Abstract
Purpose:
Maintaining systolic blood pressure (SBP) at 100% of baseline is best for fetal and maternal outcome. We hypothesized that irrespective of the vasopressor used, maintaining SBP at 100% of baseline with phenylephrine (P), metaraminol (M), or ephedrine (E) will produce the best fetal pH after cesarean section (LSCS) under subarachnoid block (SAB).
Materials and Methods:
Ninety ASA 1 women scheduled for elective LSCS were randomly allocated to receive P, M, or E. SAB was established with patient in left lateral position using 2.5 cc of 0.5% hyperbaric bupivacaine. Immediately following SAB, patients received a bolus of the study drug (E = 5 mg, M = 0.5 mg, P = 30 mcg) followed by infusion (E = 2.5 mg/min, M = 0.25 mg/min, P = 15 mcg/min) to maintain SBP at 100% baseline. Umbilical blood gases, maternal hemodynamic parameters, and complications were recorded.
Results:
The umbilical pH was comparable in all the three groups (P > 0.05). The mean SBP from spinal block until delivery was similar over time for all the three groups. The incidence of reactive hypertension was more in group M (P < 0.05) than in group E and group P. Total drug consumption to meet target blood pressure till delivery was 39.3 ± 14.6 mg in group E, 1.7 ± 0.9 mg in group M, and 283.6 ± 99.8 mcg in group P. The incidence of nausea and vomiting was comparable in the three groups.
Conclusion:
All the three vasopressors were equally effective in maintaining maternal blood pressure as well as umbilical pH during spinal anesthesia for cesarean section without any detrimental effects on fetal and maternal outcome.
Keywords: Cesarean section, fetal pH, spinal anaesthesia, vasopressors
Introduction
Maternal hypotension is an undesirable consequence of spinal anesthesia for cesarean delivery as it causes detrimental maternal and fetal effects.[1] The anesthesiologist does not allow hypotension to persist and the treatment often requires the use of vasopressors to maintain blood pressure.[2,3] Ephedrine has been the drug of choice for prophylaxis and treatment of post spinal hypotension for many years. It is considered to maintain uteroplacental blood flow but has been shown to cause fetal acidosis.[4,5] Recent clinical trials have shown that α-agonists such as phenylephrine and metaraminol produce better fetal acid base status than ephedrine in healthy term cesarean deliveries and clinical outcome with these vasopressors has been variable.[6–10]
In earlier studies, the vasopressor was withheld until after maternal systolic blood pressure (SBP) started to decrease and therefore there were initial periods of uncorrected hypotension resulting in placental hypoperfusion.[9,11] Recent studies have shown that any reduction in maternal blood pressure following spinal anesthesia is undesirable and the best strategy is to maximize the use of vasoconstrictors to maintain SBP at 100% of baseline.[7] This strategy gives the best outcome for the baby (highest umbilical artery pH) and the mother (less nausea).[7,9,10,12,13] Studies comparing ephedrine with metaraminol and ephedrine with phenylephrine are available.[8,10] However, there are no studies comparing metaraminol, phenylephrine, and ephedrine. We hypothesized that regardless of the vasopressor chosen, maintaining SAB at 100% baseline by early administration of a vasopressor would be more beneficial for the mother and baby. By keeping a tight control of maternal blood pressure, using ephedrine, metaraminol, or phenylephrine, we planned to study their fetal and maternal effects. The primary outcome measure of our study was therefore assessment of umbilical blood gases, whereas the secondary outcome was to assess the effect of the above vasopressors on maternal hemodynamics (hypertension, hypotension, nausea, and vomiting).
Materials and Methods
After approval by the Institute Ethics Committee we enrolled 90 ASA-I patients scheduled for elective cesarean delivery under subarachnoid block. Written informed consent was taken from all the patients. Only women with singleton pregnancy without known fetal abnormalities, pre-eclampsia, or cerebrovascular diseases were included in this randomized, double-blind study.
All patients received oral premedication with ranitidine 150 mg the night before and on the morning of the surgery. On arrival to the operation theatre, patients had three blood pressure and heart rate (HR) readings recorded at 3 min intervals, while lying comfortably in the bed before venipuncture. The mean of the three readings was recorded as the baseline value for the maternal systolic blood pressure (SBP) and HR. Monitoring was standard and included non-invasive blood pressure (NIBP), continuous electrocardiography (ECG), and pulse oximetry. Fetal heart rate was monitored by external cardiotocography until the time of surgery. A wide bore 18 G intravenous (IV) catheter was inserted after infiltration with local anesthetic. Patients were then randomly allocated to one of the three groups by drawing of sequentially numbered sealed envelopes that contained a computer generated randomization code. Before induction of spinal block, the envelope containing the randomization code for the patient was opened (NB/KJ). One of the investigators (NB/KJ) prepared phenylephrine (30 mcg/mL), ephedrine (5 mg/mL), or metaraminol (0.5 mg/mL) in a 20 ml syringe. The unlabelled 20 ml syringe was attached to the syringe pump, which was programmed and given to the investigators conducting the anaesthesia (SA and NBh). No preloading with crystalloid or colloid was done. Patients received isotonic saline at a rate of 7 ml/kg/h during the duration of the procedure.
Subarachnoid block (SAB) was performed with all patients in the left lateral position. After skin infiltration with 2% xylocaine, 26 G Quincke's needle was inserted at L2-3/L3-4 vertebral inter-space and once free flow of cerebrospinal fluid was obtained hyperbaric bupivacaine 0.5%, 12.5 mg (2.5 ml) was injected intrathecally. Patients were then immediately turned supine with left lateral tilt. The block height was assessed by response to cold sensation using alcohol swab every minute until maximum block height was achieved. The block height of T5 was considered appropriate. Oxygen 5 L/min was administered through ventimask until delivery of the child. SBP was measured at 1 min intervals beginning immediately after spinal injection. Immediately following SAB, patients received a 1 ml bolus of the study drug (E = 5 mg, M = 0.5 mg, P = 30 mcg) and thereafter infusion was started at 15 ml/hr (E = 2.5 mg/min, M = 0.25 mg/ min, P = 15 mcg/min,) using a syringe infusion pump (Pilot C, Fresenius, France). The infusion rate was maintained till the delivery of the baby according to the following protocol. If the SBP increased above 1.25 times the baseline, the infusion was stopped. If the blood pressure dropped 10% below the baseline, 1 ml bolus of the study drug was administered. If the investigator was not able to record SBP comparable to baseline value with 2 additional boluses, the code was broken and the vasopressor solution was then altered as necessary. The data was recorded by the anesthetist conducting the anesthesia (SA/NBh).
If maternal HR was <60 b/min, and SBP <80% of baseline, or if maternal HR was ≤50 and SBP <100% of baseline or maternal HR was <45 b/min regardless of the blood pressure, glycopyrrolate 0.2 mg IV was given to treat bradycardia. Nausea and vomiting were scored on a scale of 0-2 (0 = none, 1 = nausea without vomiting, 2 = vomiting). The maximum nausea and vomiting score before delivery of baby were noted. As soon as baby was delivered, the infusion was stopped and total dose of vasopressor was noted. Umbilical arterial and venous blood samples from a segment of clamped umbilical cord were obtained and analyzed using a blood gas analyzer (Bayer Rapid Lab 855). APGAR scores at 1 and 5 minutes were noted.
The primary aim of the study was assessment of umbilical blood gases after the maternal blood pressure is maintained at 100% baseline with ephedrine, metaraminol, and phenylepherine. Secondary outcome was to assess the effect of the above vasopressors on maternal hemodynamics.
Preoperative power analysis was based on the primary outcome which was defined as the umbilical artery pH (UA pH). A sample size of 25 patients per group had a 90% power to detect a difference in UA pH of 0.03 units among groups.[7] We recruited 30 patients per group to compensate for any exclusion.
Statistical analysis was done using SPSS software (version 10.0). Baseline summary statistics were calculated by using descriptive univariate statistics. For all quantitative characteristics 95% confidence intervals were given. Group comparisons between type of vasopressor used and various quantitative variables were conducted by One-way ANOVA (for independent variables) and Repeated measure ANOVA (for dependent variables). Post-hoc analyses were also carried out using Tukey's HSD (for one-way ANOVA) and Bonferroni's correction for multiple comparisons of time trend data (repeated heart rate and SBP values) in repeated-measure ANOVA. Non-parametric Kruskal Wallis test was used to compare time related variables and other non-normally distributed quantitative variables. Qualitative variables were compared using Chi-square test and Fisher exact test. All tests were two-tailed and P value < 0.05 was considered significant.
Results
Ninety parturients were enrolled into the study. In three patients there was failure of subarachnoid block (group E = 2, M = 1) and patients were administered general anesthesia. In two patients there was infusion pump failure (P = 2) so the test solution could not be administered to the patients immediately after subarachnoid block. These patients were therefore excluded from the study and 85 patients were statistically analyzed. It was not possible to study blood gases for 4 neonates (E = 1, M = 2, P = 1) because uterine artery or uterine vein or both could not be sampled. Except for the uterine arterial blood gases all other parameters were analyzed in these patients.
The three groups were well matched for weight, level of spinal block, skin incision to delivery time, uterine incision to delivery time and spinal block to delivery time [Table 1]. The mean age of the parturients in the metaraminol group was greater than parturients in the phenylephrine group (P = 0.01).
Table 1.
Patient characteristics

The primary outcome measure, umbilical blood gases was comparable in all the three groups (P > 0.05). The mean umbilical artery and venous pH in the three groups was comparable [Table 2]. None of the neonates in either of the groups had pH < 7.20. Similar number of neonates in all the groups had umbilical vein pH ≥ 7.3 (E = 24, M = 25, P = 24) [Table 2]. The umbilical arterio-venous difference was also not significant (P = 0.07). APGAR scores at 1 min were ≥ 7 and all APGAR scores at 5 min were ≥ 9.
Table 2.
Neonatal outcome
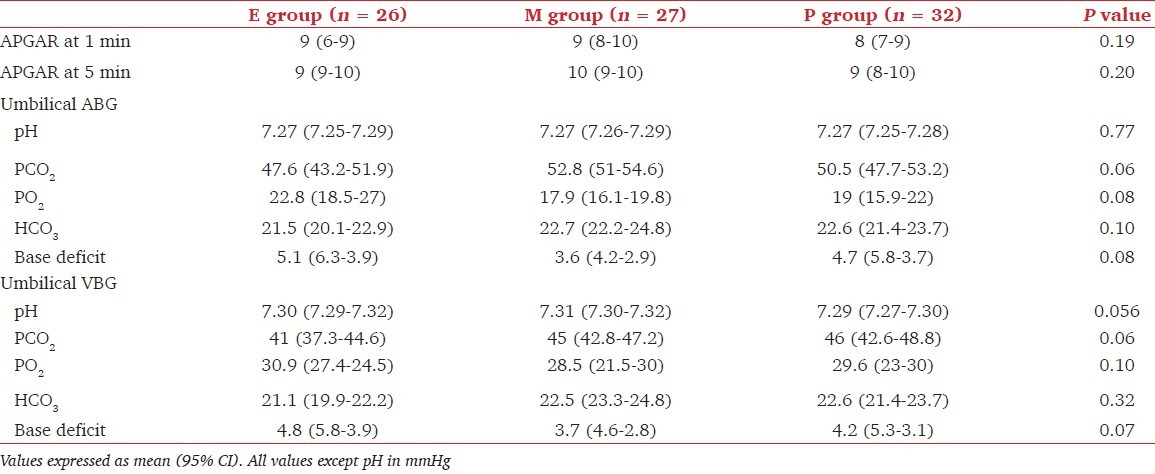
Maternal hemodynamic changes are shown in Table 3. The mean SBP from spinal block until delivery was similar over time for all the three groups. The number of patients with hypotension (less than 80% of baseline) and number of episodes of hypotension were not different among groups. Six patients in group E and four patients in group P and group M each required additional boluses to maintain target blood pressure. The incidence of reactive hypertension was more in group M (10 patients) than in group E (6 patients) and group P (3 patients) (P < 0.05). There was no significant difference between the three groups for the lowest SBP recorded and for the proportion of SBP readings below 80% of baseline [Table 4]. In no patient the code had to be broken because of a low blood pressure not responding to treatment. From 2 min onwards mean HR was higher in ephedrine group than in groups M (P = 0.001) and P (P = 0.002). No patient had bradycardia requiring treatment with glycopyrrolate.
Table 3.
Maternal heart rate and systolic blood pressure up to delivery
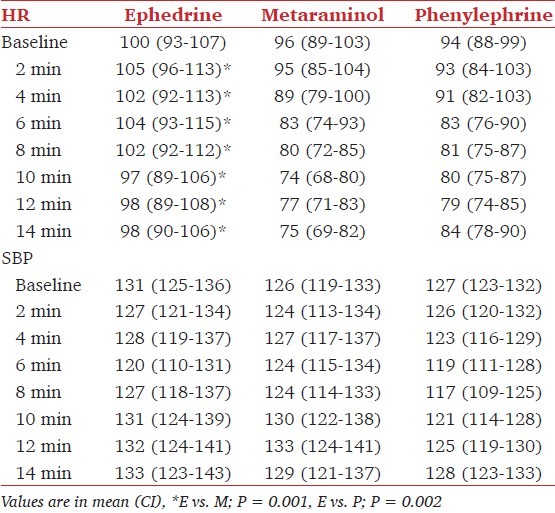
Table 4.
Baseline and post spinal hemodynamic data
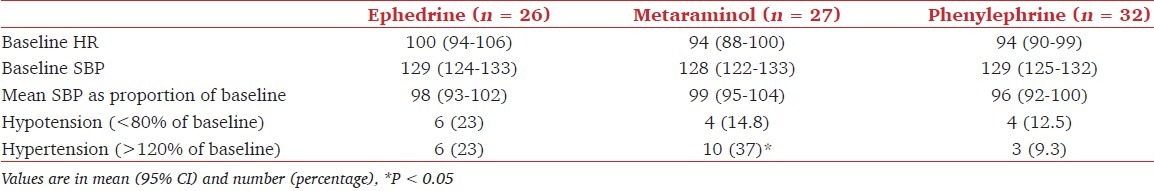
Total drug consumption to meet target blood pressure till delivery was 39.3 ± 14.6 mg in group E, 1.7 ± 0.9 mg in group M, and 283.6 ± 99.8 mcg in group P. Three patients in the ephedrine group (11.5%) had nausea and vomiting compared to none in metaraminol and phenylephrine groups but this was not statistically significant.
Discussion
The results of our study showed that regardless of the type of vasopressor, keeping a tight control of blood pressure following spinal anaesthesia for cesarean delivery was associated with beneficial effects on both mother and the baby. All the three vasopressors could be effectively titrated to the desired clinical end point (100% SBP) without breaking the code for any of the patients. There was no evidence of fetal acidosis as none of the neonates in any of the groups had a pH less then 7.20 and also the APGAR scores were good for all neonates. None of the neonates required tracheal intubation, mechanical ventilation and admission to ICU in immediate post delivery period. There was no significant incidence of maternal nausea and vomiting. The total dose of the vasopressors administered was also lower than previously reported.[7,10]
Short periods of maternal hypotension (<2 min) were not considered to be harmful to the neonate earlier and vasopressors were administered after the blood pressure started to decrease. This protocol was associated with initial periods of uncorrected hypotension, low fetal pH, and maternal symptoms. Since the activity of vasopressors administered lagged behind the physiological changes leading to hypotension, larger doses had to be given resulting in undesirable side effects.[3] Recent studies have shown that any reduction in maternal blood pressure is undesirable and the goal should be to maintain SBP at hundred percent of base line with liberal use of vasopressors.[7,12] Following this technique Kee et al. administered high dose of phenylephrine infusion to maintain blood pressure at 80%, 90%, or 100% of baseline. The authors found that maintaining SBP to 100% of baseline produced fewer episodes of hypotension, a higher mean umbilical artery pH (UA pH), and fewer episodes of maternal nausea and vomiting.[7] Similarly, aiming at a tight control of blood pressure within a range of 90 to 100% of baseline, Kee et al. reported metaraminol to be associated with less neonatal acidosis and more controlled titration of arterial pressure than ephedrine.[10]
Although various vasopressors in a variety of doses have been utilized for preventing hypotension produced by SAB, to the best of our knowledge there is no study comparing the efficacy of ephedrine, metaraminol, or phenylephrine infusion in maintaining the blood pressure at hundred percent of baseline and achieving good fetal pH.[7,10,12–14] We hypothesized that maximizing perfusion pressure by not allowing any reduction in SBP following spinal anesthesia may be more beneficial in maintaining uterine blood flow, and therefore, the fetal pH than a selection of a particular vasopressor. We found that all three vasopressors were equally effective in maintaining blood pressure when administered before the onset of hypotension. Our protocol (bolus dose followed by infusion immediately after SAB) did not allow for any fall in maternal blood pressure. This technique provided a uniform delivery of drug and so prevented long periods of uncorrected hypotension. In our study there were brief periods of hypotension (<2 min) in all the three groups, which responded to bolus vasopressor treatment. This technique also offered the advantage of reducing the total dose of the drug administered.
Many studies have reported that ephedrine may not be the ideal drug for managing spinal hypotension in obstetrics and may be associated with greater fetal acidosis than phenylephrine, metaraminol, and angiotensin.[5,6,8,10] Ephedrine possibly failed because of inadequate dose and inappropriate time of administration.
We were able to maintain good control of SBP with ephedrine probably because we attempted to increase maternal blood pressure before onset of SAB induced hypotension. The recommended dose for IV ephedrine infusion is between 0.5 mg/min to 5mg/min. We selected a higher infusion dose (2.5 mg/min), after a small dose bolus (5 mg), as our target was to keep blood pressure at baseline. We found that with this regime ephedrine was effective in maintaining blood pressure without evidence of fetal acidosis. Using the same infusion rate but a larger bolus (10 mg) after the blood pressure fell to less than 90% baseline, Kee et al. reported 39% incidence of fetal acidosis.[10] This was due to larger initial requirements of ephedrine in first 10 min after induction of spinal anesthesia. In our study, there was probably more uniform distribution of drug as we attempted to increase blood pressure before development of hypotension. Kansal et al. used 2.5 mg/min infusion after a 5 mg bolus and reported effective control of maternal blood pressure, no evidence of fetal acidosis and decreased total drug consumption.-[11] The difference in consumption of ephedrine in our study and other authors is due to different methodology. They started the vasopressor after the BP dropped to 80% and 90% of baseline.
Larger doses of ephedrine are associated with maternal symptoms like tachycardia and nausea and vomiting as reported by many authors.[5,6] We observed an increase in maternal HR in all patients receiving ephedrine but the incidence of nausea and vomiting was low (11.5%). None of the patients required treatment for this nausea and vomiting.
Metaraminol is an α-agonist and acts by increasing both systemic vascular resistance and central venous pressure, which would appear ideal as both arterial vasodilatation and venous pooling are reversed during spinal anesthesia. However, it has been found to be very potent and with a potential to cause hypertension with its associated risks. Few studies in literature are available where metaraminol has been used in women undergoing cesarean section.[9,10] We selected the dose of metaraminol based on these previous studies. Kee et al. used metaraminol bolus (0.5 mg) followed by an infusion (0.25 mg/min) to maintain arterial pressure during spinal anesthesia for elective LSCS and found that it allowed a close titration of blood pressure without any overshoot.[9] Critchley et al. reported over treatment and therefore reactive hypertension in 33% of elderly patients receiving prophylactic metaraminol infusions.[15] In our study, we also observed reactive hypertension with metaraminol infusion in 10 out of 27 patients (33.3%). The concept of tight control of blood pressure in our study may have resulted in over treatment in few of our patients, although the cumulative dose of metaraminol used by us is less (1.7 ± 0.9 mg) compared to other authors (3.1-3.6 mg).
Most of the studies on SAB induced hypotension have used phenylephrine in high doses.[7,12,13] Such high doses may be associated with maternal bradycardia and reactive hypertension. Since phenylephrine infusion in high doses (100 mcg/min) in the above studies was associated with reactive hypertension (24%-38%) we decided to lower the dose range of phenylephrine to 15ug/min. Cooper et al. reported a low incidence of maternal nausea, vomiting, or fetal acidosis when phenylephrine was started at an initial rate of 33 mcg min and the rate adjusted up or down by factor of 2.[6] At our selected dose, we found effective maintenance of blood pressure without frequent pump settings, which can be cumbersome with rigid study protocols. Kee et al. administered prophylactic phenylephrine either as a bolus or infusion (100 mcg/min) immediately after spinal anesthesia.[16] Despite almost tripling the dose of phenylephrine infusion the authors found it to be beneficial for both mother and the baby.[12] The incidence of hypertension in their study was 38%. Similarly Kee et al. in another study used large dose of phenylephrine (1520 mcg) to maintain SBP at 100% of baseline and showed the incidence of hypertension to be 24%.[7] Although most of the authors found good fetal umbilical gases in healthy pregnancy in spite of using high doses, it may be disadvantageous in women with complicated pregnancy.
Saravanan et al. found a dose ratio of 1:80 between phenylephrine and ephedrine for prevention of hypotension in women undergoing cesarean section.[16] No such co-relation between metaraminol and phenylephrine or ephedrine has been studied. A dose-response study to find equipotent doses of the three vasopressors is required.
Maternal hypotension should be aggressively managed specially for the first 5-10 minutes, when the spinal block is evolving. This minimizes the risk of fetal acidosis as a definite correlation exists between the maximum decrease in systolic blood pressure and umbilical arterial pH. We found that all the three vasopressors were similarly effective in maintaining maternal blood pressure at 100% baseline during spinal anesthesia for elective cesarean section. This resulted in good fetal (pH > 7.20) and maternal outcome. However, the effect of complicated obstetrics on neonatal outcome using this vasopressor regime remains to be defined. More dose finding and dose equivalence studies of the three vasopressors is required.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Wright RG, Shnider SM. Hypotension and regional anaesthesia. In: Shnider SM, Levison G, editors. Anaesthesia for obstetrics. 3rd ed. Baltimore: Williams and Wilkins; 1993. pp. 397–406. [Google Scholar]
- 2.Rout CC, Rocke DA. Prevention of hypotension following spinal anaesthesia for cesarean section. Int Anaesthesiol Clin. 1994;32:117–35. [PubMed] [Google Scholar]
- 3.Macarthur A, Riley ET. Obstetric Anesthesia controversies: Vasopressor choice for postspinal hypotension during cesarean delivery. Int Anesthesiol Clin. 2007;45:115–32. doi: 10.1097/AIA.0b013e31802b8d53. [DOI] [PubMed] [Google Scholar]
- 4.Ralston DH, Shnider SM, DeLorimier AA. Effects of equipotent ephedrine, metaraminol, mephenteramine and methoxamine on uterine blood flow in the pregnant ewe. Anesthesiology. 1974;40:354–70. doi: 10.1097/00000542-197404000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Lee A, Ngan Kee WD, Gin T. Prophylactic ephedrine prevents hypotension during spinal anesthesia for cesarean delivery but does not improve neonatal outcome: A quantitative systematic review. Can J Anaesth. 2002;49:588–99. doi: 10.1007/BF03017387. [DOI] [PubMed] [Google Scholar]
- 6.Cooper DW, Carpenter M, Mowbray P, Desira WR, Ryall DM, Kokri MS. Fetal and maternal effects of phenylepherine and ephedrine during spinal anaesthesia for cesarean delivery. Anesthesiology. 2002;97:1582–90. doi: 10.1097/00000542-200212000-00034. [DOI] [PubMed] [Google Scholar]
- 7.Ngan Kee WD, Khaw KS, Ng FF. Comparison of phenylephrine infusion regimens for maintaining maternal blood pressure during spinal anaesthesia for Caesarean section. Br J Anaesth. 2004;92:469–74. doi: 10.1093/bja/aeh088. [DOI] [PubMed] [Google Scholar]
- 8.Lee A, Ngan Kee WD, Gin T. A quantitative systematic review of randomized controlled trials of ephedrine versus phenylepherine for the management of hypotension during spinal anaesthesia for cesarean delivery. Anesth Analg. 2002;91:920–6. doi: 10.1097/00000539-200204000-00028. [DOI] [PubMed] [Google Scholar]
- 9.Ngan Kee WD, Khaw KS, Lee BB, Wong MMS, Ng FF. Metaraminol infusion for maintenance of arterial blood pressure during spinal anaesthesia for cesarean delivery-The effect of crystalloid bolus. Anesth Analg. 2001;93:703–8. doi: 10.1097/00000539-200109000-00033. [DOI] [PubMed] [Google Scholar]
- 10.Ngan Kee WD, Lau TK, Khaw KS, Lee BB. Comparison of metaraminol and ephedrine infusion for maintaining arterial pressure during spinal anaesthesia for elective cesarean section. Anesthesiology. 2001;95:307–13. doi: 10.1097/00000542-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Kansal A, Mohta M, Sethi AK, Tyagi A, Kumar P. Randomised trial of intravenous infusion of ephedrine or mephentermine for management of hypotension during spinal anaesthesia for caesarean section. Anaesthesia. 2005;60:28–34. doi: 10.1111/j.1365-2044.2004.03994.x. [DOI] [PubMed] [Google Scholar]
- 12.Ngan Kee WD, Khaw KS, Ng FF, Lee BB. Prophylactic phenylepherine infusion for preventing hypotension during spinal anaesthesia for cesarean delivery. Anesth Analg. 2004;98:815–21. doi: 10.1213/01.ane.0000099782.78002.30. [DOI] [PubMed] [Google Scholar]
- 13.Ngan Kee WD, Khaw KS, Ng FF. Prevention of hypotension during spinal anesthesia for cesarean delivery.An effective technique using combination phenylepherine infusion and crystalloid co-hydration. Anesthesiology. 2005;103:744–50. doi: 10.1097/00000542-200510000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Nagan Kee WD, Khaw KS, Lee BB, Lau TK, Gin T. A dose response study of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anaesthesia for cesarean delivery. Anesth Analg. 2000;90:1390–5. doi: 10.1097/00000539-200006000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Critchley LA, Conway F. Hypotension during subarachnoid anaesthesia: Haemodynamic effects of colloid and metaraminol. Br J Anaesth. 1996;76:734–736. doi: 10.1093/bja/76.5.734. [DOI] [PubMed] [Google Scholar]
- 16.Saravanan S, Kocarev M, Wilson RC, Watkins E, Columb MO, Lyons G. Equivalent dose of ephedrine and phenylephrine in the prevention of post-spinal hypotension in caesarean section. Br J Anaesth. 2006;96:95–9. doi: 10.1093/bja/aei265. [DOI] [PubMed] [Google Scholar]


