Abstract
Although, opioids are advocated in various guidelines their use for chronic non-cancer pain is controversial because evidence of long term benefit is weak. The potential for serious adverse effects and local regulations promote caution in both the prescribers and users. However, opioids have a place in the management of chronic non-cancer pain in carefully selected patients with regular monitoring and as a part of the multimodal therapy. It is important for the treating physician to be up-to-date with this form of therapy, in order to have the necessary confidence to prescribe opioids and manage adverse effects. The common adverse effects should be treated promptly to improve patient compliance. We believe that opioid therapy in low doses is beneficial to some patients. It should not be denied but carefully considered on case by case basis.
Keywords: Chronic non cancer pain, musculoskeletal pain, neuropathic pain, opioids
Introduction
In the past two decades, there has been increasing use of opioids in cancer and chronic non-cancer pain in the western world. Opioids are recommended by the World Health Organization as a part of the analgesic ladder for cancer pain. For chronic non-cancer pain including neuropathic pain, case series and randomized controlled trials demonstrates high quality evidence for a weak recommendation for opioids when used in the short term.[1] The role of the low dose opioids is increasingly recognized[2,3] and has been included as a second or third line treatment according to several international guidelines.[3–6] However, recent epidemiological studies fail to demonstrate the improvements in many “essential” outcomes including pain, function and quality of life in patients who have taken these drugs for many months or years.[7,8] Furthermore, as the number of prescriptions for strong opioids increases, so does the risk of serious adverse effects including inadvertent overdose and death.[9,10] Therefore, patient selection and outcome assessment is essential and long term use should be preceded by a trial in which the goals of treatment are agreed with the patient.
Opioid pharmacology
Opioids have been classified by strength [Table 1], duration of action [Table 2], or source (natural, semi-synthetic or synthetic). Opioids exert their effects via endogenous opioid receptors. These receptors are widespread throughout the central and peripheral nervous system. A number of endogenous opioid receptors have been described, Mu opioid receptor (MOR), Delta opioid receptor (DOR) and Kappa opioid receptor (KOR). The binding characteristics and therefore overall effects of opioids vary but by far the most important is mu receptor binding. The significance of this variation in clinical practice for treating persistent chronic pain is not known. However, this variation in binding characteristics may be helpful in managing opioid tolerance and side effects. Very recently some evidence has been published where delta opioid receptor function is enhanced in chronic pain and an agonist at DOR[11,12] may help persistent pain. This aspect however, needs further evaluation.
Table 1.
Common weak and strong opioids[2]

Table 2.
Some common long and short acting opioids

There is little evidence to support the recommendation of one opioid to another in terms of quality of analgesia. Some medication augments their overall effects via receptors other than opioid receptors (Tramadol, Methadone). They may be more effective in conditions (e.g., Tramadol in fibromyalgia) where pure opioid agonists may be ineffective. The potency of common opioids is shown in Table 3.
Table 3.
Conversion table for commonly prescribed opioid medications
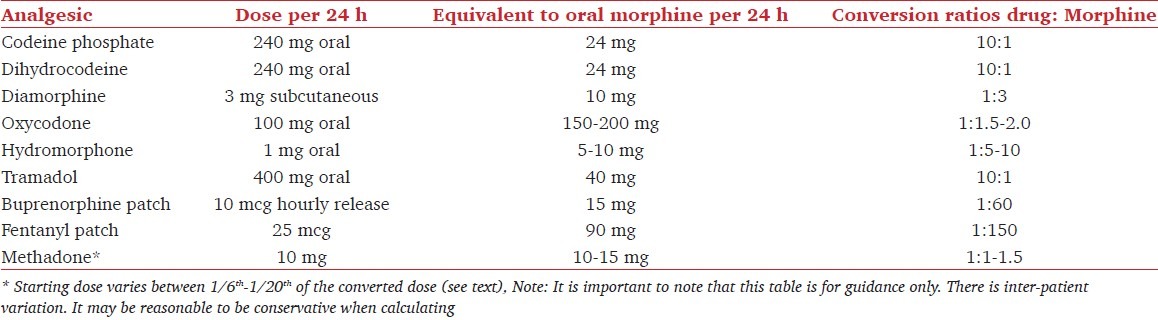
The analgesic effect of Codeine phosphate requires conversion to morphine in the liver. Nine percent of Caucasians[13] lack the enzymes needed for this conversion.
Morphine is metabolized in the liver by glucuronidation to morphine 3 glucuronide (inactive) and morphine 6 glucuronide (pro-analgesic effects). These metabolites are renally excreted and M6G may accumulate in renal impairment.
Hydromorphone, a semi-synthetic derivative of morphine and 5 times more potent, differs structurally from morphine with a keto group replacing the hydroxyl group at position 6 of the benzyl ring. This results in a glucuronidation at position 3 only into a non-active metabolite. Therefore, hydromorphone may be preferred in patients with renal impairment[14] and needs further studies for its validation.
Methadone,[15] an opioid and an N methyl-D-aspartate receptor antagonist and serotonin reuptake inhibitor, has an unusually variable elimination half life (4.5-130 h), which can lead to accumulation. Use of methadone has been associated with prolonged QTc interval[16] (in moderate doses), torsade de pointes[17] (high doses), sleep apnoea and sudden death.[18] Methadone has been used for analgesia, opioid dependence, tolerance or as a part of opioid rotation (described later). Its advantages are long duration of action, inexpensive, no dose adjustment required for renal and hepatic insufficiency and no active metabolites.
Tramadol is a weak MOR receptor agonist, releases serotonin and prevents the reuptake of nor-adrenaline.
Opioids can be administered via the intranasal, buccal, sublingual, oral, transdermal, rectal, parentral, epidural and intrathecal route. There is little evidence to suggest superiority of one route to the other in chronic non-cancer pain.[19] However, a change of route may improve analgesia, adverse effects and patient compliance. We do not recommend the use of parentral preparation to check the opioid responsiveness.
A regular long acting preparation has been recommended over repeated short acting (immediate release) preparations. This may improve pain control, reduce adverse effects and reduce the risk of addiction. However, patients may have more opioid related concerns while taking the drugs regularly rather than an as needed basis.[20,21] Furthermore, there is little evidence to support the use of sustained release preparation as opposed to immediate release medications.
Adverse effects
Opioid medications are associated with substantial adverse effects,[22] commonly being drowsiness, constipation, nausea, vomiting, itching, sweating and mood change. Eighty percent of individuals on opioids will experience at least one side effect.[2] These contribute to a high rate of discontinuation of medication. Respiratory depression, addiction and drug misuse cause caution and apprehension to opioid use. Other adverse effects include hyperalgesia and endocrinological suppression.
Opioid induced constipation
Opioids reduce gut motility and secretions, which leads to gastro-intestinal (GI) fluid absorption.[23–25] This is mediated principally through GI opioid receptors in the gut submucosa and to a lesser extent through central mechanisms. There is generally no tolerance observed with constipation on continued use. Treatment strategies include adequate hydration, high fibre content diet and encouraging physical activity. Laxatives (oral and rectal) and bulk forming agents have been used extensively. Opioid switch[26] (e.g., codeine to Tramadol) and change of route of administration (morphine to transdermal buprenorphine patches) can help some patients. To improve results opioid antagonists have been used successfully without reducing the analgesic effects. These include naloxone[27] (used in combination with oxycodone), methylnaltrexone and Alvimopan. Two percent of Naloxone is absorbed systemically and has extensive first pass metabolism. However, there are some case reports where high dose combination of oxycodone and naloxone has resulted in reduced pain relief. Costs preclude the routine use of these drug combinations however, they can be considered in certain circumstances, especially in the elderly.
Nausea vomiting and sedation
These are common adverse effects with initiation or dose escalation but tolerance develops in most patients after a few days and persistent effects are infrequent. Anti-emetics in the trial phase, slow titration and patient education are important considerations. Opioids are known to cause dizziness, drowsiness, lack of concentration, confusion, can affect an individual's ability to drive or work and higher doses increase the risk of falls (and fracture) in the elderly. Research[28,29] suggests that there is no evidence of deterioration of psychomotor and cognitive skills once the patient is on stable doses. Recently, some questions have been raised on the above interpretation and some authors have added some prerequisites.[30] However, caution should be exercised on initiation of the therapy and dose titration.
Opioid induced hyperalgesia
Opioid induced hyperalgesia (OIH) is defined as a state of nociceptive sensitization caused by exposure to opioids.[31] It results in worsening pain state, especially with certain pain stimuli (mechanical allodynia and cold perception) and is seen frequently for patient's long term opioids for surgical postoperative pain. Various mechanisms have been proposed such as NMDA activation, descending facilitation, spinal dynorphins. It probably uses similar pathways as neuropathic pain.[32] Therefore, neuropathic pain patients might be more susceptible to this phenomenon. Clinically it is difficult to diagnose OIH as the cause of deteriorating pain perception but it should be suspected if the pain has a different quality, site and distribution compared to the pre-existing pain. Pain may be worse on opioid escalation. The best treatment if suspected is to reduce opioids in consultation with the patient.
Endocrine effects
The most prevalent endocrine disorder associated with opioids is a deficiency of gonadotrophins leading to reduction in sex hormones, in particular testosterone.[33–35] This may occur with any route of administration and is more likely with doses above 100 mg daily morphine equivalent.[36] It may occur within few weeks of opioid use and is reversible if opioids are withdrawn. Diagnosis is made by the presence of symptoms (e.g., reduced libido, sexual dysfunction, fatigue, mood change) and signs (e.g., infertility, reduced hair growth, testicular atrophy, menstrual disorder) and the presence of reduced hormone levels. However, the symptoms and signs are not exclusive to androgen deficiency and minimum levels of testosterone are not clearly defined. Replacement therapy is available and will correct the abnormality but is not without risks. Therefore, our practice is to refer patients with suspected androgen deficiency to a specialist endocrine unit for evaluation, treatment and long-term follow-up.
Addiction/death
Opioids are associated with risk of addiction and unintentional death.[9] This pattern is seen in North America where there was a 3-fold increase in opioid related deaths during the years 1999-2007, similarly there was a twofold increase in deaths from methadone and codeine in United Kingdom. According to a report by the International Narcotics Control Board, 6.2 million American and 1.4-1.9 million Germans are addicted to pharmaceutical medication.[37] According to this report, young adults are the most vulnerable group. It is therefore important to have a robust patient selection criteria and clear outcome measures from the outset.
Tolerance and physical dependence are normal physiological features that happen on regular drug use. They are often seen in patients who are addicted or abuse drugs. These should not be confused with addiction.[38]
Patient selection and outcomes for opioids in chronic pain management
Appropriate patient selection requires a thorough assessment and only then should the patient have a trial of opioids. This should be tailored to the patient by selecting agreed goals or outcome measures. An opioid trial should be undertaken only after other treatments with good evidence base have been tried, for example, tricyclic antidepressants and anticonvulsants for neuropathic pain or as a part of multimodal therapy.
Assessment
Opioids should only be used for pain control, and pain scores can be a helpful indication of severity of pain, particularly if the patient maintains a pain diary (records daily average pain scores over time, for example, 1-2 weeks). Opioids should not be used if the primary indication is anxiolysis, depression or for a sedative effect.
The physician should seek to confirm a patho-physiological diagnosis that is proportional to the pain and disability reported by the patient. Failure to acknowledge this principle may lead to inappropriate prescribing particularly to patients exhibiting high levels of distress that may have a substantial psychological basis.
Functional impairment due to pain and impact on an individual should be recorded. Functional assessment may include work, ability to do activities of daily living, sleep and social and family activities. A psychological assessment will help to identify important psychological factors that may not only contribute to the initial presentation but also predict a poor outcome with isolated medical treatment, whether it be opioids, injection therapy or adjuvant medication. Identification of depression and anxiety is important and missed frequently without direct questioning and the routine use of a patient questionnaire such as the Hospital Anxiety and Depression scale or Beck Depression Inventory is recommended. Underlying psychological distress may be associated with an increased level of pain perception, bodily awareness and multiple physical symptoms (chronic fatigue, difficulty concentrating, irritability, muscle tension). Such patients usually respond poorly[39] if treated with opioids because the treatment fails to address the underlying psychological cause.
A history of drug and alcohol abuse which often coexist does not prohibit[40] the use of opioids for pain management. However, the presence of on-going drug abuse may make it impossible to prescribe opioids safely without a substantial risk of overdose. It is important to recognize patients who develop drug abuse and they may exhibit aberrant behaviors [Table 4]. Questionnaires such as the Opioid Risk Tool are available that helps to predict which patients are at risk from developing drug-related aberrant behaviors.[41,42]
Table 4.
Behavior that may indicate problem drug use

Outcome measures
Given the uncertainty of the long term benefit of opioids for chronic non-malignant pain, it is very helpful to conduct a trial of opioids with clear goals that have been agreed with the patient before the trial begins. A formal contract, signed by the patient and doctor, may be helpful in some patients particularly if the doctor has concern about the risk of drug misuse. There is no general consensus on outcome measures for opioids.
Goals for use of opioids
Pain reduction is the primary requirement of opioid therapy. Pain relief goal should be realistic and complete pain relief is rarely achieved. Documenting pain scores and maintaining a pain diary before and during the trial may give a more accurate estimate of pain severity and benefit.
Sleep. Lack of sleep can contribute to the maintenance of pain. It is important to help the patient sleep and document how often sleep is disturbed due to pain. Quantify the number of times per night and the number of nights per week.
Mood. Changes in the mood in a pain clinic are most easily measured using a tool such as the Hospital Anxiety and Depression scale, or the Beck Depression Inventory.
Return to work. Caution should be exercised when using return to work as an outcome measure for an individual who has not worked for many months, especially if there is no job to return to after a period of sick leave. Consider also work days lost due to pain; this may be a useful measure for patients who remain at work.
Physical function. It is important to be precise in the desired outcomes. For example, physical improvement should be measured in the ability to undertake physical tasks that could not be achieved without the drugs, rather than a vague outcome such as "can move about more easily". This may include ability to get dressed or wash independently; undertake specific household tasks or sitting comfortably for sufficient time to undertake tasks (eating a meal, hobbies, reading the newspaper). It is unlikely that physical function of a more strenuous nature will improve with opioids.
Social function. This may partly reflect improvement in physical function but also mood and sense of general well-being. It may be extended to include improvement in relationships with family members.
To measure above goals various tools have been used. The Brief Pain Inventory is a tool used widely to assess pain and treatment. It measures pain intensity and the effects of pain on sleep, mood, physical and social function. It exhibits reliability and validity across different cultures and languages, and it is quick and easy to complete and administer. Other indices like quality of life measures lack sensitivity to measure changes in an individual and are more helpful for population studies. However, it may be reasonable to measure a “global perception of change” which is a useful guide to overall change and allows the patient to describe overall benefit or harm. Adverse effects should be measured at each assessment and can be quantified simply on a VAS for severity.
Duration of trial and use of opioids after successful trial
A trial will typically take several weeks unless adverse effects intervene. “Start low and go slow” is a useful principle but tailored to the patient. There is rarely an urgency to increase the dose more frequently for chronic non-malignant pain and a slow increase in dose will usually avoid distressing adverse effects. In our experience, pain is very unlikely to respond with higher doses if there is no appreciable response at 100 mg morphine daily or equivalent doses.
It is important to know and document the nature of the response. In our practice, some of the responses have been better sleep or takes mind off or being drowsy but no pain relief. In these circumstances it may be reasonable to review the patient more frequently and appropriate decision made on the continuation of the opioids in discussion with the patient. We do not recommend routine use of injectable form of opioids for persistent chronic non-cancer pain to check the opioids responsiveness.
Follow-up
During the trial the clinic follow-up may be monthly for further prescriptions and more frequently by telephone. Once the stable dose is established, we discharge the patient to the general practitioner, who becomes the sole prescriber. Dose escalation beyond about 25-50% of this stable dose should be avoided without further assessment by the Pain Clinic.
Side effects should be treated appropriately and some medication (anti-emetics, laxatives) can be prescribed at the same time to maximize compliance.
In patients who are responders but have become tolerant or responders in whom side effects preclude further escalation, it may be reasonable to undertake an opioid switch or rotation. Vadalauca and others[43] reviewed the general principle on opioid rotation in cancer patients. In our view these principle can be extrapolated to non-cancer patients. These include consistent practice/method, good patient assessment, use of dose conversion tables (used as guidelines only), 24 h opioid requirements and opioid selection.
If the patient is on high opioid dosage, we reduce the opioid to maximally tolerated dose or try to reduce it to 100-200 mg equivalent morphine before we switch to a different opioid. Some authors[44] have discontinued any prior analgesic medications for a period of few weeks before switching to a different opioid. The starting dose should be around 50% of the estimated converted dose except methadone (e.g., 100 mg morphine is equivalent to 60 mg oxycodone; the starting dose will be 30 mg oxycodone). Methadone should be started at 1/6th to 1/20th the converted dose (e.g., 100 mg morphine is equivalent to 60 mg methadone; the starting dose should be around 3-5 mg methadone).
Opioids and travel
People on regular opioids should inform the country they are visiting via their respective embassy.[45] In the United Kingdom, an individual who is travelling abroad has to carry a letter from the prescribing doctor or drug case worker if the travel for abroad is less than 3 months. A license from the home office is mandatory with the prescriber's letter if the travel for abroad is for more than 3 months. The letter should confirm the name, travel itinerary, names of prescribed controlled drugs, dosages and total amounts of each to be carried.
Conclusion
Strong opioids have a place in the management of chronic persistent non-cancer pain. The decision to initiate this treatment has to be done with a fully informed individual on a background of limited long term efficacy and potential severe adverse effects. It has an associated mortality. Opioids may benefit only a small proportion of patients. Patient selection is important and it is very difficult to predict a responder from non-responder. There is no substitution for a good medical review.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Trescot AM, Helm S, Hansen H, Benyamin R, Glaser SE, Adlaka R, et al. Opioids in the management of chronic non-cancer pain: An update of American Society of the Interventional Pain Physicians’ (ASIPP) Guidelines. Pain Physician. 2008;11:S5–62. [PubMed] [Google Scholar]
- 2.2010. Jan, [Last accessed on 2011 Nov 20]. The British Pain society. Opioids for persistent pain: Good practice. A consensus Statement. Available from: http://www.britishpainsociety.org/book_opioid_main.pdf . [Google Scholar]
- 3.Furlan AD, Reardon R, Weppler C National Opioid Use Guideline Group. Opioids for chronic noncancer pain: A new Canadian practice guideline. Can Med Assoc J. 2010;182:923–30. doi: 10.1503/cmaj.100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dworkin RH, O’Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, et al. Recommendations for the Pharmacological Management of Neuropathic Pain: An Overview and Literature Update. Mayo Clin Proc. 2010;85:S3–14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain. 2007;132:237–51. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CPN, Sessle BJ, et al. Pharmacological management of chronic neuropathic pain-Consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12:13–21. doi: 10.1155/2007/730785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain. Clin J Pain. 2008;24:469–78. doi: 10.1097/AJP.0b013e31816b2f26. [DOI] [PubMed] [Google Scholar]
- 8.Eriksen J, Sjøgren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non.cancer pain: An epidemiological study. Pain. 2006;125:172–9. doi: 10.1016/j.pain.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Dhalla IA, Persaud N, Juurlink DN. The prescription opioid crisis. BMJ. 2011;343:d5142. doi: 10.1136/bmj.d5142. [DOI] [PubMed] [Google Scholar]
- 10.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescription for chronic pain and overdose.A cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahill CM, Taylor A. A piece of the puzzle is revealed for delta opioid receptor-mediated analgesia. Pain. 2011;152:1217–8. doi: 10.1016/j.pain.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Gaveriaux-Ruff C, Nozaki C, Nadal X, Hever XC, Weibel R, Matifas A, et al. Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain. 2011;152:1238–48. doi: 10.1016/j.pain.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Yue QY, Hasselström J, Svensson JO, Säwe J. Pharmacokinetics of codeine and its metabolites in Caucasian healthy volunteers: Comparisons between extensive and poor hydroxylators of debrisoquine. Br J Clin Pharmacol. 1991;31:635–42. doi: 10.1111/j.1365-2125.1991.tb05585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felden L, Walter C, Harder S, Treede RD, Kayser H, Drover D, et al. Comparative clinical effects of hydromorphone and morphine: A meta-analysis. Br J Anaesth. 2011;107:319–28. doi: 10.1093/bja/aer232. [DOI] [PubMed] [Google Scholar]
- 15.McNicol ED, Haroutiunian S, Lipman AG. Methadone for chronic non-cancer pain in adults (Protocol) Cochrane Database Syst Rev. 2009;4:CD008025. doi: 10.1002/14651858.CD008025.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruciani RA, Sekine R, Homel P, Lussier D, Yap Y, Suzuki Y, et al. Measurement of QTc in Patients Receiving Chronic Methadone Therapy. J Pain Symptom Manage. 2005;29:385–91. doi: 10.1016/j.jpainsymman.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Krantz MJ, Lewkowiez L, Hays H, Woodroffe MA, Robertson AD, Mehler PS. Torsade de Pointes Associated with Very-High-Dose Methadone. Ann Intern Med. 2002;137:501–4. doi: 10.7326/0003-4819-137-6-200209170-00010. [DOI] [PubMed] [Google Scholar]
- 18.Chugh SS, Socoteanu C, Reinier K, Waltz J, Jui J, Gunson K. A Community-Based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med. 2008;121:66–71. doi: 10.1016/j.amjmed.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballantyne JC, Carwood C. Comparative efficacy of epidural, subarachnoid, and intracerebroventricular opioids in patients with pain due to cancer. Cochrane Database Syst Rev. 2005;1:CD005178. doi: 10.1002/14651858.CD005178. [DOI] [PubMed] [Google Scholar]
- 20.Ballantyne JC. Opioids around the clock? Pain. 2011;152:1221–2. doi: 10.1016/j.pain.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 21.Von Korff M, Merrill JO, Rutter CM, Sullivan M, Campbell CI, Weisner C. Time-scheduled vs. pain-contingent opioid dosing in chronic opioid therapy. Pain. 2011;152:1256–62. doi: 10.1016/j.pain.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic non cancer pain: A meta.analysis of effectiveness and side effects. Can Med Assoc J. 2006;174:1589–94. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camilleri M. Opioid-Induced Constipation: Challenges and Therapeutic Opportunities. Am J Gastroenterol. 2011;106:835–42. doi: 10.1038/ajg.2011.30. [DOI] [PubMed] [Google Scholar]
- 24.McNicol E, Horowicz-Mehler N, Fisk RA, Bennett K, Gialeli-Goudas M, Chew PW, et al. Management of opioid side effects in cancer-related and chronic noncancer pain: A Systemic Review. J Pain. 2003;4:231–56. doi: 10.1016/s1526-5900(03)00556-x. [DOI] [PubMed] [Google Scholar]
- 25.Tamayo AC, Diaz-Zuluaga PA. Management of opioid-induced bowel dysfunction in cancer patients. Support Care Cancer. 2004;12:613–8. doi: 10.1007/s00520-004-0649-7. [DOI] [PubMed] [Google Scholar]
- 26.Leppert W. The role of opioid receptor antagonists in the treatment of opioid-induced constipation: A review. Adv Ther. 2010;27:714–30. doi: 10.1007/s12325-010-0063-0. [DOI] [PubMed] [Google Scholar]
- 27.Smith K, Hopp M, Mundin G, Bond S, Bailey P, Woodward J, et al. Naloxone as part of a prolonged release oxycodone/naloxone combination reduces oxycodone-induced slowing of gastrointestinal transit in healthy volunteers. Expert Opin Investig Drugs. 2011;20:427–39. doi: 10.1517/13543784.2011.563236. [DOI] [PubMed] [Google Scholar]
- 28.Fishbain DA, Cutler RB, Rosomoff HL, Rosomoff RS. Can patients taking opioids drive safely. A structured evidence-based review? J Pain Palliat Care Pharmacother. 2002;16:9–28. [PubMed] [Google Scholar]
- 29.Fishbain DA, Cutler RB, Rosomoff HL, Rosomoff RS. Are opioid-dependent/tolerant patients impaired in driving-related skills? A structured evidence-based review. J Pain Symptom Manage. 2003;25:559–77. doi: 10.1016/s0885-3924(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 30.Kress HG, Kraft B. Opioid medication and driving ability. Eur J Pain. 2005;9:141–4. doi: 10.1016/j.ejpain.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A Comprehensive Review of Opioid-Induced Hyperalgesia. Pain Physician. 2011;14:145–61. [PubMed] [Google Scholar]
- 32.Mao J. Opioid-Induced Hyperalgesia. IASP Pain Clin Updat. 2008;16:1–4. [Google Scholar]
- 33.Fraser LA, Morrison D, Morley-Forster P, Paul TL, Tokmakejian S, Larry Nicholson R, et al. Oral Opioids for Chronic Non.cancer Pain: Higher Prevalence of Hypogonadism in Men than in Women. Exp Clin Endocrinol Diabetes. 2009;117:38–43. doi: 10.1055/s-2008-1076715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aloisi AM, Ceccarelli I, Carlucci M, Suman A, Sindaco G, Mameli S, et al. Hormone replacement therapy in morphine-induced hypogonadic male chronic pain patients. Reprod Biol Endocrinol. 2011;9:26. doi: 10.1186/1477-7827-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy RG, Aung T, Karavitaki N, Wass JA. Opioid induced hypogonadism. BMJ. 2010;341:c4462. doi: 10.1136/bmj.c4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith HS, Elliott JA. Opioid-Induced Androgen Deficiency (OPIAD) Pain Physician. 2012;15:ES145–56. [PubMed] [Google Scholar]
- 37.United Nations. Report of the International Narcotics Control Board for 2009. [Last accessed on 2012 Sep 3]. Available from: http://www.incb.org/pdf/annual.report/2009/en/AR_09 .
- 38.Raith K, Hochhaus G. Drugs used in the treatment of opioid tolerance and physical dependence: A review. Int J Clin Pharmacol Ther. 2004;42:191–203. doi: 10.5414/cpp42191. [DOI] [PubMed] [Google Scholar]
- 39.Jensen MK, Thomsen AB, Højsted J. 10-year follow-up of chronic non-malignant pain patients: Opioid use, health related quality of life and health care utilization. Eur J Pain. 2006;10:423–33. doi: 10.1016/j.ejpain.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Watson CP, Watt-Watson JH. Chronic non cancer pain and the long term utility of opioids. Pain Res Manage. 2004;9:19–24. doi: 10.1155/2004/304094. [DOI] [PubMed] [Google Scholar]
- 41.Webster LR, Webster RM. Predicting aberrant behaviours in opioid treated patients: Preliminary validation of the opioid risk tool. Pain Med. 2005;6:432–42. doi: 10.1111/j.1526-4637.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 42.Ballantyne JC, LaForge KS. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007;129:235–55. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 43.Vadalouca A, Moka E, Argyra E, Sikioti P, Siafaka I. Opioid rotation in patients with cancer: A review of the current literature. J Opioid Manag. 2008;4:213–50. doi: 10.5055/jom.2008.0027. [DOI] [PubMed] [Google Scholar]
- 44.Lange B, Kuperwasser B, Okamoto A, Steup A, Häufel T, Ashworth J, et al. Efficacy and safety of tapentadol prolonged release for chronic osteoarthritis pain and low back pain. Adv Ther. 2010;27:381–99. doi: 10.1007/s12325-010-0036-3. [DOI] [PubMed] [Google Scholar]
- 45.Home Office. Personal licences. [Last accessed 2011 Nov 20]. Available from: personal/?view=Standard .


