Abstract
Neuregulin-1 binds to ErbB3 and ErbB4 and regulates cancer proliferation and differentiation. Neuregulin-1 had been suggested to also react with ErbB2, but this argument becomes controversial. Here, we re-evaluated the cellular responses and ErbB2 interaction of neuregulin-1 in ErbB2 overexpressing cell lines. In a competitive ligand-binding assay, we detected significant replacement of [35S]-labeled neuregulin-1 with nano molar ranges of cold neuregulin-1 in L929 cells expressing ErbB2 alone and SKOV3 cells carrying sulf-1 cDNA but not in these parental cells. The concentration of neuregulin-1 significantly decreased thymidine incorporation and phosphorylation of ErbB2 (Tyr877, Tyr1396, and Tyr1121) in ErbB2-overexpressing cancer cells as well as in L929 cells expressing ErbB2. A crosslinking assay ascertained the presence of neuregulin-1 immunoreactivity in the ErbB2 immune complexes of L929 expressing ErbB2 alone. These results suggest that the higher concentrations of neuregulin-1 exert an anti-oncogenic activity to attenuate ErbB2 auto-phosphorylation potentially through its low-affinity interaction with ErbB2.
A receptor molecule for neuregulin-1 (neu-differentiation factor or heregulin) was initially suggested to be not only ErbB3 and ErbB4 but also ErbB2 (Her2, Neu)1,2. The following studies demonstrate that the high affinity interaction of ErbB2 with neuregulin-1 involves ErbB3 hetrodimerization and tone down the argument for the direct ErbB2−neuregulin-1 interaction3,4,5,6. ErbB2 is often overexpressed with or without gene amplification in various cancer cells, such as mammary cancer cells and ovarian cancer cells7,8,9,10. ErbB2 overexpression results in ligand-independent self-dimerization and subsequent auto-phosphorylation, leading to downstream signaling linked to cell proliferation and migration. Thus, the extent of ErbB2 overexpression in cancer cells is sometimes correlated with malignancy and/or a poor prognosis11,12. Recently, various anti-cancer drugs that target ErbB2 have been developed, including anti-ErbB2 antibodies that inhibit ErbB2 self-dimerization or induce its down regulation13,14. In this regard, ErbB2 auto-phosphorylation play key roles in the proliferation of the cancer cell lines overexpressing ErbB215,16,17.
After two initial reports demonstrated an interaction between neuregulin-1 and ErbB2, subsequent studies questioned the receptor function of ErbB2. There are two major reasons why ErbB2 cannot function as a high-affinity receptor for neuregulin-1. First, many ovarian cancer cell lines that express high levels of ErbB2 do not exhibit an increase in ErbB2 phosphorylation in response to neuregulin-13. Secondly, [125I]-labeled neuregulin-1 exhibits a low or no binding affinity for these cells4,18. However, other studies still indicate that the direct interaction between ErbB2 and neuregulin-1 might be real despite the weak affinity3,19. In addition, subsequent studies further increase the complexity of this argument; there are positive and negative regulators for ErbB receptor function and subcellular localization. For example, sulfatase-1 (Sulf-1), which removes O-sulfate groups of heparin or heparan sulfate proteoglycans, significantly influence the molecular interaction of neuregulin-1 with ErbB receptors20,21. Of note, approximately 70% of ovarian cancer cells lost the expression of sulf-1 and exhibit higher grades of malignancy often with ErbB2 overexpression21,22. With this respect, the affinity and biological role of the neuregulin-1–ErbB2 receptor interaction remains to be controversial and needs to be re-evaluated.
To clarify this issue, we carefully assessed the molecular response of an ovarian cancer line, SKOV3, and a breast cancer line, BT-474, to neuregulin-1, both of which highly express ErbB2. We also used L929 mouse fibroblasts that lack the expression of ErbBs or those transfected with erbB2 cDNA. Molecular interactions of ErbB2 with neuregulin-1 were examined with novel procedures for ligand labeling and immunoprecipitation. In particular, we manipulated the expression of heparan sulfatase-1 in the ovarian cancer cells as the O-sulfate structures markedly alter receptor interactions of the heparin-binding growth factors such as neuregulin-120,21. With the obtained results, we discuss the involvement of ErbB2 in neuregulin-1 signaling and functions.
Results
Expression of ErbB1-4 proteins in L929, SKOV3, and BT-474 cells
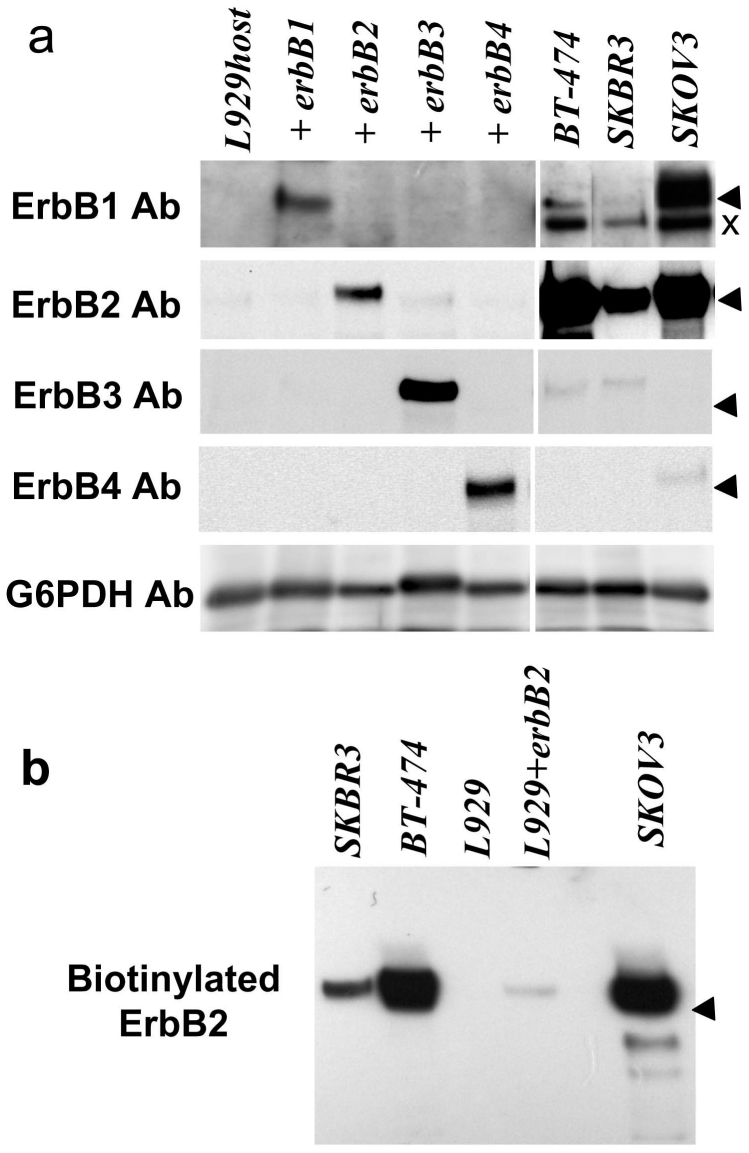
The primary amino acid sequences of the ErbB molecular family members share high structural homology and are often cross-recognized by antibodies raised against different subtypes. As the previous reports demonstrate that mouse fibroblast L929 cells have no binding to neuregulin-1 and harbor the limited expression of ErbB2, we employed parental L929 cells as a negative control and transfected these cells with erbB1, B2, B3 or B4 cDNA expression vectors as positive controls23,24. We examined and confirmed endogenous ErbB1-4 expression in the cell lines (i.e., L929, SKOV3, SKBR3 and BT-474 cells) used in the present study (Fig. 1a). Although L929 cells expressed trace amounts of ErbB2, these cells lacked detectable levels of ErbB1, ErbB3 and ErbB4 as described previously23,24,25. SKOV3 cells expressed high levels of ErbB2 and ErbB1 and low levels of ErbB4, whereas BT-474 expressed high levels of ErbB2 and low levels of ErbB3 and ErbB1 when we compared those with ErbB1-4 levels in transfected L929 cells.
Figure 1.
Cellular and surface expression of ErbB1-4 in L929 and cancer cells (a) Proteins were extracted from untransfected L929 cells, L929 cells transfected with erbB1-4 cDNA, or BT-474, SKBR3, and SKOV3 cancer cells and were probed with antibodies raised against ErbB1, ErbB2, ErbB3, ErbB4, and G6PDH (control).(b) Living cells were exposed to Sulfo-NHS-biotin to label surface proteins. Biotinylated proteins were subjected to Western blotting for ErbB2. Arrowheads marks the position of 175 kDa molecular marker and × represents the non-specific immunoreactivity that commonly appears in many types of cells.
Although these cell lines contained ErbB2 proteins, they could be nonfunctional if they are not located at the cell surface. We examined the cell surface expression of ErbB2 in the above cell lines by surface biotinylation (Fig. 1b). Except for the parental L929, there were significant surface levels of the ErbB2 protein in all cell lines, which appeared to be correlated with the total ErbB2 levels.
To mimic the ErbB2-overexpression state of the cancer cells in L929 cells, we established the L929 cell line that stably carries erbB2 cDNA through neomycin selection. By transiently transfecting an erbB2-expression vector, we further boosted ErbB2 expression in this cell line and used these ErbB2-overexpressing L929 cells (referred to as ErbB2-L929 hereafter) in the following experiments. Again, we confirmed that the transfection of erbB2 cDNA and G418 selection did not induce the expression of ErbB3 and ErbB4 in ErbB2-L929 cells (Supplemental Figure S1).
Responses of ErbB2-overexpressing cells to neuregulin-1
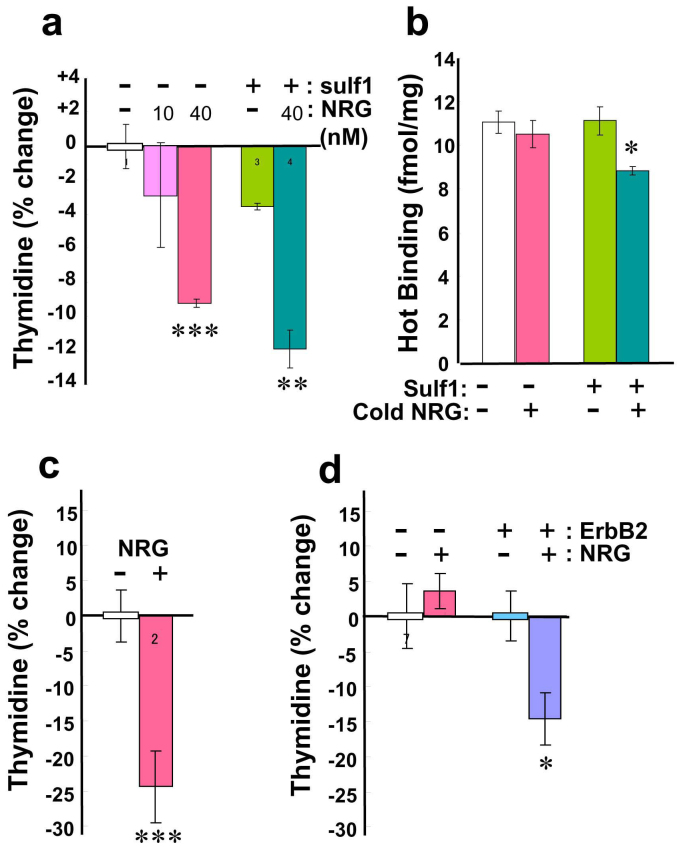
The ovarian cancer cell line, SKOV3, is reported not to have a high affinity for neuregulin-12,3. We re-examined the cellular responses of SKOV3 cells to relatively high concentrations of neuregulin-1, monitoring their cell proliferation and competitive binding activity. The rates of cell proliferation were measured using [3H] thymidine incorporation in the presence or absence of 10 nM and 40 nM type I neuregulin-1 (a soluble form comprising the extracellular domain; ECD) as well as with or without the transfection of sulf-1 (sulfatase 1) cDNA (Fig. 2a). As blood serum contains high levels of neuregulin-1, SKOV3 cells were extensively washed prior to the assay26. The concentration of 10 nM neuregulin-1 had no apparent influence on thymidine incorporation, whereas 40 nM neuregulin-1 significantly decreased incorporation compared with untreated controls. SKOV3 cells lack the enzyme Sulf-1, which selectively removes the 6-O-sulfate group from heparan sulfate27 and regulates neuregulin-1 binding to ErbB receptors20. We also tested the effect of Sulf-1 expression on the thymidine incorporation of SKOV3 cells. The transfection of sulf-1 cDNA further increased the response to neuregulin-1. In addition, we assessed the effects of 40 nM neuregulin-1 on BT-474 cells, ErbB2-L929 cells, and parental L929 cells (Fig. 2c, d). We observed a similar anti-proliferative activity of neuregulin-1 in BT-474 cells and ErbB2-L929 cells, but not in parental L929 cells.
Figure 2.
Effects of neuregulin-1 on cell proliferation and binding to [35S]-labeled neuregulin-1 (a) SKOV3 cells that were transfected with sulf-1 cDNA were serum-starved and then challenged with neuregulin-1 (10 nM or 40 nM).% ratios of changes in [3H] thymidine incorporation were calculated compared to that of untreated and mock-transfected SKOV3 cells.(b) Binding of [35S]-labeled neuregulin-1 to SKOV3 cells was measured in the presence or absence of 40 nM cold neuregulin-1. (c) The effects of 40 nM neuregulin-1 on [3H] thymidine incorporation in BT-474 cells were assessed. (d) [3H] thymidine incorporation in parental L929 cells or ErbB2-L929 cells was determined. *p < 0.05, **p < 0.01, ***p < 0.001, compared to control cells.
We recently developed a simple method that enables us to synthesize and label authentic neuregulin-1 with the radioisotope [35S] methionine28. This procedure does not involve additional chemical modification, such as iodination and/or oxidization of the target protein, we can avoid the structural hindrance of the additional atom in the receptor-binding assay. In fact, we found that the iodination of neuregulin-1 proteins (i.e., NRG1-ECD and an EGF domain peptide of neuregulin-1; eNRG1) significantly reduced their biological activity (Supplemental Figure S2). In the present study, therefore, we prepared a [35S]-labeled ligand of neuregulin-1 by this in vitro translation system. We performed the competitive ligand-binding assay in SKOV3 cells with this [35S]-labeled ligand (Fig. 2b). As reported previously, there was no significant replacement of [35S]-labeled neuregulin-1 in SKOV3 cells3. Again, we examined the effects of sulf-1 cDNA transfection on neuregulin-1 binding to this cell line. When the sulf-1 gene was transduced into these cells, there was a significant replacement of [35S]-labeled neuregulin-1 with 40 nM of cold neuregulin-1. The absence of Sulf-1 protein expression in the parental SKOV3 cells as well as its presence after transfection was confirmed by Western blotting (Supplemental Figure S3).
ErbB2 dephosphorylation following neuregulin-1 challenge
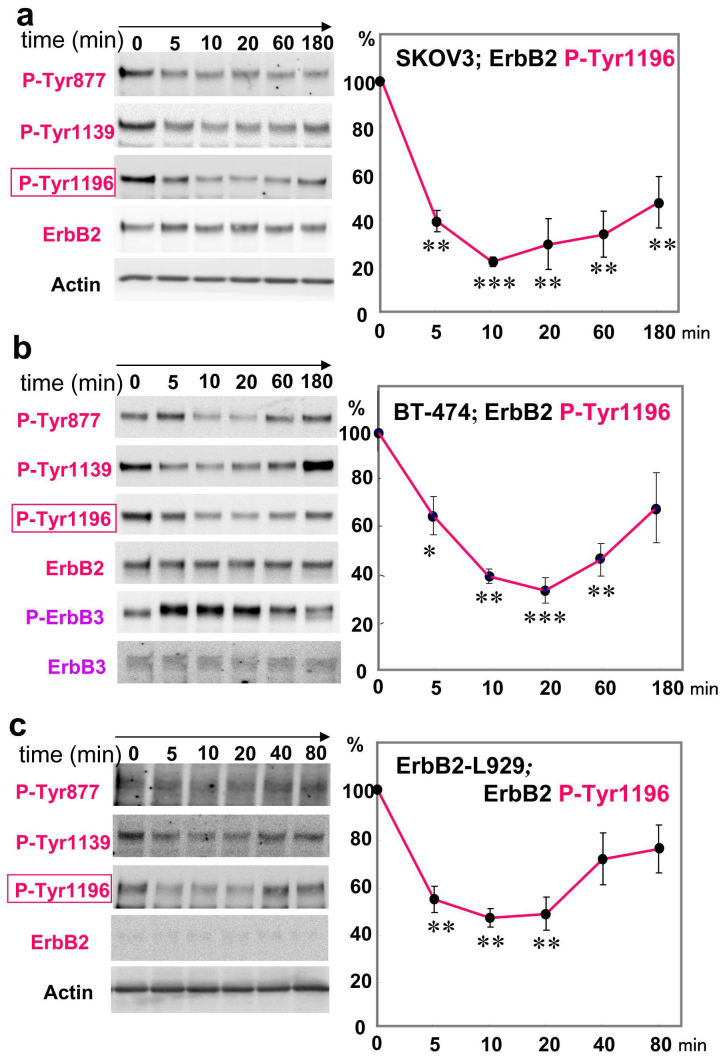
The above results suggest the biological implication of ErbB2 signaling in the anti-proliferative responses to neuregulin-1. We examined time-dependent alterations in ErbB2 phosphorylation (Tyr877, Tyr1139, and Tyr1196) of SKOV3 cells following neuregulin-1 challenge (Fig. 3a). Western blotting with anti-phospho ErbB2 antibodies suggested that exposing SKOV3 cells to 40 nM neuregulin-1 rapidly decreased the phosphorylation levels of ErbB2 at Tyr877, Tyr1139, and Tyr1196. Statistical analyses revealed that the decrease in Tyr1196 phosphorylation was statistically significant and continued at least for 3 h. In contrast, there were no apparent alterations in the total protein levels of ErbB2 and β-actin.
Figure 3.
Neuregulin-dependent and time-dependent decreases in ErbB2 phosphorylation (a) SKOV3 cells that had been transfected with sulf-1 cDNA and subjected to serum starvation were challenged with 40 nM neuregulin-1.Time-dependent alterations in ErbB2 phosphorylation at Tyr877, Tyr1139, or Try1196 as well as in authentic ErbB2 levels were examined by Western blotting. (b) Gel loading was controlled with β-actin levels. Changes in ErbB2 and ErbB3 phosphorylation were also assessed in BT-474. (c) Changes in ErbB2 phosphorylation were similarly assessed in ErbB2-L929 cells. *p < 0.05, **p < 0.01, ***p < 0.001, compared to the values of 0 time point (n = 3).
Similarly, we examined time-dependent ErbB2 dephosphorylation in another ErbB2-overexpressing cell line, BT-474 (Fig. 3b). BT-474 cells exhibited anti-proliferative responses to neuregulin-1 (see Fig. 2) whereas these cells also express ErbB3 whose signaling promotes cell proliferation29,30. Neuregulin-1 treatment indeed elevated ErbB3 phosphorylation in BT-474 cells. In parallel, neuregulin-1 challenge triggered the rapid dephosphorylation of ErbB2 at Tyr877, Tyr1139, and Tyr1196 as was seen in SKOV3 cells. In contrast to the effects on SKOV3, ErbB2 dephosphorylation at Tyr1196 did not continue more than 180 min, however.
It was not evident that the observed dephosphorylation of ErbB2 might involve the interaction of neuregulin-1 with ErbB4 and ErbB3 receptors expressed by SKOV3 and BT-474 cells, respectively. We examined ErbB2 phosphorylation in ErbB2-L929 cells (Fig. 3c). When we challenged ErbB2-L929 cells with neuregulin-1, we found similar rapid decreases in ErbB2 phosphorylation at Tyr877, Tyr 1139, and Tyr 1196. Statistical analysis of Tyr 1196 phosphorylation verified that there was a significant reduction in ErbB2 tyrosine phosphorylation. In comparison with the duration of the dephosphorylation in SKOV3 and BT-474 cells, the ErbB2 dephosphorylation had a shorter duration and did not continue more than 20 min in ErbB2-L929 cells.
Competitive binding of neuregulin-1 to L929 cells overexpressing ErbB2
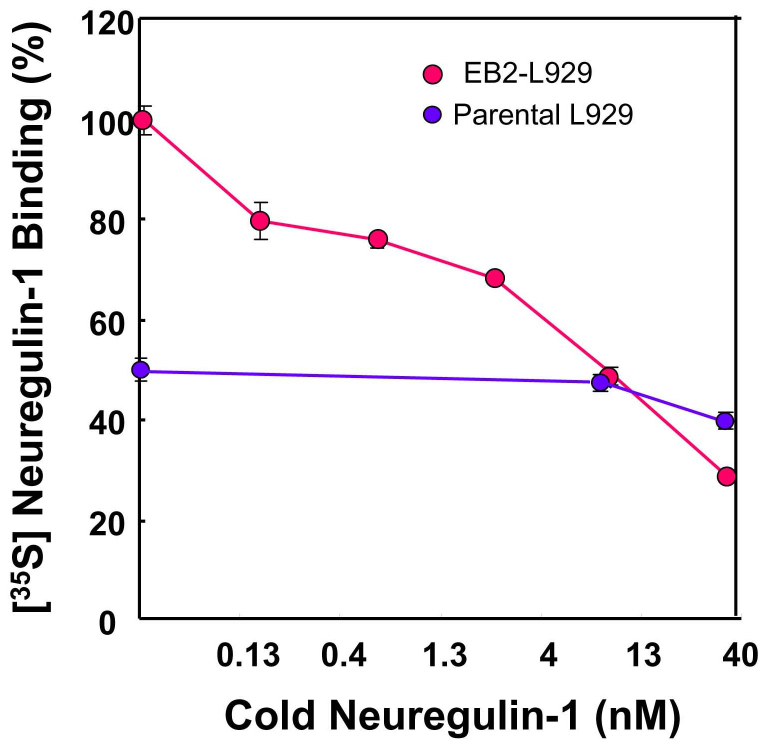
Does ErbB2 dephosphorylation stem from the molecular interaction or association of ErbB2 with neuregulin-1? To challenge this question, ErbB2-L929 cells as well as parental L929 cells were examined in a competitive ligand-binding assay (Fig. 4). The total binding of [35S]-labeled neuregulin-1 to ErbB2-L929 cells was gradually diminished with increasing concentrations of cold neuregulin-1. IC50 of the neuregulin-1 binding was approximately 10 nM. In contrast, parental L929 cells exhibited reduced total binding to [35S]-labeled neuregulin-1 and failed to show any significant replacement with 10 nM cold neuregulin-1. This result agrees with the report that the cytotoxicity of toxin-conjugated neuregulin-1 is undetectable in parental L929 cells24.
Figure 4. Competitive binding assay of [35S]-labeled neuregulin-1 to ErbB2-overexpressing L929 cells.
The tracer of neuregulin-1 was synthesized using the rabbit reticulocyte lysate system in the presence of [35S]-methionine. [35S]-labeled neuregulin-1 (1.7 kBq, 1.9 nM) was incubated in the presence of various concentrations of cold neuregulin-1 (0–40 nM). The ratios relative to the maximum binding with no competitor (100%) were determined in triplicate (n = 3). Data represent mean ± SE. Note; the total binding of the tracer was decreased to 54.3 ± 3.1% in parental L929 cells that were not exposed to cold neuregulin-1. The putative background binding found in parental L929 cells was not compensated as parental L929 cells express a low level of ErbB2.
Since we did not modify the chemical structure of the ligand, [35S]-labeled neuregulin-1 should have the same affinity to ErbB receptors as authentic neuregulin-1. Thus we can assume that Ki is equal to Kd in the Cheng-Prusoff equation. According to this equation, we estimate that Kd of neuregulin-1 binding was ~12 nM or less with the given IC50 (approximately 10 nM) and the concentration of the tracer (i.e. 1.9 nM) in this assay.
Appearance of the ErbB2 complexes crosslinked to cold neuregulin-1
Using chemical crosslinking of cold neuregulin-1 to ErbB2, we attempted to visualize the receptor complexes of neuregulin-1 and ErbB2 using Western blotting with the anti-neuregulin-1 antibody. In our preliminary experiment, we first tested the immunoprecipitation performance of anti-ErbB2 antibodies. SKBR3 cells, which express both ErbB2 and ErbB3, were exposed to a chemical crosslinker. Then we attempted to immunoprecipitate the ErbB2 complexes with four distinct anti-ErbB2 antibodies using the conventional immunoprecipitation procedure of the non-denatured condition (Supplemental Figure S4). However, all the anti-ErbB2 antibodies failed to immunoprecipitate the ErbB2 complexes when the cells were treated with the chemical crosslinker, even though all these antibodies were able to immunoprecipiate authentic ErbB2 protein (Supplemental Figure S4). These results suggest that the amine-reactive chemical crosslinker masks the epitopes for the anti-ErbB2 antibodies in the non-denatured condition.
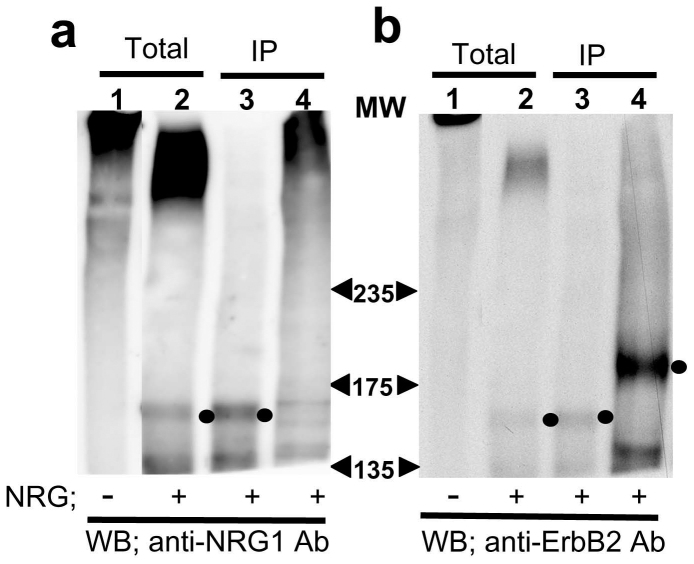
Alternatively, we took the SDS-denatured condition for ErbB2 extraction and then immunoprecipitated the ErbB2 complexes (Fig. 5a, b). In the ErbB2 immune complexes as well as in the total cell lysate, we detected the neuregulin-1-like immunoreactivities slightly below the 175 kDa maker as well as above the 235 kDa marker (lanes 2, 3 in a) whereas there were no apparent neuregulin-1-immunoreactive bands in the absence of neuregulin-1 (lane 1 in A). In contrast, the neuregulin-1-like immunoreactivity in the complexes disappeared when the chemical crosslinkage was digested, suggesting that these immunoreactivities indeed represent the molecular complexes containing neuregulin-1. When we probed the membrane with the anti-ErbB2 antibody, there were ErbB2 immunoreactivities in the immunoprecipitates (lanes 3 in b). However, the molecular size of the immunoreactivities was smaller than the authentic size of ErbB2. The digestion of the crosslinkers uncovered the masked ErbB2 epitopes and re-emerged the ErbB2 immunoreactivity at the authentic size (lane 4 in b). We found similar gel mobility shift of the ErbB2 immunoreactivity when BT474 cells were treated with a chemical crosslinker (data not shown). The phenomenon agrees with the fact that the intramolecular crosslinkage is known to fold and pack a bulky polypeptide chain and increases its gel mobility31.
Figure 5. A crosslink study of neuregulin-1 in ErbB2-overexpressing L929 cells ErbB2-L929 cells were incubated with or without 40 nM cold neuregulin-1 and then exposed to the amine-reactive crosslinker sulfo-EGS.
Total cell lysates (Total) and ErbB2 immunoprecipites (IP) were subjected to Western blotting with the anti-neuregulin-1 antibody (a) or anti-ErbB2 antibody (b). Prior to the electrophoresis, half of the immune complexes were treated with 1 M hydroxylamine to cleave the crosslinkage (lane 4). Arrowheads indicate the positions of molecular weight markers (kDa) Note; the cleavage of the crosslinker conversely elevated ErbB2 immunoreactivity (lanes 4 in b).
Thus, we speculate that that the mobility of ErbB2 was increased by chemical crosslinkage and shifted to a lower molecular range. These results suggest that the high concentration of neuregulin-1 can associate with ErbB2.
Discussion
In the present investigation, we demonstrated the biological actions of neuregulin-1 in distinct cell lines harboring high ErbB2 expression. Treating with 40 nM neuregulin-1 resulted in a significant decrease in thymidine incorporation as well as in ErbB2 phosphorylation in cancer cells with erbB2 amplification. The lower dose of 10 nM neuregulin-1 did not significantly affect thymidine incorporation of SKOV3 cells, however. The anti-proliferative reaction was also found in SKOV3, BT-474 and L929 cells overexpressing ErbB2, but not in the parental L929 cells. The dose-dependency of neuregulin-1 in thymidine incorporation roughly agrees with the profile of the competitive ligand-binding assay; nano molar concentrations of neuregulin-1 were able to replace the cell binding of [35S]-labeled neuregulin-1. The competitive interaction of neuregulin-1 in SKOV3 cells required additional expression of Sulf-1, however. The low-affinity interaction between neuregulin-1 and ErbB2 was ascertained by the crosslink study as well; there was neuregulin-1 immunoreactivity in the ErbB2 immune complexes in ErbB2-L929 cells. Therefore, the present findings suggest that neuregulin-1 challenge exerts anti-proliferative effects, which presumably involve the direct or indirect interaction of neuregulin-1 with ErbB2. However, we confirmed that the biological interaction of neuregulin-1 is significantly influenced by the structures of heparan sulfate moiety on cell surfaces of ErbB2-overexpressing cells20.
There were significant levels of basal tyrosine phosphorylation of the ErbB2 kinase domain in ErbB2-overexpressing cell lines. The high level of ErbB2 phosphorylation in L929 cells was achieved by both transient and permanent transfection of erbB2 cDNA. ErbB2-overexpressing L929 cells responded to neuregulin-1 and exhibited a consistent decrease in ErbB2 phosphorylation at multiple tyrosine residues. In contrast, the parental L929 cells exhibited almost no response to neuregulin-1 in the cell proliferation test and competitive binding assay. There results support the previous findings that the parental L929 cells express a limited level of ErbB2 but no detectable levels of authentic receptors for neuregulin-124,25. With the given proliferative action of ErbB2 auto-phosphorylation7,8,13,14, therefore, we assume that the neuregulin-1-triggered ErbB2 dephosphorylation plays a key role in the attenuation of the cell proliferation observed.
However, these findings and/or the data explanations appear to be contradictory against the previous report that the transfection of erbB2 cDNA to L929 cells fail to induce their interaction with [125I]-labeled neuregulin-1 or alter neuregulin-1-triggered ErbB2 phosphorylation3. The differences from the present results might stem from the fact that serum contains so high concentrations of neuregulin-1 (i.e. ~200 pM for human serum) that serum-supplemented culture media can perturb these assays27. In fact, extensive cell wash or starvation prior to these assays was essential in the present study. Alternatively, it might be illustrated by the fact that the iodination of neuregulin-1 limits its interaction to ErbB2 receptors and perturbs the ligand-binding assay (see Supplemental Figure S2).
Still there is another argument against our conclusion; ErbB4 signaling has been suggested to underlie the anti-proliferative action of neuregulin-1 in some cancer cell lines and might contribute to the observed anti-proliferative phenomena. This scenario might potentially illustrate the results from the SKOV3 cells harboring a low level of ErbB4. However, the lack of ErbB4 expression in ErbB2-expressing L929 and BT-474 limits this explanation32,33. Alternatively, the anti-proliferative action of neuregulin-1 might be ascribed to ErbB2 down-regulation34,35. However, we did not find decreases in total ErbB2 immunoreactivity in SKOV3, BT-474, and ErbB2-L929 cells following acute neuregulin-1 challenge when we used fresh blotting membranes for ErbB2 detection (see Material and Methods). Accordingly, we assume that the neuregulin-1-depedent decrease in the proliferation of SKOV3, BT-474, and ErbB2-L929 cells involves attenuated ErbB2 auto-phosphorylation.
There are many novel molecules identified that regulate homo or hetero dimerization of ErbB2 and potentially modify direct and indirect interaction with neuregulin-1 as well; erbin, decorin, and RALT (receptor-associated late transducer)36. Erbin binds to the carboxyl terminal of ErbB2 and anchors ErbB2 to cytoskeletal networks to regulate its dimerization and protein stability37,38,39. RALT also interacts with the tyrosine kinase domain of ErbB2 and promote its lysosomal degradation40,41,42, although Anastasi et al.41 report the limited expression of RALT in BT-474 and SKBR3. Decorin is suggested to attenuate ErbB2 phosphorylation but involving ErbB4 interaction43. These findings indicate the possibility that the homomeric interaction of ErbB2 or its autophosphorylation can be markedly influenced by these ErbB2-interacting molecules. Thus, the involvement of these ErbB2-interacting molecules might illustrate the previous controversy of the anti-proliferative action of neuregulin-1 among literatures32,33,34,35.
The present immunoprecipitation results provide circumstantial evidence that ErbB2 functions as a low-affinity receptor for neuregulin-1. The given epitope masking of ErbB2 by chemical crosslinkage made our experiments difficult. However, this argument is consistent with a previous study using the ligand-binding assay in SKOV3 cells. [125I]-labeled type 1 neuregulin-1 is indeed replaced with 40 nM cold neuregulin-13. The surface plasmon technique demonstrates the direct interaction between ErbB2 and neuregulin-1; immobilized neuregulin-1 on a solid phase bound to the recombinant extracellular domain of ErbB2 with a calculated dissociation constant (Kd) of 850 nM19, which is one digit larger than the present value, however. The variation in Kd is presumably ascribed to the difference of the experimental procedures; the in vitro solid phase system vs. the natural cell-based binding system. In the ErbB2 immunoprecipiates, we additionally detected the neuregulin-1-like immunoreactivities that carried much larger molecular sizes (>250 kDa). In this context, we do not rule out that the observed dephosphorylation of ErbB2 or the attenuation of cell proliferation may involve unknown receptors for neuregulin-1, which might form the molecular complexes with ErbB244. For example, cell-ubiquitous proteins, integrins are reported to interact directly with neuregulin-145,46. Alternatively, ErbB2 forms the hetero-oligomer with the other receptor tyrosine kinases as well47. Thus, several possibilities and controversies still remain regarding the molecular nature of the neuregulin-1 receptor complexes found in the ErbB2-expressing cells. However the present results, at least, suggest that neuregulin-1 triggers ErbB2 dephosphorylation and the anti-proliferative action in an ErbB2 expression-dependent manner. Thus, our present study reveals and confirms an anti-oncogenic role of neuregulin-1 in ErbB2-overexpressing cancer cells.
Methods
Plasmid construction
cDNA for the human type I neurergulin-1 beta3 isoform (i.e. extracellular domain neuregulin-1; NRG1-ECD) was chemically synthesized by GenScript USA Inc. (Piscataway, NJ, USA) and subcloned into the pET-22b(+) plasmid (EMD Chemical Darmstadt, Germany) or pcDNA TM 3.1-TOPO TA plasmid (Invitrogen Corporation, Carlsbad, CA, USA), both of which carry T7 and/or cytomegalovirus promoters. cDNA for human Sulf-1 was synthesized from human brain RNA by RT-PCR and subcloned into the pcDNA TM 3.1-TOPO TA plasmid (Invitrogen). The expression vectors carrying human erbB1 cDNA and erbB2 cDNA were obtained from a Riken genome bank (Wako, Saitama, Japan)48 and those for erbB3 and erbB4 cDNAs were prepared as reported previously49.
Cell culture
We obtained cell lines, SKOV3, SKBR3, BT-474 and L929 cells, from the American Type Culture Collection (ATCC, Manassas, VA, USA) and SKBR3 cells from Dr. Sakamaki (Niigata University of Pharmacy and Applied Life Sciences). Cells that overexpress ErbB2 were grown in DMEM/F12 medium (Invitrogen, Tokyo, Japan) with 10% heat-inactivated fetal calf serum (Hyclone; Thermo Scientific, Yokohama, Japan). These cells were subjected to experiments within six passages. Mouse L929 cells were transfected with a human erbB2-expression vector and grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% heat-inactived calf serum (CS) (Invitrogen, Tokyo, Japan) for two days (see below). Some of the cells were exposed to genestin G418 (0.4 mg/ml; Sigma-Aldrich Japan, Tokyo, Japan) for three weeks. Cells that were resistant to G418 and thus expressed ErbB2 were cloned. The expression of ErbB2 in the erbB2 transformants was further boosted with the transient transfection of a human erbB2-expression vector for the binding assay or phosphorylation assay.
Cell surface biotinylation
Cells were extensively washed with Hank's balanced salt solution (HBSS) and incubated with 2 mM Sulfo-NHS-biotin (Pierce/Thermo Scientific, Yokohama, Japan) at room temperature for 20 min, after which the reaction was quenched with 50 mM Tris HCl and 10 mM ethanol amine (pH 8.0). Cells were lysed with PIPA buffer [25 mM Tris HCl pH 7.6, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS] containing a protease inhibitor cocktail (Boehringer Ingelheim Pharma Japan, Tokyo, Japan) and mixed with streptavidin beads (Sigma-Aldrich). The biotinylated proteins that were trapped with the beads were liberated by boiling with SDS sample buffer (see below).
Competitive binding assay
According to our method23, [35S]-methionine-labeled neuregulin-1 (type I NRG1-ECD; 2300 dpm/fmol) was synthesized using the reticulocyte lysate translation system (Promega). The pET-22b(+) vector (1 μg) was directly transcribed and translated in vitro in the presence of [35S]-methionine (740 kBq, 43500 TBq/mol; American Radiolabeled Chemical Inc., Saint Louis, MO, USA). The incorporation of [35S] methionine into the protein fraction was estimated using alkaline treatment followed by trichloroacetic acid precipitation. The specificity and authenticity of translated neuregulin-1 was fully evaluated previously28.
L929 cells and SKOV3 cells during the growth phase were plated at a density of 5 × 104 cells/well in poly D-lysine-coated 48-well plates. Cells were extensively washed with Leibovitz -15 Medium (L-15; pH 7.4; Sigma-Aldrich, Japan) containing 0.1% bovine serum albumin (immunoglobulin-free grade; Sigma-Aldrich, Japan) and 10 mM HEPES. [35S]-methionine-labeled neuregulin-1 (16.7 kBq/mL, 8900 Bq/pmol) was mixed with 0–40 nM of a cold competitor of human recombinant neuregulin-1 (type 1 NRG1-ECD; R&D Systems, Minneapolis, MN, USA). Cells were incubated with the tracer mixture on ice for 2 h. After washing with L-15 buffer, L929 cells were lysed with 0.5 N NaOH. The radioactivity of the cell lysates was determined using the Liquid Scintillation System LSC-3050 (Aloka, Tokyo, Japan).
Western blotting
Protein samples were prepared from cultured cells for Western blot analyses. These samples were homogenized in cell lysis buffer [25 mM Tris HCl pH 7.6, and 2% SDS] by sonication and denatured in the presence of 100 μM dithiothreitol (Wako Chemicals, Tokyo, Japan) at 85°C for 5 min. In parallel, the protein concentrations were determined using a micro-BCA kit (Protein Assay Reagent; Pierce/Thermo Scientific). Typically, 20 μg of protein was subjected to SDS-polyacrylamide gel electrophoresis and then transferred onto a nitrocellulose membrane (Advantec, Tokyo, Japan). The membrane was probed with anti-ErbB1 (Eptomics Inc., Burlingame, CA, USA), anti-ErbB2 (Eptomics Inc.), anti-ErbB3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-ErbB4 (Cell Signaling Technology, Danvers, MA, USA), or anti-phospho ErbB2 or anti-phospho ErbB3 antibodies (Cell Signaling; Eptomics Inc.). We used fresh blotting membranes to detect total and phosphorylated ErbB2 proteins as the conventional stripping procedure did not allow us to reuse the membrane that had been treated with the anti-phospho ErbB2 antibodies (data not shown). A blotting membrane was also probed with the anti-Sulf-1 antibody (Abcam, Cambridge, UK). After extensive washing, the immunoreactivity of the membrane was detected with a horseradish peroxidase-conjugated anti-rabbit immunoglobulin followed by a chemiluminescence reaction (ECL kit, GE Healthcare Japan Co., Tokyo, Japan or Immuno Star LD, Wako Chemical, Tokyo, Japan). The intensity of an immunoreactive band, whose size matched the authentic molecular weight, was measured by a CCD camera and an image processing software, GENETOOLS (Syngene, Cambridge, UK). Comparable protein loading was confirmed by Western blotting using an anti-β-actin antibody (Merck Millipore Japan, Tokyo, Japan).
Cell proliferation assay
L929, SKOV3, and BT-474 cells were maintained at a growth phase. Growing cells were plated at a density of 1 × 105 cells/well in 24-well dishes that had been coated with 0.1 mg/ml poly-D-lysine hydrobromide (Sigma-Aldrich, Japan). The cells were allowed to adhere for 20–40 h, and then starved in serum-free DMEM medium containing 10 mM HEPES for 14-20 h. Thirty-seven kilo Bq/ml of [3H]thymidine (Perkin Elmer, Waltham, MA, USA) and various concentrations of recombinant neuregulin-1 (R&D Systems) were added to each well. After 4-h incubation, the cells were washed once with HBSS and then fixed with 5% trichloroacetic acid at 4°C for 45 min. The cells were solubilized in 0.2 ml of 0.5 N NaOH at room temperature. One tenth of the cell lysates was mixed with 5 ml of Ready Value scintillation fluid (Beckman Instruments, Fullerton, CA, USA) and the radioactivity was measured using a liquid scintillation counter LSC-3050 (Aloka).
Chemical crosslinking of neuregulin-1
L929 cells expressing ErbB2 (0.5 − 1.0 × 106 cells) or SKBR3 cells were harvested or incubated with 40 nM human recombinant neuregulin-1 (NRG1-ECD, R&D Systems) at 20°C for 15 min in HBSS. The neuregulin-1 solution was then replaced with 2.0 mM of the crosslinker bis(sulfosuccinimidyl) substrate (BS3; Pierce/Thermo Scientific) or ethylene glycol bis-sulfosuccinimidylsuccinate (Sulfo-EGS; Pierce/Thermo Scientific) and then quenched with 50 mM Tris HCl and 10 mM ethanol amine (pH 8.0). The cells were lysed with PIPA buffer and then immunoprecipitated for ErbB2. Alternatively, cell lysates were prepared by homogenizing cells with a 1% SDS solution at 70°C and then incubated with 0.5M potassium chloride in the presence of 1% NP-40 and a protease inhibitor cocktail (Boehringer Ingelheim) to replace the detergent. The ErbB2 complexes in PIPA buffer were treated with anti-ErbB2 antibodies (C-18; Santa Cruz Biotechnology, Ab-3; Merck Millipore Japan, trastuzumab; Roche, Swiss, or polyclonal 06-562; Merck Millipore Japan) for overnight and precipitated with Protein-G Sepharose (GE Healthcare, Tokyo, Japan). Alternatively, the complexes in the denaturing condition were immunoprecipitated with the ErbB2 antibody used in Western blotting (Eptomics Inc.).
The total cell lysates and the immunoprecipitates were separated using 6% SDS-PAGE and probed with an anti-neuregulin-1 antibody (Santa Cruz Biotechnology) or with an anti-ErbB2 antibody (Eptomics Inc.). To control the efficacy of the ErbB2 immunoprecipitation, some cells were not exposed to the crosslinker and directly subjected to Western blotting for ErbB2. To cleave the chemical crosslinkage of Sulfo-EGS, ErbB2 immunoprecipitates in the Protein-G Sepharose beads were treated with 1 M hydroxylamine (pH 8.5), prior to gel loading.
Author Contributions
R.W. and H.N. conceived and designed the experiments and coordinated the work presented. Y.I., K.A. and N.T. performed the experiments. K.M., M.M., X.W. and S.H. provided materials and commented on the manuscript.
Supplementary Material
Supplementary Figures
Acknowledgments
The authors are grateful to Dr. Sakamaki for SKBR3 cells. This study was, in part, supported by MEXT KAKENHI (HN; No.24116010), JSPS KAKENHI (HN; No. 22300107), and a grant for Promotion of Niigata University Research Projects. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Peles E. et al. Isolation of the neu/HER-2 stimulatory ligand: a 44 kd glycoprotein that induces differentiation of mammary tumor cells. Cell 69, 205–216 (1992). [DOI] [PubMed] [Google Scholar]
- Holmes W. E. et al. Identification of heregulin, a specific activator of p185erbB2. Science 256, 1205–1210 (1992). [DOI] [PubMed] [Google Scholar]
- Peles E. et al. Cell-type specific interaction of Neu differentiation factor (NDF/heregulin) with Neu/HER-2 suggests complex ligand-receptor relationships. EMBO J 12, 961–971 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwkowski M. X. et al. Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J Biol Chem 269, 14661–14465 (1994). [PubMed] [Google Scholar]
- Karunagaran D. et al. ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer. EMBO J 15, 254–264 (1996). [PMC free article] [PubMed] [Google Scholar]
- Graus-Porta D., Beerli R. R., Daly J. M. & Hynes N. E. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J 16, 1647–1655 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve R. M. et al. Effects of oncogenic ErbB2 on G1 cell cycle regulators in breast tumour cells. Oncogene 19, 1647–1656 (2000). [DOI] [PubMed] [Google Scholar]
- Clinchy B. et al. The growth and metastasis of human, HER-2/neu-overexpressing tumor cell lines in male SCID mice. Breast Cancer Res Treat 61, 217–228 (2000). [DOI] [PubMed] [Google Scholar]
- Olayioye M. A. Update on HER-2 as a target for cancer therapy: intracellular signaling pathways of ErbB2/HER-2 and family members. Breast Cancer Res 3, 385–389 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuel E., Akita R. W. & Sliwkowski M. X. Identification of a region within the ErbB2/HER2 intracellular domain that is necessary for ligand-independent association. J Biol Chem 277, 28468–28473 (2002). [DOI] [PubMed] [Google Scholar]
- Slamon D. J. et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177–182 (1987). [DOI] [PubMed] [Google Scholar]
- Ursini-Siegel J., Schade B., Cardiff R. D. & Muller W. J. Insights from transgenic mouse models of ERBB2-induced breast cancer. Nature Reviews Cancer 7, 389–397 (2007). [DOI] [PubMed] [Google Scholar]
- Drebin J. A., Link V. C., Stern D. F., Weinberg R. A. & Greene M. I. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell 41, 697-706 (1985). [DOI] [PubMed] [Google Scholar]
- Nagy P. et al. Lipid rafts and the local density of ErbB proteins influence the biological role of homo- and heteroassociations of ErbB2. J Cell Sci 115, 4251–4262 (2002). [DOI] [PubMed] [Google Scholar]
- Santin A. D., Bellone S., Roman J. J., McKenney J. K. & Pecorelli S. Trastuzumab treatment in patients with advanced or recurrent endometrial carcinoma overexpressing HER2/neu. Int J Gynaecol Obstet 102, 128–131 (2008). [DOI] [PubMed] [Google Scholar]
- Roy V. & Perez E. A. Beyond trastuzumab: small molecule tyrosine kinase inhibitors in HER-2-positive breast cancer. Oncologist 14, 1061–1069 (2009). [DOI] [PubMed] [Google Scholar]
- Tan M. & Yu D. Molecular mechanisms of erbB2-mediated breast cancer chemoresistance. Adv Exp Med Biol 608, 119–129 (2007). [DOI] [PubMed] [Google Scholar]
- Fitzpatrick V. D., Pisacane P. I., Vandlen R. L. & Sliwkowski M. X. Formation of a high affinity heregulin binding site using the soluble extracellular domains of ErbB2 with ErbB3 or ErbB4. FEBS Lett 431, 102–106 (1998). [DOI] [PubMed] [Google Scholar]
- Tzahar E. et al. Bivalence of EGF-like ligands drives the ErbB signaling network. EMBO J 16, 4938–4950 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankonin M. S., Gallagher J. T. & Loeb J. A. Specific structural features of heparan sulfate proteoglycans potentiate neuregulin-1 signaling. J Biol Chem 280, 383–388 (2005). [DOI] [PubMed] [Google Scholar]
- Lafky J. M., Wilken J. A., Baron A. T. & Maihle N. J. Clinical implications of the ErbB/epidermal growth factor (EGF) receptor family and its ligands in ovarian cancer. Biochim Biophys Acta 1785, 232–265 (2008). [DOI] [PubMed] [Google Scholar]
- Khurana A., Beleford D., He X., Chien J. & Shridhar V. Role of heparan sulfatases in ovarian and breast cancer. Am J Cancer Res 3, 34–45 (2013). [PMC free article] [PubMed] [Google Scholar]
- Kihara A. & Pastan I. Cytotoxic activity of chimeric toxins containing the epidermal growth factor-like domain of heregulins fused to PE38KDEL, a truncated recombinant form of Pseudomonas exotoxin. Cancer Res 55, 71–77 (1995). [PubMed] [Google Scholar]
- Schneider B. et al. Potent antitumoral activity of TRAIL through generation of tumor-targeted single-chain fusion proteins. Cell Death Dis 1, e68 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y. et al. Qualitative and quantitative re-evaluation of epidermal growth factor-ErbB1 action on developing midbrain dopaminergic neurons in vivo and in vitro: target-derived neurotrophic signaling (Part 1). J Neurochem 118, 45–56 (2011). [DOI] [PubMed] [Google Scholar]
- Shibuya M. et al. Measurement and comparison of serum neuregulin 1 immunoreactivity in control subjects and patients with schizophrenia: an influence of its genetic polymorphism. J Neural Transm 117, 887–895 (2010). [DOI] [PubMed] [Google Scholar]
- Nagamine S. et al. Organ-specific sulfation patterns of heparan sulfate generated by extracellular sulfatases Sulf1 and Sulf2 in mice. J Biol Chem 287, 9579–9590 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. et al. In vitro production of an active neurotrophic factor, neuregulin-1: qualitative comparison of different cell-free translation systems. Neurosci Lett 497, 90–93 (2011). [DOI] [PubMed] [Google Scholar]
- Chan R., Hardy W. R., Dankort D., Laing M. A. & Muller W. J. Modulation of Erbb2 signaling during development: a threshold level of Erbb2 signaling is required for development. Development 131, 5551–5560 (2004). [DOI] [PubMed] [Google Scholar]
- Brockhoff G., Heiss P., Schlegel J., Hofstaedter F. & Knuechel R. Epidermal growth factor receptor, c-erbB2 and c-erbB3 receptor interaction, and related cell cycle kinetics of SK-BR-3 and BT474 breast carcinoma cells. Cytometry 44, 338–348 (2001). [DOI] [PubMed] [Google Scholar]
- Lodish H. F. & Kong N. The secretory pathway is normal in dithiothreitol-treated cells, but disulfide-bonded proteins are reduced and reversibly retained in the endoplasmic reticulum. J Biol Chem 268, 20598–20605 (1993). [PubMed] [Google Scholar]
- Sartor C. I. et al. Her4 mediates ligand-dependent antiproliferative and differentiation responses in human breast cancer cells. Mol Cell Biol 21, 4265–4275 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka-Cook R. S. et al. Heregulin-dependent delay in mitotic progression requires HER4 and BRCA1. Mol Cell Biol 26, 6412–6424 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. M. et al. Neu differentiation factor induces ErbB2 down-regulation and apoptosis of ErbB2-overexpressing breast tumor cells. Cancer Res 57, 3804–3811 (1997). [PubMed] [Google Scholar]
- Ouyang X., Gulliford T. & Epstein R. J. The duration of phorbol-inducible ErbB2 tyrosine dephosphorylation parallels that of receptor endocytosis rather than threonine-686 phosphorylation: implications for the physiological role of protein kinase C in growth factor receptor signaling. Carcinogenesis 19, 2013–2019 (1998). [DOI] [PubMed] [Google Scholar]
- Segatto O., Anastasi S. & Alemà S. Regulation of epidermal growth factor receptor signaling by inducible feedback inhibitors. J Cell Sci 124, 1785–1793 (2011). [DOI] [PubMed] [Google Scholar]
- Jaulin-Bastard F. et al. Interaction between Erbin and a Catenin-related protein in epithelial cells. J Biol Chem 277, 2869–2875 (2002). [DOI] [PubMed] [Google Scholar]
- Tao Y. et al. Erbin regulates NRG1 signaling and myelination. Proc Natl Acad Sci USA 106, 9477–9482 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslekås C. et al. The inhibitory effect of ErbB2 on epidermal growth factor-induced formation of clathrin-coated pits correlates with retention of epidermal growth factor receptor-ErbB2 oligomeric complexes at the plasma membrane. Mol Biol Cell 16, 5832–5842 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino L. et al. Inhibition of ErbB-2 mitogenic and transforming activity by RALT, a mitogen-induced signal transducer which binds to the ErbB-2 kinase domain. Mol Cell Biol 20, 7735–7750 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasi S. et al. Feedback inhibition by RALT controls signal output by the ErbB network. Oncogene 22, 4221–4234 (2003). [DOI] [PubMed] [Google Scholar]
- Frosi Y. et al. A two-tiered mechanism of EGFR inhibition by RALT/MIG6 via kinase suppression and receptor degradation. J Cell Biol 189, 557–571 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra M., Eichstetter I. & Iozzo R. V. An anti-oncogenic role for decorin. Down-regulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J Biol Chem 275, 35153–35161 (2000). [DOI] [PubMed] [Google Scholar]
- Nakano N. et al. The N-terminal region of NTAK/neuregulin-2 isoforms has an inhibitory activity on angiogenesis. J Biol Chem 279, 11465–11470 (2004). [DOI] [PubMed] [Google Scholar]
- Falcioni R. et al. Alpha 6 beta 4 and alpha 6 beta 1 integrins associate with ErbB-2 in human carcinoma cell lines. Exp Cell Res 236, 76–85 (1997). [DOI] [PubMed] [Google Scholar]
- Ieguchi K. et al. Direct binding of the EGF-like domain of neuregulin-1 to integrins ({alpha}v{beta}3 and {alpha}6{beta}4) is involved in neuregulin-1/ErbB signaling. J Biol Chem 285, 31388–31398 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanizaki J., Okamoto I., Sakai K. & Nakagawa K. Differential roles of trans-phosphorylated EGFR, HER2, HER3, and RET as heterodimerisation partners of MET in lung cancer with MET amplification. Br J Cancer 105, 807–813 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T. et al. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature 319, 230–234 (1986). [DOI] [PubMed] [Google Scholar]
- Kinugasa Y. et al. Neuroglycan C, a novel member of the neuregulin family. Biochem Biophys Res Commun 321, 1045–1049 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures