Abstract
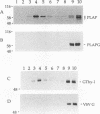
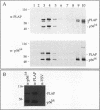
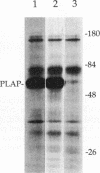
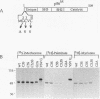
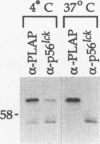
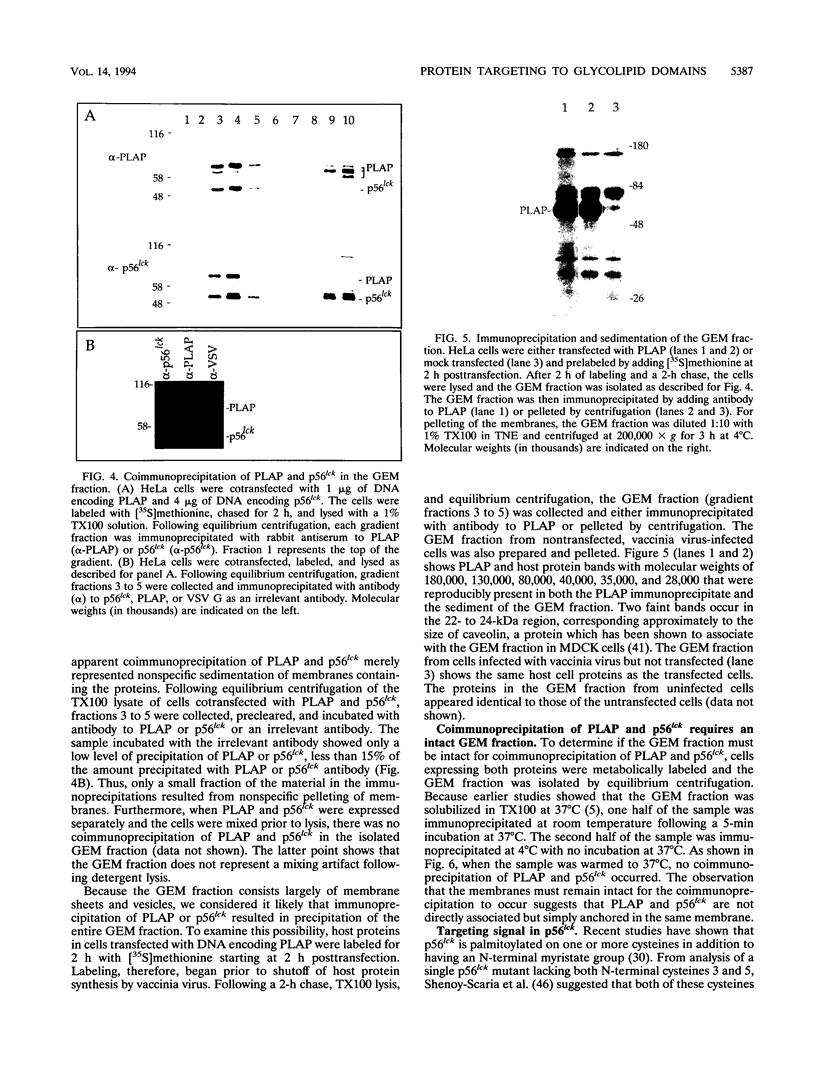
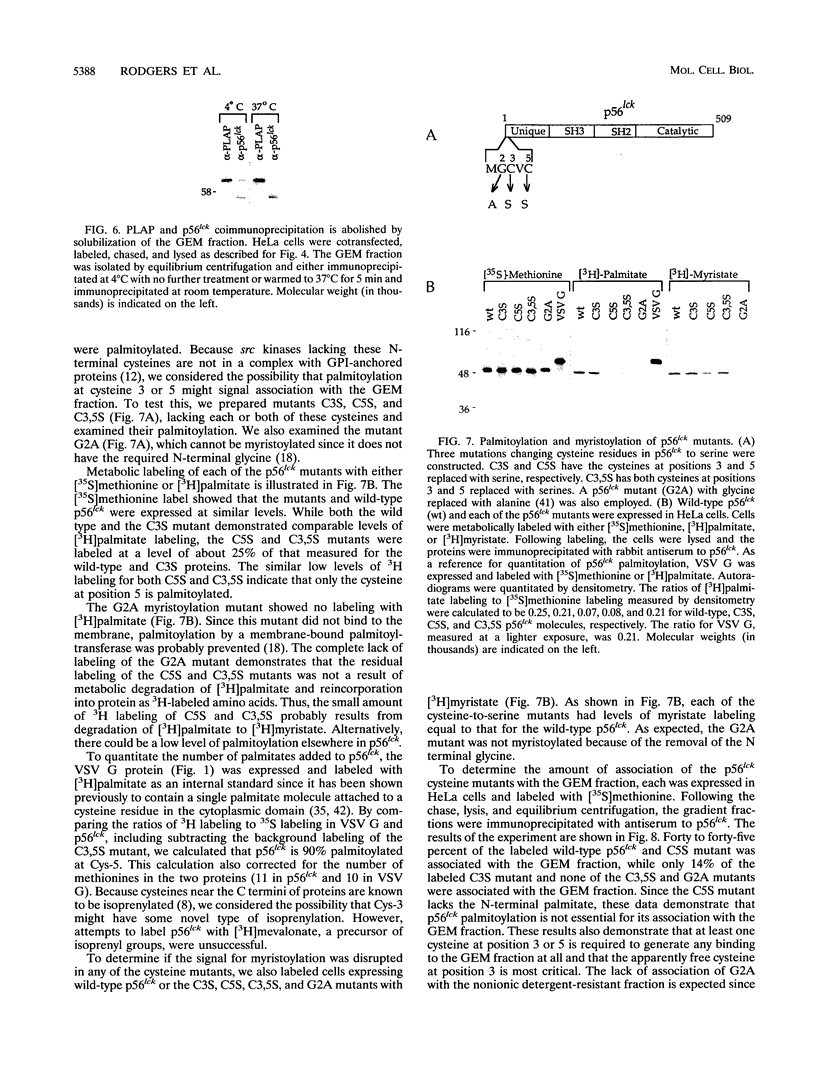
Glycosyl-phosphatidylinositol (GPI)-anchored membrane proteins and certain protein tyrosine kinases associate with a Triton X-100-insoluble, glycolipid-enriched membrane fraction in MDCK cells. Also, certain protein tyrosine kinases have been shown to associate with GPI-anchored proteins in other cell types. To characterize the interaction between GPI-anchored proteins and protein tyrosine kinases, GPI-anchored proteins were coexpressed with p56lck in HeLa cells. Both proteins were shown to target independently to the glycolipid-enriched membranes. Coimmunoprecipitation of GPI-anchored proteins and p56lck occurred only when both proteins were located in the glycolipid-enriched membranes, and gentle disruption of these membranes abolished the interaction. The GPI anchor was found to be the targeting signal for this membrane fraction in GPI-anchored proteins. Analysis of mutants indicated that p56lck was nearly quantitatively palmitoylated at Cys-5 but not palmitoylated at Cys-3. The nonpalmitoylated cysteine at position 3 was very important for association of p56lck with the membrane fraction, while palmitoylation at Cys-5 promoted only a low level of interaction. Because other src family protein tyrosine kinases that are associated with GPI-anchored proteins always contain a Cys-3, we propose that this residue, in addition to the N-terminal myristate, is part of a common signal targeting these proteins to a membrane domain that has been linked to transmembrane signaling.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Berger J., Micanovic R., Greenspan R. J., Udenfriend S. Conversion of placental alkaline phosphatase from a phosphatidylinositol-glycan-anchored protein to an integral transmembrane protein. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1457–1460. doi: 10.1073/pnas.86.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohuslav J., Cinek T., Horejsí V. Large, detergent-resistant complexes containing murine antigens Thy-1 and Ly-6 and protein tyrosine kinase p56lck. Eur J Immunol. 1993 Apr;23(4):825–831. doi: 10.1002/eji.1830230409. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Crise B., Rose J. K. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989 Sep 29;245(4925):1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992 Feb 7;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Cinek T., Horejsí V. The nature of large noncovalent complexes containing glycosyl-phosphatidylinositol-anchored membrane glycoproteins and protein tyrosine kinases. J Immunol. 1992 Oct 1;149(7):2262–2270. [PubMed] [Google Scholar]
- Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- Crise B., Ruusala A., Zagouras P., Shaw A., Rose J. K. Oligomerization of glycolipid-anchored and soluble forms of the vesicular stomatitis virus glycoprotein. J Virol. 1989 Dec;63(12):5328–5333. doi: 10.1128/jvi.63.12.5328-5333.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. S., Patel S. S., Atkinson J. P., Lipsky P. E. Decay-accelerating factor functions as a signal transducing molecule for human T cells. J Immunol. 1988 Oct 1;141(7):2246–2252. [PubMed] [Google Scholar]
- Devaux P. F., Seigneuret M. Specificity of lipid-protein interactions as determined by spectroscopic techniques. Biochim Biophys Acta. 1985 Jun 12;822(1):63–125. doi: 10.1016/0304-4157(85)90004-8. [DOI] [PubMed] [Google Scholar]
- Dráberová L., Dráber P. Thy-1 glycoprotein and src-like protein-tyrosine kinase p53/p56lyn are associated in large detergent-resistant complexes in rat basophilic leukemia cells. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3611–3615. doi: 10.1073/pnas.90.8.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund P. T. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu Rev Biochem. 1993;62:121–138. doi: 10.1146/annurev.bi.62.070193.001005. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M., Mirre C., Quaroni A., Reggio H., Le Bivic A. GPI-anchored proteins associate to form microdomains during their intracellular transport in Caco-2 cells. J Cell Sci. 1993 Apr;104(Pt 4):1281–1290. doi: 10.1242/jcs.104.4.1281. [DOI] [PubMed] [Google Scholar]
- Gascard P., Sauvage M., Sulpice J. C., Giraud F. Characterization of structural and functional phosphoinositide domains in human erythrocyte membranes. Biochemistry. 1993 Jun 15;32(23):5941–5948. doi: 10.1021/bi00074a004. [DOI] [PubMed] [Google Scholar]
- Haverstick D. M., Glaser M. Visualization of domain formation in the inner and outer leaflets of a phospholipid bilayer. J Cell Biol. 1988 Jun;106(6):1885–1892. doi: 10.1083/jcb.106.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James G., Olson E. N. Fatty acylated proteins as components of intracellular signaling pathways. Biochemistry. 1990 Mar 20;29(11):2623–2634. doi: 10.1021/bi00463a001. [DOI] [PubMed] [Google Scholar]
- Kawakami T., Pennington C. Y., Robbins K. C. Isolation and oncogenic potential of a novel human src-like gene. Mol Cell Biol. 1986 Dec;6(12):4195–4201. doi: 10.1128/mcb.6.12.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klages S., Adam D., Eiseman E., Fargnoli J., Dymecki S. M., Desiderio S. V., Bolen J. B. Molecular cloning and analysis of cDNA encoding the murine c-yes tyrosine protein kinase. Oncogene. 1993 Mar;8(3):713–719. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Caras I. W., Davitz M. A., Rodriguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989 Nov;109(5):2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Tang Z. L., Sargiacomo M. Caveolin forms a hetero-oligomeric protein complex that interacts with an apical GPI-linked protein: implications for the biogenesis of caveolae. J Cell Biol. 1993 Nov;123(3):595–604. doi: 10.1083/jcb.123.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maino V. C., Norcross M. A., Perkins M. S., Smith R. T. Mechanism of Thy-1-mediated T cell activation: roles of Fc receptors, T200, Ia, and H-2 glycoproteins in accessory cell function. J Immunol. 1981 May;126(5):1829–1836. [PubMed] [Google Scholar]
- Malek T. R., Ortega G., Chan C., Kroczek R. A., Shevach E. M. Role of Ly-6 in lymphocyte activation. II. Induction of T cell activation by monoclonal anti-Ly-6 antibodies. J Exp Med. 1986 Sep 1;164(3):709–722. doi: 10.1084/jem.164.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D. Lipid-protein interactions in membranes. FEBS Lett. 1990 Aug 1;268(2):371–375. doi: 10.1016/0014-5793(90)81288-y. [DOI] [PubMed] [Google Scholar]
- Martinez R., Mathey-Prevot B., Bernards A., Baltimore D. Neuronal pp60c-src contains a six-amino acid insertion relative to its non-neuronal counterpart. Science. 1987 Jul 24;237(4813):411–415. doi: 10.1126/science.2440106. [DOI] [PubMed] [Google Scholar]
- Mumby S. M., Kleuss C., Gilman A. G. Receptor regulation of G-protein palmitoylation. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2800–2804. doi: 10.1073/pnas.91.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanaswami V., Kim J., McNamee M. G. Protein-lipid interactions and Torpedo californica nicotinic acetylcholine receptor function. 1. Spatial disposition of cysteine residues in the gamma subunit analyzed by fluorescence-quenching and energy-transfer measurements. Biochemistry. 1993 Nov 23;32(46):12413–12419. doi: 10.1021/bi00097a020. [DOI] [PubMed] [Google Scholar]
- Paige L. A., Nadler M. J., Harrison M. L., Cassady J. M., Geahlen R. L. Reversible palmitoylation of the protein-tyrosine kinase p56lck. J Biol Chem. 1993 Apr 25;268(12):8669–8674. [PubMed] [Google Scholar]
- Pelchen-Matthews A., Boulet I., Littman D. R., Fagard R., Marsh M. The protein tyrosine kinase p56lck inhibits CD4 endocytosis by preventing entry of CD4 into coated pits. J Cell Biol. 1992 Apr;117(2):279–290. doi: 10.1083/jcb.117.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S. K., Cunningham B. A., Edelman G. M., Rodriguez-Boulan E. Targeting of transmembrane and GPI-anchored forms of N-CAM to opposite domains of a polarized epithelial cell. Nature. 1991 Sep 5;353(6339):76–77. doi: 10.1038/353076a0. [DOI] [PubMed] [Google Scholar]
- Rodgers W., Glaser M. Characterization of lipid domains in erythrocyte membranes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1364–1368. doi: 10.1073/pnas.88.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W., Glaser M. Distributions of proteins and lipids in the erythrocyte membrane. Biochemistry. 1993 Nov 30;32(47):12591–12598. doi: 10.1021/bi00210a007. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Adams G. A., Gallione C. J. The presence of cysteine in the cytoplasmic domain of the vesicular stomatitis virus glycoprotein is required for palmitate addition. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2050–2054. doi: 10.1073/pnas.81.7.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Altered cytoplasmic domains affect intracellular transport of the vesicular stomatitis virus glycoprotein. Cell. 1983 Sep;34(2):513–524. doi: 10.1016/0092-8674(83)90384-7. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Buonocore L., Whitt M. A. A new cationic liposome reagent mediating nearly quantitative transfection of animal cells. Biotechniques. 1991 Apr;10(4):520–525. [PubMed] [Google Scholar]
- Rothberg K. G., Heuser J. E., Donzell W. C., Ying Y. S., Glenney J. R., Anderson R. G. Caveolin, a protein component of caveolae membrane coats. Cell. 1992 Feb 21;68(4):673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M., Sudol M., Tang Z., Lisanti M. P. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993 Aug;122(4):789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979 Aug;17(4):813–819. doi: 10.1016/0092-8674(79)90321-0. [DOI] [PubMed] [Google Scholar]
- Shaw A. S., Amrein K. E., Hammond C., Stern D. F., Sefton B. M., Rose J. K. The lck tyrosine protein kinase interacts with the cytoplasmic tail of the CD4 glycoprotein through its unique amino-terminal domain. Cell. 1989 Nov 17;59(4):627–636. doi: 10.1016/0092-8674(89)90008-1. [DOI] [PubMed] [Google Scholar]
- Shaw A. S., Chalupny J., Whitney J. A., Hammond C., Amrein K. E., Kavathas P., Sefton B. M., Rose J. K. Short related sequences in the cytoplasmic domains of CD4 and CD8 mediate binding to the amino-terminal domain of the p56lck tyrosine protein kinase. Mol Cell Biol. 1990 May;10(5):1853–1862. doi: 10.1128/mcb.10.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy-Scaria A. M., Gauen L. K., Kwong J., Shaw A. S., Lublin D. M. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol Cell Biol. 1993 Oct;13(10):6385–6392. doi: 10.1128/mcb.13.10.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy-Scaria A. M., Kwong J., Fujita T., Olszowy M. W., Shaw A. S., Lublin D. M. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn 1. J Immunol. 1992 Dec 1;149(11):3535–3541. [PubMed] [Google Scholar]
- Slotte J. P., Ostman A. L., Kumar E. R., Bittman R. Cholesterol interacts with lactosyl and maltosyl cerebrosides but not with glucosyl or galactosyl cerebrosides in mixed monolayers. Biochemistry. 1993 Aug 10;32(31):7886–7892. doi: 10.1021/bi00082a008. [DOI] [PubMed] [Google Scholar]
- Stefanová I., Horejsí V., Ansotegui I. J., Knapp W., Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991 Nov 15;254(5034):1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Thomas P. M., Samelson L. E. The glycophosphatidylinositol-anchored Thy-1 molecule interacts with the p60fyn protein tyrosine kinase in T cells. J Biol Chem. 1992 Jun 15;267(17):12317–12322. [PubMed] [Google Scholar]
- Thompson T. E., Tillack T. W. Organization of glycosphingolipids in bilayers and plasma membranes of mammalian cells. Annu Rev Biophys Biophys Chem. 1985;14:361–386. doi: 10.1146/annurev.bb.14.060185.002045. [DOI] [PubMed] [Google Scholar]
- Voronova A. F., Sefton B. M. Expression of a new tyrosine protein kinase is stimulated by retrovirus promoter insertion. Nature. 1986 Feb 20;319(6055):682–685. doi: 10.1038/319682a0. [DOI] [PubMed] [Google Scholar]
- Whitt M. A., Rose J. K. Fatty acid acylation is not required for membrane fusion activity or glycoprotein assembly into VSV virions. Virology. 1991 Dec;185(2):875–878. doi: 10.1016/0042-6822(91)90563-q. [DOI] [PubMed] [Google Scholar]
- Yi T. L., Bolen J. B., Ihle J. N. Hematopoietic cells express two forms of lyn kinase differing by 21 amino acids in the amino terminus. Mol Cell Biol. 1991 May;11(5):2391–2398. doi: 10.1128/mcb.11.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurzolo C., Lisanti M. P., Caras I. W., Nitsch L., Rodriguez-Boulan E. Glycosylphosphatidylinositol-anchored proteins are preferentially targeted to the basolateral surface in Fischer rat thyroid epithelial cells. J Cell Biol. 1993 Jun;121(5):1031–1039. doi: 10.1083/jcb.121.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]