghersa P., DeRaemy-Schenk A. M., Chen B. P., Hai T., DeLamarter J. F., Whelan J. Cyclic AMP-independent ATF family members interact with NF-kappa B and function in the activation of the E-selectin promoter in response to cytokines. Mol Cell Biol. 1993 Nov;13(11):7180–7190. doi: 10.1128/mcb.13.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. D., Ransone L. J., Wamsley P., Schmitt M. J., Boyer T. G., Zhou Q., Berk A. J., Verma I. M. Association between proto-oncoprotein Rel and TATA-binding protein mediates transcriptional activation by NF-kappa B. Nature. 1993 Sep 30;365(6445):412–419. doi: 10.1038/365412a0. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Jeang K. T., Glick A. B., Sporn M. B., Roberts A. B. Promoter sequences of the human transforming growth factor-beta 1 gene responsive to transforming growth factor-beta 1 autoinduction. J Biol Chem. 1989 Apr 25;264(12):7041–7045. [PubMed] [Google Scholar]
- Kitajima I., Shinohara T., Bilakovics J., Brown D. A., Xu X., Nerenberg M. Ablation of transplanted HTLV-I Tax-transformed tumors in mice by antisense inhibition of NF-kappa B. Science. 1992 Dec 11;258(5089):1792–1795. doi: 10.1126/science.1299224. [DOI] [PubMed] [Google Scholar]
- Kunsch C., Ruben S. M., Rosen C. A. Selection of optimal kappa B/Rel DNA-binding motifs: interaction of both subunits of NF-kappa B with DNA is required for transcriptional activation. Mol Cell Biol. 1992 Oct;12(10):4412–4421. doi: 10.1128/mcb.12.10.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Getz M. J., Moses H. L. Transforming growth factor type beta regulation of actin mRNA. J Cell Physiol. 1986 Apr;127(1):83–88. doi: 10.1002/jcp.1041270111. [DOI] [PubMed] [Google Scholar]
- Massagué J. The TGF-beta family of growth and differentiation factors. Cell. 1987 May 22;49(4):437–438. doi: 10.1016/0092-8674(87)90443-0. [DOI] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- McIntyre K. W., Lombard-Gillooly K., Perez J. R., Kunsch C., Sarmiento U. M., Larigan J. D., Landreth K. T., Narayanan R. A sense phosphorothioate oligonucleotide directed to the initiation codon of transcription factor NF-kappa B p65 causes sequence-specific immune stimulation. Antisense Res Dev. 1993 Winter;3(4):309–322. doi: 10.1089/ard.1993.3.309. [DOI] [PubMed] [Google Scholar]
- Narayanan R., Higgins K. A., Perez J. R., Coleman T. A., Rosen C. A. Evidence for differential functions of the p50 and p65 subunits of NF-kappa B with a cell adhesion model. Mol Cell Biol. 1993 Jun;13(6):3802–3810. doi: 10.1128/mcb.13.6.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl H. L., Rosmarin A. G., Tenen D. G. Characterization of the myeloid-specific CD11b promoter. Blood. 1992 Feb 15;79(4):865–870. [PubMed] [Google Scholar]
- Perez J. R., Li Y., Stein C. A., Majumder S., van Oorschot A., Narayanan R. Sequence-independent induction of Sp1 transcription factor activity by phosphorothioate oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5957–5961. doi: 10.1073/pnas.91.13.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins N. D., Edwards N. L., Duckett C. S., Agranoff A. B., Schmid R. M., Nabel G. J. A cooperative interaction between NF-kappa B and Sp1 is required for HIV-1 enhancer activation. EMBO J. 1993 Sep;12(9):3551–3558. doi: 10.1002/j.1460-2075.1993.tb06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben S. M., Narayanan R., Klement J. F., Chen C. H., Rosen C. A. Functional characterization of the NF-kappa B p65 transcriptional activator and an alternatively spliced derivative. Mol Cell Biol. 1992 Feb;12(2):444–454. doi: 10.1128/mcb.12.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt R. M., Bruyns E., Snodgrass H. R. Hematopoietic development of embryonic stem cells in vitro: cytokine and receptor gene expression. Genes Dev. 1991 May;5(5):728–740. doi: 10.1101/gad.5.5.728. [DOI] [PubMed] [Google Scholar]
- Sokoloski J. A., Sartorelli A. C., Rosen C. A., Narayanan R. Antisense oligonucleotides to the p65 subunit of NF-kappa B block CD11b expression and alter adhesion properties of differentiated HL-60 granulocytes. Blood. 1993 Jul 15;82(2):625–632. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Stein B., Baldwin A. S., Jr, Ballard D. W., Greene W. C., Angel P., Herrlich P. Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 1993 Oct;12(10):3879–3891. doi: 10.1002/j.1460-2075.1993.tb06066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B., Baldwin A. S., Jr Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-kappa B. Mol Cell Biol. 1993 Nov;13(11):7191–7198. doi: 10.1128/mcb.13.11.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Prorock C., Ishikawa H., Maldonado E., Ito Y., Gélinas C. Functional interaction of the v-Rel and c-Rel oncoproteins with the TATA-binding protein and association with transcription factor IIB. Mol Cell Biol. 1993 Nov;13(11):6733–6741. doi: 10.1128/mcb.13.11.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]