Abstract
Due to their tunable surface plasmon and photothermal effects, gold nanorods (AuNRs) have proved to be promising in a wide range of biomedical applications such as imaging, hyperthermia therapy and drug delivery. All these applications can be remotely controlled by near infrared (NIR) light which can penetrate deep into human tissues with minimal lateral invasion. AuNRs thus hold the potential to combine both imaging diagnosis and therapeutic treatment into one single system and function as a NIR light-mediated theranostic platform. Herein we review recent progress in diagnostic and therapeutic applications of AuNRs with a highlight on combined applications for theranostic purposes.
Keywords: Gold nanorods, theranostics, cancer therapy, imaging, hyperthermia, drug delivery.
Introduction
In the classical Chinese epic novel Journey to the West, the most powerful weapon of Monkey King to fight demons is Golden-bound Cudgel which can change its size, stretching to reach the sky, or shrinking to the size of an embroidery needle. Today, scientists in the field of nanotechnology have gained the realistic ability to make gold particles at nanoscale which have proven to be a “magic weapon” to fight human diseases. These gold nanoparticles (AuNPs), such as nanospheres, nanorods, nanoshells, nanostars, and nanocages, are of great interest for bio-imaging and disease therapy due to their remarkable capacity to absorb and scatter light, i. e. due to the localized surface plasmon resonance (SPR) effects 1-2. Light scattering is used primarily in imaging techniques such as dark-field microscopy 3-4 and optical coherence tomography (OCT) 5-7. AuNPs also support nonlinear optical imaging, such as two-photon luminescence (TPL) imaging 8-12. Another unique property of AuNPs is that they can efficiently convert absorbed light to heat through the so called “photothermal effect” 13-15. The generated heat can be used to enhance contrast in photoacoustic tomography (PAT) imaging 16-19, for hyperthermia therapy 20-22, or to trigger thermo-sensitive release in drug delivery systems 2, 23-24. In addition, AuNPs are well-known for their nontoxicity, biocompatibility, and chemical inertness 25-26. All of these properties render AuNPs as a promising platform for various biomedical applications, particularly in cancer diagnosis and therapy.
Due to their anisotropic shapes, gold nanorods (AuNRs) exhibit two distinct SPR bands, a weak transverse SPR band at ~ 520 nm, similar to that of gold nanospheres , and an intense longitudinal SPR band which can be tuned from visible to near infrared (NIR, 650 - 900 nm) regions by increasing their aspect ratios 2. The latter forms the basis for AuNRs' in vivo applications, as NIR light only have minimal absorption by skin and tissue, leading to minimal tissue invasion and deeper (up to 10 cm) tissue penetration 13, 27-29. Compared to most other type of nanostructures, AuNRs have larger extinction coefficients (108-1010 M-1 cm-1) and narrower line-widths, with consequently higher photothermal conversion efficiencies and higher sensitivity to changes in the local dielectric constant 2. These properties have given rise to many exciting possibilities to employ AuNRs for NIR-resonant biomedical imaging modalities such as TPL, PAT, OCT, and X-ray computed tomography (X-ray CT), and for hyperthermia therapy and gene/drug delivery.
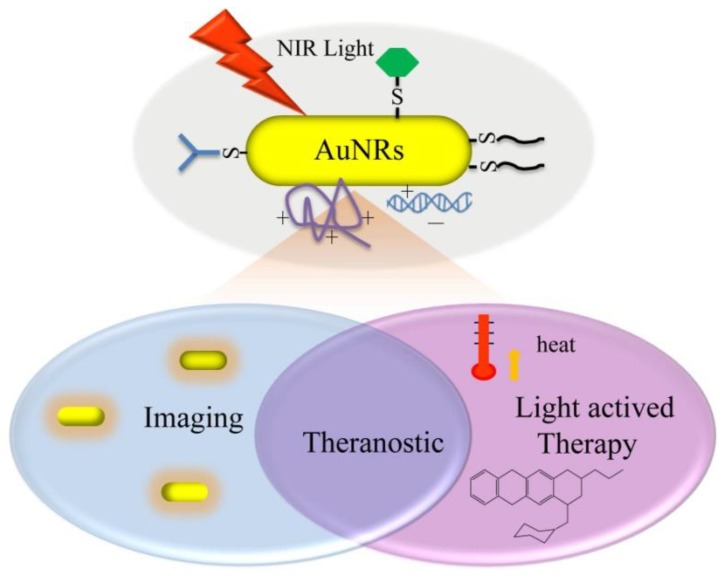
With multiple imaging and therapeutic modalities, AuNRs provide a platform to realize both diagnosis and therapeutic treatment simultaneously in one single system 30-31. These systems follow on the concept of theranostics, the fusion of therapy and diagnostics for optimizing efficacy and safety of therapeutic regimes (Figure 1). One outstanding advantage of AuNRs-based theranostics over other theranostic platforms is that all the functional modalities can be mediated remotely by NIR light with high spatial and temporal precision.
Fig 1.
Illustration of various light-mediated biomedical applications of AuNRs.
In this article, we will first discuss the methods for surface modification and functionalization of AuNRs. The imaging applications of AuNRs will only be reviewed briefly since they have recently been discussed in a few excellent review articles 1-2, 15-16, 25, 27, 32. The applications for hyperthermia therapy, as drug delivery vehicles and combined applications with theranostic implications will be discussed in more details.
Surface modification and functionalization
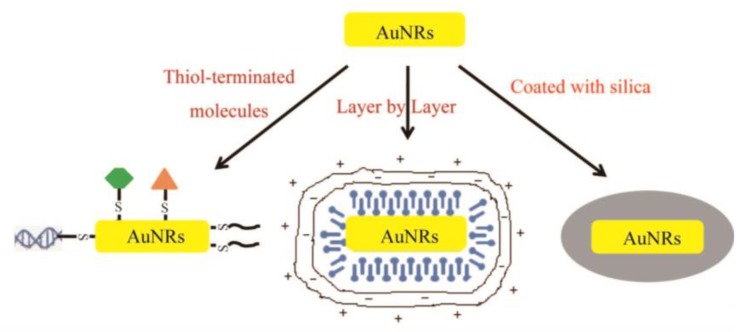
The wet chemical seed-mediated synthesis is the most widely used method for AuNRs preparation because it is facile and offers flexible and fine control over aspect ratios 33-35. AuNRs prepared by this method are coated with the structure-directing surfactant of cetyltrimethylammonium bromide (CTAB) which provides the surface of AuNRs positively charged and thus prevents their aggregation in certain aqueous media via electrostatic repulsion. However, the CTAB-coated AuNRs (CTAB-AuNRs) aggregate rapidly in solutions with high ionic strength such as PBS and cell culture media and CTAB is known to disrupt bio-membrane integrity and thus causes cytotoxicity 36. Therefore, it is necessary to remove or overcoat the residual CTAB and surface modification is required to prepare stable AuNRs dispersions for various biomedical applications. Dispersion and toxicity issues aside, surface functionalization is often needed to add desirable functionalities such as specific targeting, additional imaging modalities and incorporation of an outer layer for drug delivery. The three most widely used methods for surface modification and functionalization are illustrated in Figure 2.
Fig 2.
Three most widely used methods for surface modification and functionalization of AuNRs.
Due to the strong affinity between thiol and Au, the surface modification of AuNRs for detoxification and stable dispersion is overwhelmingly conducted via CTAB displacement with thiolated species 25, 31. The displacement is relatively straightforward, requiring a few hours incubation of AuNRs with an excess amount of thiolated species, such as the most often used thiolated polyethyleneglycol (PEG) 37-39. As a common strategy for preparing biocompatible nanomaterials, PEG coating helps prevent particle aggregation and non-specific protein adsorption, and allows for longer circulation time in blood stream for greater accumulation in tumors. Our group has found that PDDAC-coated AuNRs exhibit more than 10 folds cellular uptake than CTAB-AuNRs with almost no cytotoxicity 36. Some typical cytotoxicity results of AuNRs with different surface coatings are summarized in Table 1. Targeting ligands such as RGD peptide 40, folic acids (FA) and deltorphin 41 can be conjugated at the distal end of thiolated PEG for specific interaction with cells and biomolecules to minimize nonspecific cellular uptake which may cause adverse effects to healthy cells. Some other thiolated species have also been used for surface functionalization of AuNRs, such as thiolated DNA for gene therapy 23,42, thiolated peptides for nuclear targeting 43.
Table 1.
Summary of cytotoxicity of AuNRs with different surface coatings.
| Surface Coatings | Exposure (concentration*, duration) | Cell Types# | Cell Responses | Ref |
|---|---|---|---|---|
| PDDAC, CTAB, PSS | 70 pM, 72 h | MCF-7 | uptake: PDDAC>CTAB>PSS; cytotoxicity: CTAB>PSS>PDDAC |
36 |
| CTAB, PEG | 200 μM (Au atoms), 72 h | A549, 16HBE, MSC |
CTAB-AuNRs: all cell death for A549, 75% of cell viability to 16HBE, nontoxicity to MSC; nontoxicity of PEG-AuNRs | 56 |
| PSS, PEG-SH | 100 pM, 24 h | SKBR3, CHO, C2C12, HL60 |
cell viability: PSS-AuNRs: ~30%; PEG-AuNRs: >90% for SKBR3, CHO and C2C12 cells, 20% for HL60 cells |
57 |
| PDADMAC, PSS, PAH | 150 μM(Au atoms), 6 h | HeLa | PDADMAC coating: highest uptake; >90% of cell viability; no gene overexpression | 58 |
| PAA, PAH | 0.4 nM, 4days | HT-29 | cell viability: PAA-AuNRs: ~90%; PAH-AuNRs: ~90% | 59 |
| PAA | 120 μg/mL, 7 h | SW1222 | cell viability: PAA-AuNRs: >75% | 62 |
| thioalkyl-tri- azole-peptide |
1 nM, 2 h | HaCaT, HSC 3 | much higher cellular uptake of peptide-AuNRs than CTAB-AuNRs; more concentration in nucleus of peptide-AuNRs for cancer cells | 43 |
| mesoporous silica | 122 μM(Au atoms), 72 h | A549 | nontoxicity | 55 |
*: The concentration refers to the amount of AuNRs except the annotations. #: MCF-7: human breast adenocarcinoma cell line; A549: human pulmonary adenocarcinoma cell line; 16HBE: human bronchial epithelial cell line; MSC: mesenchymal stem cells; SK-BR-3: human breast adenocarcinoma cell line; HeLa: human cervical cancer cells; HT-29: human colon carcinoma cells; SW1222: colon adenocarcinoma cell line; HaCaT: human keratinocytes; HSC3: human oral squamous cell carcinoma; SKBR3: mammary adeno -carcinoma; CHO: Chinese hamster ovary, C2C12: mouse myoblast; HL60: human leukemia cell lines.
Another widely used method for surface modification of AuNRs is layer-by-layer polyelectrolyte absorption that relies on the sequential deposition of anionic, then cationic polyelectrolytes to the AuNRs surface 2,44. In this method, negatively charged polystyrenesulfonate (PSS) is often used as a mild detergent and absorbent to remove CTAB by ultrafiltration and ligand exchange. Cationic polyelectrolytes such as poly(diallyldimethylammonium chloride) (PDDAC or PDADMAC) will then be absorbed on the surface of PSS-coated AuNRs. This method allows for easy control over surface groups for attachment of antibodies, proteins, or other targeting molecules, and surface charges which have a significant effect on cellular uptake and bio-distribution of AuNRs. Our group has found that PDDAC-coated AuNRs exhibit negligible cytotoxicity and much greater cell internalization ability than CTAB-AuNRs 36.
A more recent and promising surface modification/functionalization approach is to form mesoporous silica layer around AuNRs. In 2008, Gorelikov and Matsuura reported a single-step synthesis method to coat individual CTAB-capped AuNRs with a thin layer of mesoporous silica 45. In the coating reaction, the localized CTAB molecules on individual AuNRs functioned as the template for the three dimensional polymerization of tetraethyl orthosilicate and thus help to form the mesoporous -structured silica layer. As is well known, mesoporous silica nanoparticles are ideal drug carriers with high surface area, large pore volume, excellent biocompatibility, good chemical and thermal stability, and ease for further functionalization 46-48. Therefore, the silica coating not only solves the CTAB toxicity and dispersion issue of AuNRs but also endows AuNRs with drug delivery and functionalization potentials, opening an exciting window for more versatile applications of AuNRs in biomedicine. So far, a few applications of mesoporous silica-coated AuNRs (Au@SiO2) in imaging 49-52, hyperthermia therapy 53 and drug delivery 54 have been reported separately by different groups. Our group has recently developed Au@SiO2 as a light-mediated multifunctional theranostic platform for cancer treatment 55. More details about this work will be discussed below.
AuNRs as imaging agents
In contrast to conventional organic dyes and fluorophores for NIR imaging applications, AuNRs have several pronounced advantages such as much larger absorption cross sections and more robust chemical and photophysical stabilities. The applications of AuNRs in various imaging techniques have been intensively investigated. In this review, however, we focus on those very recent developments in TPL imaging, PAT, OCT and X-ray CT, which is currently under active research.
Two-photon luminescence imaging
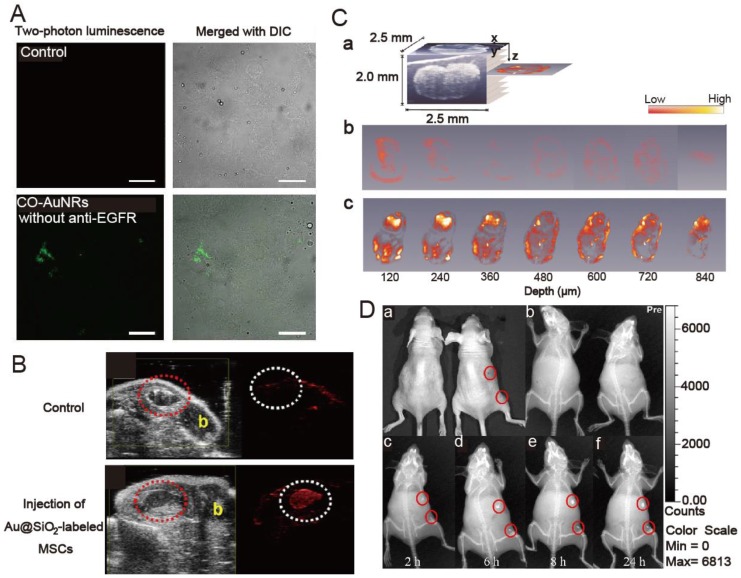
TPL imaging has recently gained popularity due to increased light-penetration depth, submicron spatial resolution, low background fluorescence, and reduced photodamage to living tissues. AuNRs have large two-photon absorption cross sections in the NIR region. Upon ultra-fast laser irradiation, AuNRs emit a strong TPL that can be detected under confocal conditions. The use of AuNRs for TPL imaging has been demonstrated in cultured cells, tissue phantoms, and blood vessels close to the skin surface, such as those in a mouse ear. Recently, TPL imaging was used to demonstrate effective binding of epidermal growth factor receptor (EGFR)-targeted AuNRs (anti-EGFR-AuNRs) both in human epithelial carcinoma cell line cells (A431) in vitro and A431 tumors in vivo 60. The anti-EGFR-AuNRs were observed at depths up to 100 μm in the tumors. Selective targeting and accumulation of chitosan oligosaccharide-modified AuNRs were also observed in vitro via TPL imaging studies in human oral adenosquamous carcinoma cell line (CAL 27) 11, consistent with in vivo targeting studies performed via NIR laser irradiation in CAL 27 xenograft tumors (Figure 3A).
Fig 3.
(A) Two-photon luminescence and merged image with differential interference contrast (DIC) of chitosan oligosaccharide-modified AuNRs with and without anti-EGFR conjugation in Cal 27cells. Cells were incubated with 0.1 μg gold nanorods for 4 hours. Scale bar, 10 μm. Adapted with permission from 11 (B) In vivo backscatter mode ultrasound (gray scale) and PAT (red) images of the intramuscular injection of a negative control (0 nM Au@SiO2, no cells), and 800 000 Au@SiO2-labeled MSCs all in 50% matrigel/PBS into hind limb muscle of an athymic mouse. The “b” in panels indicates bone and the red dashed circle highlights the injection. Adapted with permission from 17. (C) Cross-sectional photothermal OCT images obtained at different depths below the surface by slicing the 3D data cube (shown in (a)) acquired from the SLN at the time point of (b) 12 and (c) 96 h after AuNRs injection. Adapted with permission from 7. (D) In vivo X-ray imaging of mice after subcutaneous injection without and with folic acids-conjugated Au@SiO2 at different time points. (a) The photograph of mice; the X-ray images at (b) 0 h, (c) 2 h, (d) 6 h, (e) at 8 h, and (f) 24 h. Adapted with permission from 51.
Photoacoustic tomography
PAT, an emerging imaging technique analogous to ultrasound, can offer non-invasive optical imaging at even greater depth into tissue than TPL imaging. AuNRs can also support optoacoustic properties that rely on absorption of pulsed laser to create an acoustic shockwave owing to transient superheating and thermoelastic expansion. The acoustic signal will be measured and converted into an image by a scanning transducer. Very recently, Yang et al. reported intracellular AuNRs imaging using their homemade photoacoustic microscopy. The time-dependent uptake and distribution of the AuNRs in human breast adenocarcinoma cell line were successfully monitored 61. Jokerst et al. reported PAT imaging of mesenchymal stem cells in living mice via Au@SiO2 (Figure 3B) 17. The authors found that the silica layer both enhanced the photoacoustic signal of AuNRs and increased uptake of the AuNRs into the cells. This Au@SiO2-mediated PAT imaging method has low background and high spatial and temporal resolutions, both of which are significant advantages over traditional cell imaging techniques like positron emission tomography and magnetic resonance imaging.
Optical coherence tomography
OCT is a relatively recent imaging modality that captures micrometer resolution, three-dimensional cellular and subcellular tissue morphology using the coherence-gated detection of scattered NIR light. AuNRs accumulated in biological tissue of interest can significantly enhance contrast due to intense optical absorption or scattering. Recently, Jung et al. reported OCT imaging of AuNRs uptake in sentinel lymph node (SLN) of mice in situ (Figure 3C) 7. Compared to conventional scattering OCT, the AuNRs-mediated OCT system obtained 3-D images with higher resolution, based on which different time-dependent accumulation of AuNRs within several SLN structures were demonstrated. The average concentration of AuNRs within the whole SLN could be calculated by summing the OCT signal strength.
X-ray computed tomography
As one of the most widely used diagnostic tools in hospitals today, X-ray CT is an imaging technique based on X-ray absorption difference (absorption contrast) of the body compositions. Due to their high atomic weight and X-ray absorption coefficient, small iodinated molecules are generally used as contrast agents to improve the visibility of internal body structures in X-ray CT procedures. However, these contrast agents only allow very short time imaging due to rapid renal excretion, and may also cause renal toxicity. Recently, AuNRs have attracted much attention for the development of new CT imaging agents because gold has a higher atomic weight and X-ray absorption coefficient than iodine. It has been demonstrated that PEG-coated AuNRs (PEG-AuNRs) have a much longer circulation time than a typical iodine-based molecular contrast agent. More importantly, AuNRs conjugated with targeting ligand such as peptides or antibodies can enable visualization of disease -specific biomarkers at the molecular levels through CT procedure. Luo et al. reported indocyanine green (ICG)-loaded Au@SiO2 for the dual capability of X-ray CT and fluorescence imaging 52. It was demonstrated that Au@SiO2 could enhance CT contrast significantly and multiplexed images can be easily obtained by using this dual mode imaging agent. In another paper, the same group also showed that X-ray imaging could be employed to monitor the tumor targeting ability of folic acid-conjugated Au@SiO2 during the whole blood circulation (Figure 3D) 51.
AuNRs for hyperthermia therapy
The major therapeutic application of AuNRs is to treat cancers as hyperthermia agents. Upon high power laser irradiation, AuNRs can raise the temperature of its immediate environment by hundreds of degrees due to high efficient and extremely fast photothermal conversion 1. This localized superheating effect can be directed to eradicate the diseased tissue such as cancer, providing a noninvasive alternative to surgery. The photothermal effects of AuNRs have been well established on cultured tumor cells and tumor xenografts in mice, as well on parasitic protozoans, macrophage and bacterial pathogens. Some typical experiment conditions and therapeutic effects of AuNRs induced hyperthermia are summarized in Table 2.
Table 2.
Selective targeting and therapeutic effects of AuNRs induced hyperthermia.
| Materials with target molecules | Dose | Incubation/ Circulation time |
Laser Parameters | Hyperthermia Effects | Ref |
|---|---|---|---|---|---|
| AuNR-folate | 105 cells/mL, 100 μL 0.2 nM AuNRs | N/A | fs*-pulsed laser, 765nm, 3 mW, 61.5 s | membrane blebbing; cell necrosis | 21 |
| AuNR-PEG | direct injection: 15 μL (ODλmax800 = 40); intravenous administration: 100 μL (ODλmax800 = 120) | intramural injection: 2 min; intravenous administration: 24 h |
cw*, 808 nm, 6mm diameter spot,10min. direct injection: .9-1.1W/cm2; intravenous administration:1.7-1.9W/cm2 |
intramural injection: resorption of >57% (13days) intravenous administration: resorption of the tumors of 25% (13days) |
20 |
| A33scFv-PAA-AuNR | N/A | 5 h | cw, 808nm, 5.1W/cm2, 5 min | >62% cell death | 62 |
| anti-EGFR-PSS- AuNRs |
106 cells, 6.023×1011 AuNRs | N/A | cw, 650nm, 0.3mW/cm2, 3 min | 92% mortality of the carcinoma cells; unclear boundary, membranous vesicles and fragmentation of cells | 63 |
| RGD-AuNRs | in vitro: 100 μg/mL AuNRs; in vivo: 200 μg, intravenous administration | in vivo: 6 h | in vitro: 808 nm, 4-20W/cm2, 4min; in vivo: 808 nm, 24W/cm2, 5 min, irradiation for four times per month, once every week | all cancer cells were killed within the laser spots; disappearance of tumors implanted in four sample mice from test group of ten | 64 |
*: A laser can be classified as operating in either continuous or pulsed mode, depending on whether the power output is continuous over time or whether its output takes the form of pulses of light on one or another time scale. CW is the abbreviation of continuous wave. fs-pulsed laser means that a laser can generate pulses of light as short as a few femtoseconds (10−15 s).
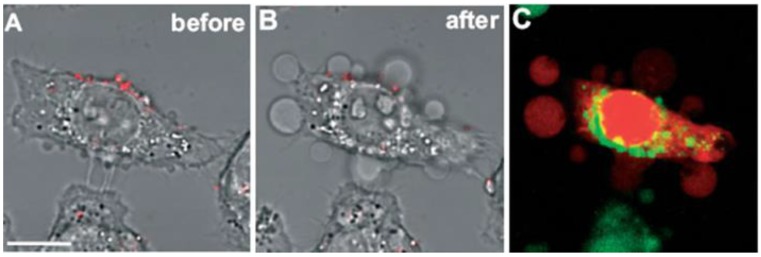
Tong et al. did excellent work to study the mechanism of photo-induced cell necrosis by AuNRs 1, 21. The authors found that photothermolysis of cells was most effective when AuNRs were adsorbed to the cell surface prior to uptake, and an influx of extracellular Ca2+ was required to produce membrane bleb formation that was in strong correlation with a gross redistribution of the cytoskeletal infrastructure. They thus attributed the cell death to the disruption of the plasma membrane which was most likely a consequence of AuNRs-mediated cavitation resulted from the photoacoustic effects (Figure 4).
Fig 4.
Membrane blebbing is induced by Ca2+ influx during AuNRs-mediated photothermolysis. (A,B) Cells with membrane-bound AuNRs (red) in PBS containing 0.9 mM Ca2+ exhibited blebbing after exposure to fs-pulsed laser irradiation at 3 mW for 61.5 s. (C) Incubation with 2.5 μM EB (red) and 2 μM Oregon Green 488 for 20 min indicated a compromise in membrane integrity and an elevation in intracellular Ca2+ , respectively. Scale bar = 10 μm. Adapted with permission from 21.
Dickerson et al. reported the potential of using AuNRs for in vivo photothermal therapy by using PEG-AuNRs 20. The nanoparticles were delivered to subcutaneous squamous cell carcinoma xenografts grown in nude (nu/nu) mice by both direct and intravenous injection. Specific tumor growth suppression from both direct and intravenous NIR laser induced photothermal therapy treatments were observed with no statistically significant differences. Obvious resorption of the tumors indicated that the potential curative applications of AuNRs-mediated photothermal therapy in pre-clinical settings.
AuNRs-mediated hyperthermia is inherently localized, but specifically targeted AuNRs are expected to be much more effective at avoiding photothermal ablation of healthy cells which may result from a nonspecific uptake of AuNRs. Kirui et al. prepared poly(acrylic acid) (PAA)-coated AuNRs with an antibody (A33scFv, a single-chain antibody) conjugated for selective colorectal carcinoma targeting 62. Selective targeting and uptake of AuNRs in antigen expressing cells compared to antigen free cells were observed by using fluorescence microscopy. Upon irradiation with NIR laser for 5 min, >62% cells were killed through AuNRs-mediated photothermal therapy, while the viability of HT29 cells remained almost unchanged, indicating that the targeted A33-AuNRs conjugates are potent photothermal absorbers in cells that express A33 antigen. Rejiya et al. demonstrated the anti-cancer potential of PSS-coated AuNRs with EGFR antibody conjugated (anti-EGFR-AuNRs) 63. The efficient uptake of anti-EGFR-AuNRs by A431 cells was confirmed by inductively coupled plasma atomic emission spectroscopy (ICP-AES) and immunofluorescence studies. Results show that about 87.5% of anti-EGFR conjugated gold nanorods was extensively took up by cell within 3 h when compared to unconjugated gold nanorods i.e. 64% in 3 h. The efficacy of the anti-EGFR- AuNRs coupled with laser treatment was evident with 92% mortality of the carcinoma cells, while cells given unconjugated AuNRs and laser treatment exhibited only 9% cell death. Flow cytometry analysis showed that anti-EGFR-AuNRs could selectively destruct the cancer cells and induce its apoptosis through ROS-mediated mitochondrial pathway under low power laser exposure.
The in vivo tumor targeting and photothermal therapy was demonstrated by Li et al. using RGD-conjugated dendrimer-modified AuNRs (RGD-AuNRs) 64. It was demonstrated that RGD-AuNRs could specifically target ανβ3 integrin overexpressed tumor cells both in vitro and in vivo and thus selective destructive effects on solid tumors and even complete tumor removal under NIR laser irradiation were achieved. Results show that 17% of the RGD-AuNRs accumulated in local tumor tissues at 6 h after injection and only 7% of RGD free AuNRs reached the tumor tissues at the same time, which proved the active targeting effects of RGD-AuNRs. Therefore, the RGD-AuNRs based targeting photothermal therapy hold the potential to extend to various tumors with overexpressed ανβ3 integrins.
Although the targeting on cancer cells is generally achieved by adding active ligands for specific recognition of membrane biomarkers, Zhou at al. reported zwitterionic phosphorylcholine (PC) as a new ligand for AuNRs stabilization with unexpected specific cancer targeting abilities 65. It was demonstrated that the conjugation of PC onto AuNRs brought good dispersion stability and low cytotoxicity. Cell internalization studies by ICP-AES, transmission electron microscopy (TEM) and UV-Vis spectroscopy all supported that PC-coated AuNRs could selectively enhance cell uptake within cancer cells over related normal ones. The ICP-AES results also reflected that the number of PC-coated AuNRs within each cell surpassed more than two times the number of PEG-coated AuNRs. Selective accumulation made them able to kill cancer cells at a low laser energy which did not harm the neighboring normal ones.
AuNRs as drug and gene delivery vehicles
On-demand drug release can be triggered and mediated by the unique environments of tissues or external stimuli. Some excellent delivery systems responding to the slightly acidic pH or specific protease activity of tumor tissues have been successfully applied in tumor therapy 46, 66-67. As the external stimulations, light, magnetic fields, ultrasound, electricity and heat is the most commonly used switch/mediator for release control 68. The use of light as a remote-activation mechanism for drug delivery has received increased attention due to its advantages in highly flexible control of dosage and timing 24, 69. When AuNRs are used as drug delivery vehicles, that is, drugs are physically absorbed or chemical conjugated onto the surface of AuNRs, the dissipation of absorbed light energy as heat can help to release drugs actively. In other words, light-controlled drug delivery can be achieved through AuNRs mediated photothermal effects. For these reasons, AuNRs have been applied as drug delivery vehicles responding to NIR light irradiation.
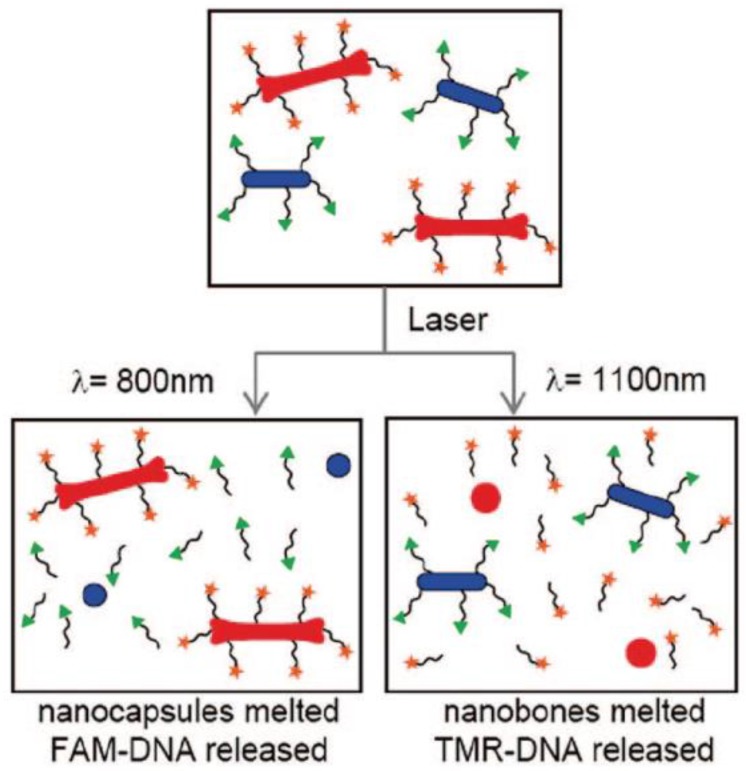
DNA and RNA are the earliest “drugs” delivered by AuNRs partly because they can be easily absorbed or chemically conjugated onto the surface of AuNRs and are able to keep their functions after being released. In 2005, Niidome and coworkers reported a Phosphatidylcholine (PC) coated AuNRs with immobilized plasmid DNA, release of which was induced by pulsed NIR laser irradiation 70. The authors attributed the reason of the release to the surface area decrease resulted from morphological changes of AuNRs (reshaping and fusion) upon laser irradiation. Later, the same group reported AuNRs-embedded N-isopropylacrylamide hydrogels, which have thermo-sensitive properties 71. Obvious phase transition behavior induced by NIR laser irradiation through the photothermal conversion characteristics was observed under optical microscopy. When a gel containing a model drug (rhodamine-labeled dextran) was irradiated, the rapid irradiation-point specific release of the drug was observed under fluorescence microscope. More recently, they reported a controlled-release system mediated by a photothermal effect induced retro Diels-Alder Reaction, which is known to proceed at 90℃ 72.Hamad-Schifferli and co-workers demonstrated the ability to load and selectively release two different DNA oligonucleotides from a mixture of two different AuNRs by using NIR laser corresponding to their SPR maximum wavelengths 23. This work suggests AuNRs' potential applications in delivering more than one drugs sequentially for combination therapy (Figure 5).
Fig 5.
Overview of selective release. Laser irradiation of DNA-conjugated nanocapsules (blue ovals) and nanobones (red bones) are exposed to 800 irradiation (left), which melts the nanocapsules and selectively releases the conjugated DNA (labeled by FAM (green triangles)). Exposure to 1100 irradiation (right) melts the nanobones, selectively releasing the conjugated DNA (labeled by TMR (orange stars)) 23.
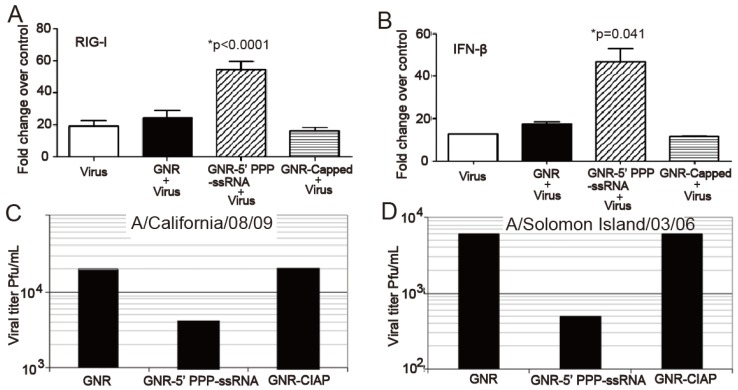
Chakravarthy and coworkers employed AuNRs to deliver an innate immune activator (ssRNA) against type A influenza virus 73. They prepared nanoplexes by conjugating the ssRNA onto AuNRs (nanoplexes) through electrostatic interaction and evaluated their efficacy in human respiratory bronchial epithelial cells infected with type A influenza virus. The intracellular delivery of the nanoplexes could be easily observed from the strong orange-red light scattering of AuNRs using dark-field microscope. Based on TEM observations, the author postulated that the nanoplexes might be taken up by classical pinocytotic mechanisms of uptake. RT-PCR and western plot results showed that the nanoplex activated the retinoic acid-inducible gene I (RIG-I) pathogen recognition pathway, resulting in increased expression of IFN-β and other IFN-stimulated genes (ISGs) and this increase in type I IFN and ISGs resulted in a decrease in the replication of H1N1 influenza viruses (Figure 6).
Fig 6.
GNR-5′PPP-ssRNA enhances IFN-β and RIG-I expression and inhibits replication of 2009 pandemic H1N1 influenza viruses and Solomon Islands seasonal flu strain. A459 cells (3.5×105cells/well) in a six-well tissue culture plate were mock transfected or transfected with 3 μg of RNAs complexed with 2.5 μg of GNRs per well for 48 h and then infected with A/California/08/09 or A/Solomon Islands/03/06 at an MOI of 1. Lysates to determine mRNA levels by qRT-PCR (A and B). The viral titers were determined from the supernatants collected 24 h later (C and D) 73. (GNRs: gold nanorods; CIAP: calf intestinal alkaline phosphatase)
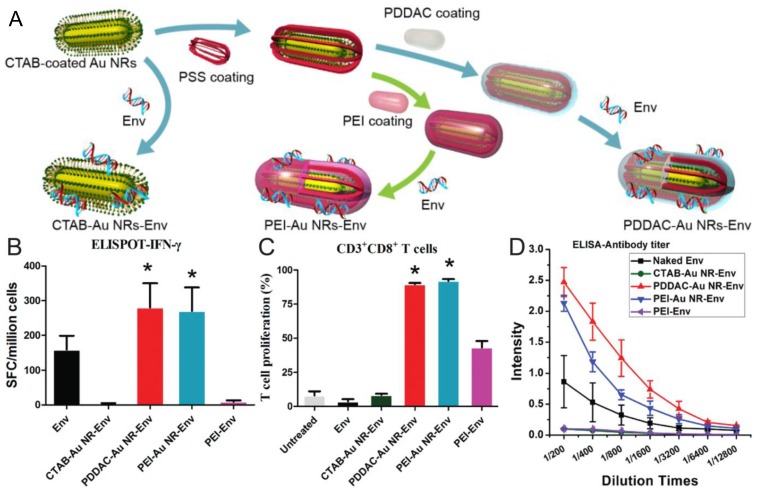
Recently, our group demonstrated that PDDAC- and PEI-coated AuNRs could be used as DNA vaccine adjuvant for HIV treatment, that is, HIV-1 Env plasmid DNA was delivered into host cells by these two surface-modified AuNRs for enhanced immunity 48. Our in vitro results demonstrated that PDDAC- and PEI-coated AuNRs had good transfection capability. Both cellular and humoral immunity were enhanced significantly when mice were immunized with these two DNA-conjugated AuNRs. For the PDDAC group, the type of immune response was Th2-biased. Based on the findings that both the two AuNRs could promote the maturation of dendritic cells (DCs) while CTAB-AuNRs could not, we postulated that DC maturation might be the mechanism for the positive effects of PDDAC- and PEI-coated AuNRs as vaccine adjuvant/delivery systems. To the best of our knowledge, this is the first report on the application of AuNRs as DNA vaccine adjuvants (Figure 7). These findings will shed light on the rational design of low-toxic nanomaterials as a versatile platform for vaccine nanoadjuvants/delivery systems.
Fig 7.
The AuNRs-HIV Env plasmid complex formed and the effects of AuNRs on the immune response and dendritic cell maturation. (A) AuNRs with different surface coatings mixed with Env plasmid. (B) IFN-γ analyzed by ELISPOT. (C) CD3+CD8+ T cells proliferation. (D) The Env 69 specific antibody titer measurement. Adapted with permission from 48.
Min et al. employed AuNRs as drug delivery vehicles for a small molecular weight drug, cisplatin 74. The drug delivery system was constructed by tethering a Pt (IV) prodrug to amine functionalized PEG-AuNRs. This delivery system is stable under physiological conditions with significantly higher cytotoxicity compared with free cisplatin on different types of cancer cells. This result provides an efficient approach to improve the efficacy of platinum drugs in cancer treatment.
Combined applications
There is currently considerable effort to develop theranostic nanoscale systems for more effective treatment of cancer. The unique physicochemical properties endow nanomaterials with great potential to integrate imaging diagnosis and therapeutic treatment into a single nanoplatform for combined cancer treatments such as imaging -guided therapy and therapeutic monitoring following treatments. Development of such multifunctional theranostic systems with individual functions acting in a coordinated way is critical to optimize therapeutic efficacy and safety of therapeutic regimes, and could provide more opportunities for on-demand therapy and pave the road toward personalized medicine.
Although AuNRs are inherently capable of various types of imaging and therapeutic functionalities, more functional modalities such as conventional fluorescence imaging, active targeting, and controlled drug release can be readily added due to various robust surface modification methods available. This provides great opportunities for the combination application of AuNRs in biomedicine. In this section, we review such combined applications for theranostic purpose such as image-guided therapy. Some recent typical combined applications of AuNRs are summarized in Table 3.
Table 3.
Typical combined applications of AuNRs.
| Surface Group | Targeting Ligand | Imaging Modalities | Drug Delivered | Therapeutic Characteristics | Ref |
|---|---|---|---|---|---|
| PSS | EGFR antibodies | light scattering of AuNRs | none | half laser energy needed for malignant cells to be photothermally destroyed than nonmalignant cells | 75 |
| PEG | folic acids | TPL | none | extensive cell membrane blebbing by hyperthermia | 76 |
| PEG | Deltorphin | AuNRs luminescence | none | selective destruction of receptor-expressing cells while sparing receptor-free cells | 41 |
| PSMA | none | ICG luminescence | none | hyperthermia combined with PDT with improved photo-destruction efficacy | 79 |
| Cy5.5 -peptide |
MMP substrate peptide | Cy5.5 fluorescence | none | simultaneous in vivo diagnosis and photothermal therapy | 78 |
| PEG | CET | NIR absorption of AuNRs | none | extensive pyknosis and cell vacuolization in tumor tissue by hyperthermia | 77 |
| PSS, PDDAC | none | light scattering of AuNRs | siRNA | significant impact on suppression of GAPDH gene expression (70% gene silencing, >10 days post-injection) | 80 |
| PDDAC | none | TEM | cisplatin | with AuNRs heating, drug dosage lowered to roughly 33% of the unheated amount to achieve comparable cytotoxicity | 81 |
| PEG, dDNA | folate | Cy3 luminescence |
DOX | selective drug delivery to target cells, effective inhibition of tumor growth through thermo-chemotherapy | 82 |
| mesoporous silica | none | TPL | DOX | two therapeutic modes of chemotherapy and hyperthermia, switchable by changing laser power density | 55 |
Combination of imaging and hyperthermia therapy
In 2006, El-Sayed and coworkers first developed AuNRs as reagents for simultaneous molecular imaging and photothermal cancer therapy 75. They prepared anti-EGFR -AuNRs and applied them to treat malignant oral epithelial cell lines. Using dark field imaging based on light scattering of AuNRs, the malignant cells were clearly visualized and diagnosed from the nonmalignant cells. Based on the extinction spectra of AuNRs in single cells, anti-EGF-AuNRs bound specifically to the surface of the malignant type cells with a much higher affinity due to the overexpressed EGFR on the membrane of the malignant cells. As a result of preferable binding of anti-EGFR -AuNRs, malignant cells required about half the laser energy to be photothermally destroyed than the nonmalignant cells upon exposure to continuous NIR laser.
Since the application in both imaging and hyperthermia therapy doesn't require AuNRs to be further functionalized, imaging-guided hyperthermia therapy has been the most investigated combination among all possible combinations of various applications of AuNRs. Targeting molecules are often conjugated to AuNRs for specific cancer detection and therapy. Huff et al. compared the cell internalization behavior of CTAB-AuNRs and FA-AuNRs using TPL imaging 76. The former was found to be internalized in to KB cells within hours while the latter accumulated on the cell surface over the same time interval. In either case, AuNRs-mediated heating could produce severe blebbing in cell membranes and render them permeable to chemical regents. Black et al. attached deltorphin, a high-affinity ligand for delta opioid receptor (δOR) to AuNRs through a thiol-terminated PEG linker and demonstrated selective imaging and destruction of receptor-expressing cells while sparing those cells that did not express the receptor in a mixed population of cells 39. Choi et al. conjugated cetuximab (CET), an epithelial cancer cells targeting molecules, to PEG-AuNRs (CET-AuNRs) and accessed their targeting, imaging and hyperthermia therapy behavior 77. ICP-AES results showed that CET-AuNRs preferably to A431 cells over MCF-7 cells. The cell internalization of CET-AuNRs in A431 cells was clearly observed by TEM. In vivo NIR absorption imaging results showed that CET-AuNRs accumulated in the tumor region after intravenously injected while CET free AuNRs did not, consistent with the results obtained by ICP-AES. After NIR laser irradiation, histological analysis of excised tumor tissue showed that CET-AuNRs-treated tumors exhibited severe cellular damage compared to non-treated controls.
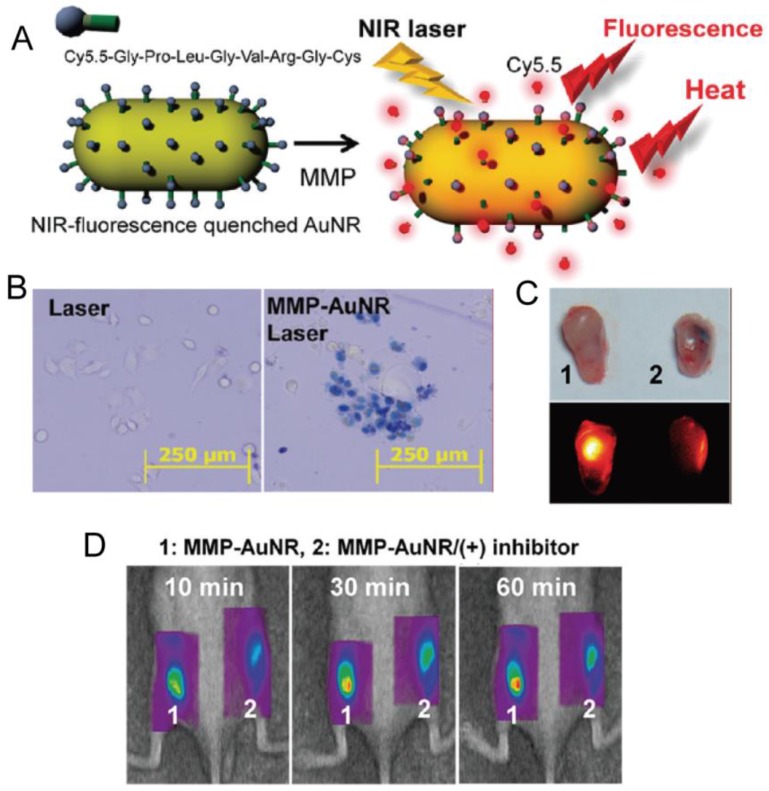
Yi et al. designed and prepared a NIR fluorescent dye coupled AuNRs through a peptide linker, which is known to be degraded by matrix metalloprotease (MMP) 78. MMP is a family of zinc-dependent proteins and the production of MMPs are distinctly involved in cancer metabolism. For the quenching effect of AuNRs, NIR fluorescence was quenched until the substrate was degraded by MMP enzymes which were secreted by cancer cells. By the recovery of quenched fluorescence, the authors determined the expression of MMP and cancer progress as well as performed hyperthermia cancer therapy simultaneously based on photothermal effects of AuNRs (Figure 8).
Fig 8.
(A) Schematic Representation of Synthesis of MMP-AuNRs for Simultaneous Imaging and Photothermal Therapy. (B) Microscopic images of HeLa cell after staining with trypan blue at different treatment conditions. (C) Optical and NIR fluorescent images of excised tumor after injection of MMP-AuNRs without and with MMP-2 inhibitor. (D) NIR fluorescent tomographic images of SCC-7 tumor-bearing mice after intratumoral injection of the MMP-AuNRs probe without (1) and with (2) inhibitor. Adapted with permission from 78.
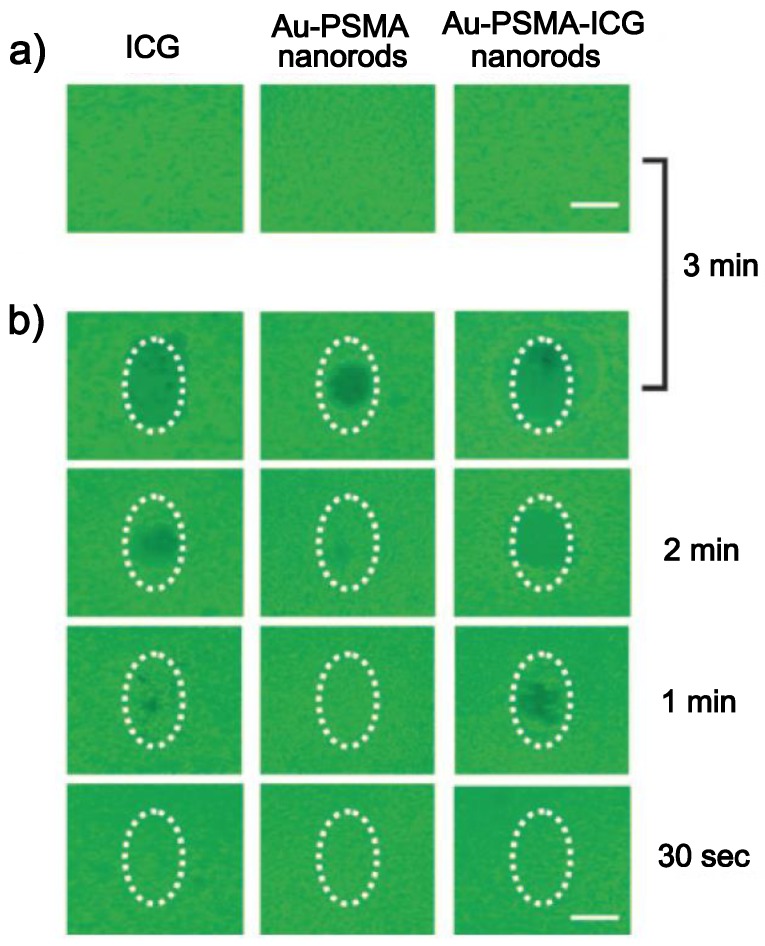
Kuo et al. coated AuNRs with poly(styrene-altmaleicacid) (PSMA) and indocyanine green (ICG), a hydrophilic and anionic photosensitizer, in sequence via electrostatic interaction, and developed them as both photodynamic therapy (PDT) and hyperthermia agents 79. It was demonstrated that combined PDT and hyperthermia could more efficiently extinguish cancer cells than PDT or hyperthermia treatment alone, and the system could also serve as an effective bio-imaging probe in the NIR region based on the fluorescence of ICG (Figure 9).
Fig 9.
Photodestruction of A549 malignant cells shown by fluorescence. ICG, Au-PSMA nanorods, and Au-PSMA-ICG nanorods a) without and b) with conjugated AbEGFR-treated A549 cells, and irradiated by an NIR laser (808 nm) at 22.5 W cm-2 power density. The dotted circles indicate the laser beam area. The cells were stained with calcein AM and incubated for 2 h in the dark after irradiation; live cells exhibited green fluorescence. Scale bar: 1 mm. Adapted with permission from 79.
Combination of imaging and drug delivery
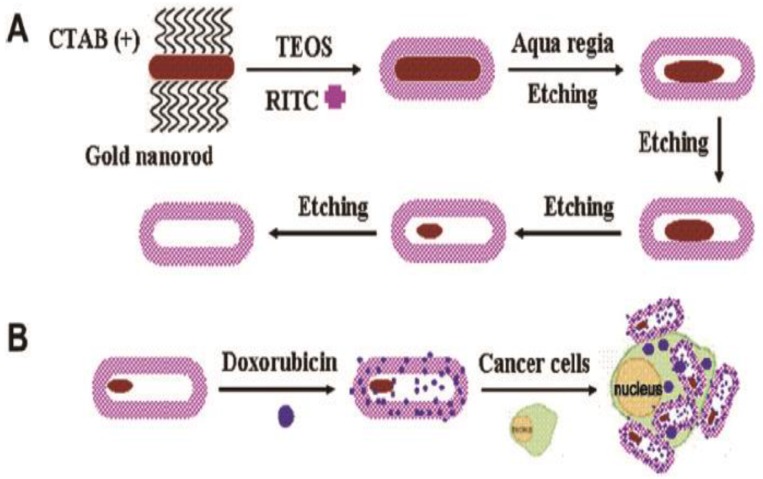
Wang et al. developed hollow and rattle-type nanocapsules composed of AuNPs as cores and fluorescent mesoporous silica shells (rattle-Au@SiO2) for simultaneous imaging and drug delivery 54. The authors coated AuNRs with mesoporous silica together with a fluorescent imaging agent, rhodamine B isothiocyanate (RITC) and then etched the AuNRs cores to spindle-shaped Au nanoparticle via a small amount of aqua regia. A well-known chemotherapy drug, doxorubicin (DOX) was loaded into the hollow interiors with high loading efficiency. Intracellular distribution of rattle-Au@SiO2 was visualized through the fluorescence of RITC. Intracellular drug release with comparable cytotoxic effect to free drug was also demonstrated (Figure 10).
Fig 10.
(A) Schematic illustration of the synthetic procedure for RITC-labeled hollow/rattle-type Au@SiO2 nanocapsules and (B) application in fluorescent drug delivery for HepG-2 cancer cells. (RITC, rhodamine B isothiocyanate). Adapted with permission from 54.
Bonoiu et al. reported siRNA carried AuNRs for in vivo gene silencing in the rat hippocampus 80. The authors modified the surface of AuNRs using the layer-by-layer method and absorbed a fluorescent labeled siRNA against glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The AuNRs-siRNA nanoplex was stereotaxically injected into the CA1 region of the hippocampus in Sprague-Dawley rats. In vivo cellular localization of nanoplexes was analyzed by immunofluorescent labeling. The nanoplexes appeared to remain localized into the CA1 region, with little diffusion to adjacent regions. Quantitative real-time PCR results showed significant suppression of GAPDH gene expression in the CA1 region after the nanoplexes were administered.
Very recently, Xiao et al. developed a PEG-AuNRs with DOX conjugated through a pH-sensitive hydrazone bond 40. Targeting ligand cRGD, and positron emission tomography (PET) imaging agent 64Cu-chelators were tethered to the distal ends of the PEG arms. Cellular uptake and cytotoxicity studies demonstrated that the cRGD-conjugated AuNRs showed a higher cellular uptake and thus a more potent cytotoxicity in ανβ3 integrin overexpressing cancer cells when compared to the cRGD-free AuNRs nanocarriers. However, according to in vivo PET imaging and biodistribution studies, the two nanocarriers had similar in vivo biodistribution, which suggested that cRGD conjugating didn't confer a significant advantage for in vivo tumor uptake.
Combination of hyperthermia and drug delivery
The combination of heat and chemotherapeutic agents is a very promising approach to optimize the efficacy of cancer treatments because of a synergistic interaction between thermal therapy and certain chemotherapeutics. Hauck and coworkers reported one of the first instances in which nanoparticle heating has been combined with chemotherapy. PDDAC-coated AuNRs and cisplatin, a chemotherapeutic drug which has a known synergy, was applied separately to cultured mammalian cells. They found that combining AuNRs heating with cisplatin lowers the cytotoxic drug dosage requirements to roughly 33% of the unheated amount to achieve comparable cytotoxicity, which has important implications to the dose-dependent renal side effects of cisplatin 81.
Very recently, Xiao and co-workers have developed a targeted NIR-responsive AuNRs-based delivery platform by a simple DNA self-assembly process. Thiolated DNA was tethered to AuNRs together with its complimentary strand. The DNA strands, which consist of sequential CG base pairs, provide loading sites for Dox. AuNRs serve as the model NIR light-to-heat transducer for cancer thermotherapy and for denaturing the DNA double helix upon NIR irradiation, leading to the triggered release of loaded drugs at target site for chemotherapy. The in vitro and in vivo results demonstrate that this platform selectively delivers anti-cancer drugs to target cells, releases them upon NIR irradiation, and effectively inhibits tumor growth through thermo-chemotherapy 82.
Combination of imaging, hyperthermia and drug delivery
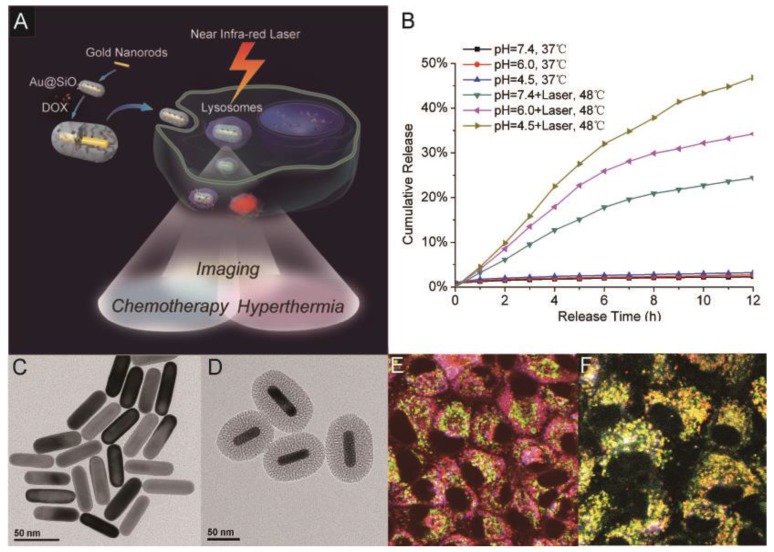
Although they have been demonstrated to be promising imaging and therapeutic agents, AuNRs only have limited specific surface area and are not appropriate to be used as an efficient carrier for drug delivery. To solve this problem, our group has recently evaluated the potential applications of Au@SiO2 in imaging, chemotherapeutics, and hyperthermia cancer therapy 55. In this work, TPL imaging was employed to visualize the intracellular co-localization of Au@SiO2 with DOX and some cellular compartments. NIR laser irradiation at a low intensity was used to enhance drug release from DOX-loaded Au@SiO2 (Au@SiO2-DOX) for chemotherapy, while at higher irradiation intensity it also induced hyperthermia effects of the AuNRs. The Au@SiO2 platform thus kept both the unique functions of mesoporous silica nanoparticles and AuNRs, and also provided a new functionality: NIR light-controlled drug release. With the unique functions of both mesoporous silica nanoparticles and gold nanorods, the new Au@SiO2 platform could have wide and promising applications in cancer theranostics (Figure 11).
Fig 11.
Multifunctional platform of imaging, hyperthermia and drug delivery. (A) TOC image of the report; TEM image of (B) AuNRs and (C) Au@SiO2; (D) DOX release profiles from Au@SiO2-DOX with and without NIR laser irradiation at different pHs; (E, F) Intracellular localization of DOX (red) and Au@SiO2 (blue) with organelle-specific probes including Lyso-Tracker (green) and Mito-Tracker (green) using two-photon confocal microscope. Adapted with permission from 55.
Perspectives
We have reviewed the research-level biomedical applications of AuNRs with a highlight on combined applications. The in vitro applications of AuNRs in bio-imaging, hyperthermia therapy and drug delivery have been well documented. A better understanding on how to optimize the properties of AuNRs for designing and preparing new AuNRs-based nanoplatforms would further help to promote their biomedical application. Currently, considerable efforts are devoted to combine both imaging and therapeutic functionalities into a single AuNRs-based system for more effective disease treatments. Surface modification and functionalization has played the key role in development of these theranostic platforms. Combined applications of AuNRs require participation and collaboration between chemists, biologists, materials scientists, and clinicians.
Though the current in vitro results are indeed encouraging, there are still issues that need to be addressed in order for AuNRs to be used for biomedical applications. More in vivo studies on their therapeutic efficacy, biocompatibility, and bio-distribution are required to make these techniques more clinically relevant.
Acknowledgments
Z. Zhang and J. Wang contributed equally to this work. We thank the Ministry of Science and Technology of China (National Basic Research Programs: 2011CB933401, 2012CB934003 and 2010CB934004), the National Science Foundation of China (31070854), and National Major Scientific Instruments Development Project (2011YQ03013406) for their financial support.
References
- 1.Tong L, Wei Q, Wei A. et al. Gold nanorods as contrast agents for biological imaging: optical properties, surface conjugation and photothermal effects. Photochem Photobiol. 2009;85:21–32. doi: 10.1111/j.1751-1097.2008.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkilany AM, Thompson LB, Boulos SP. et al. Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv Drug Deliv Rev. 2011;64:190–9. doi: 10.1016/j.addr.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Ungureanu C, Kroes R, Petersen W. et al. Light interactions with gold nanorods and cells: implications for photothermal nanotherapeutics. Nano Lett. 2011;11:1887–94. doi: 10.1021/nl103884b. [DOI] [PubMed] [Google Scholar]
- 4.Sau TK, Murphy CJ. Seeded high yield synthesis of short Au nanorods in aqueous solution. Langmuir. 2004;20:6414–20. doi: 10.1021/la049463z. [DOI] [PubMed] [Google Scholar]
- 5.Oldenburg AL, Hansen MN, Ralston TS. et al. Imaging gold nanorods in excised human breast carcinoma by spectroscopic optical coherence tomography. J Mater Chem. 2009;19:6407–11. doi: 10.1039/b823389f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chhetri RK, Kozek KA, Johnston-Peck AC. et al. Imaging three-dimensional rotational diffusion of plasmon resonant gold nanorods using polarization-sensitive optical coherence tomography. Phys Rev E Stat Nonlin Soft Matter Phys. 2011;83:040903. doi: 10.1103/PhysRevE.83.040903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung Y, Reif R, Zeng Y. et al. Three-dimensional high-resolution imaging of gold nanorods uptake in sentinel lymph nodes. Nano Lett. 2011;11:2938–43. doi: 10.1021/nl2014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Huff TB, Zweifel DA. et al. In vitro and in vivo two-photon luminescence imaging of single gold nanorods. Proc Natl Acad Sci USA. 2005;102:15752–6. doi: 10.1073/pnas.0504892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durr NJ, Larson T, Smith DK. et al. Two-photon luminescence imaging of cancer cells using molecularly targeted gold nanorods. Nano Lett. 2007;7:941–5. doi: 10.1021/nl062962v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imura K, Nagahara T, Okamoto H. Near-field two-photon-induced photoluminescence from single gold nanorods and imaging of plasmon modes. J Phys Chem B. 2005;109:13214–20. doi: 10.1021/jp051631o. [DOI] [PubMed] [Google Scholar]
- 11.Shobhit C, Sanjiv K, Singh N. et al. Development of chitosan oligosaccharide-modified gold nanorods for in vivo targeted delivery and non-invasive imaging by NIR irradiation. Bioconjug Chem. 2012;23:2173–82. doi: 10.1021/bc3001276. [DOI] [PubMed] [Google Scholar]
- 12.Li T, Li Q, Xu Y. et al. Three-dimensional orientation sensors by defocused imaging of gold nanorods through an ordinary wide-field microscope. ACS Nano. 2012;6:1268–77. doi: 10.1021/nn203979n. [DOI] [PubMed] [Google Scholar]
- 13.Niidome T, Shiotani A, Akiyama Y. et al. Theragnostic approaches using gold nanorods and near infrared light. Yakugaku Zasshi. 2010;130:1671–7. doi: 10.1248/yakushi.130.1671. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, El-Sayed IH, El-Sayed MA. Applications of gold nanorods for cancer imaging and photothermal therapy. Methods Mol Biol. 2010;624:343–57. doi: 10.1007/978-1-60761-609-2_23. [DOI] [PubMed] [Google Scholar]
- 15.Choi WI, Sahu A, Kim YH. et al. Photothermal cancer therapy and imaging based on gold nanorods. Ann Biomed Eng. 2012;40:534–46. doi: 10.1007/s10439-011-0388-0. [DOI] [PubMed] [Google Scholar]
- 16.Manohar S, Ungureanu C, Van Leeuwen TG. Gold nanorods as molecular contrast agents in photoacoustic imaging: the promises and the caveats. Contrast Media Mol Imaging. 2011;6:389–400. doi: 10.1002/cmmi.454. [DOI] [PubMed] [Google Scholar]
- 17.Jokerst JV, Thangaraj M, Kempen PJ. et al. Photoacoustic imaging of mesenchymal stem cells in living mice via silica-coated gold nanorods. ACS Nano. 2012;6:5920–30. doi: 10.1021/nn302042y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li PC, Wei CW, Liao CK. et al. Photoacoustic imaging of multiple targets using gold nanorods. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54:1642–7. doi: 10.1109/tuffc.2007.435. [DOI] [PubMed] [Google Scholar]
- 19.Li PC, Wang CR, Shieh DB. et al. In vivo photoacoustic molecular imaging with simultaneous multiple selective targeting using antibody-conjugated gold nanorods. Opt Express. 2008;16:18605–15. doi: 10.1364/oe.16.018605. [DOI] [PubMed] [Google Scholar]
- 20.Dickerson EB, Dreaden EC, Huang X. et al. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008;269:57–66. doi: 10.1016/j.canlet.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong L, Zhao Y, Huff TB. et al. Gold nanorods mediate tumor cell death by compromising membrane integrity. Adv Mater. 2007;19:3136–41. doi: 10.1002/adma.200701974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norman RS, Stone JW, Gole A. et al. Targeted photothermal lysis of the pathogenic bacteria, Pseudomonas aeruginosa, with gold nanorods. Nano Lett. 2008;8:302–6. doi: 10.1021/nl0727056. [DOI] [PubMed] [Google Scholar]
- 23.Wijaya A, Schaffer SB, Pallares IG. et al. Selective release of multiple DNA oligonucleotides from gold nanorods. ACS Nano. 2009;3:80–6. doi: 10.1021/nn800702n. [DOI] [PubMed] [Google Scholar]
- 24.Rai P, Mallidi S, Zheng X. et al. Development and applications of photo-triggered theranostic agents. Adv Drug Deliv Rev. 2010;62:1094–124. doi: 10.1016/j.addr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cobley CM, Chen J, Cho EC. et al. Gold nanostructures: a class of multifunctional materials for biomedical applications. Chem Soc Rev. 2011;40:44–56. doi: 10.1039/b821763g. [DOI] [PubMed] [Google Scholar]
- 26.Stone J, Jackson S, Wright D. Biological applications of gold nanorods. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:100–9. doi: 10.1002/wnan.120. [DOI] [PubMed] [Google Scholar]
- 27.Ratto F, Matteini P, Centi S. et al. Gold nanorods as new nanochromophores for photothermal therapies. J Biophotonics. 2011;4:64–73. doi: 10.1002/jbio.201000002. [DOI] [PubMed] [Google Scholar]
- 28.Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19:316–7. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 29.Wu G, Mikhailovsky A, Khant HA. et al. Synthesis, characterization, and optical response of gold nanoshells used to trigger release from liposomes. Methods Enzymol. 2009;464:279–307. doi: 10.1016/S0076-6879(09)64014-3. [DOI] [PubMed] [Google Scholar]
- 30.Sailor MJ, Park JH. Hybrid nanoparticles for detection and treatment of cancer. Adv Mater. 2012;24:3779–802. doi: 10.1002/adma.201200653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents. Adv Drug Deliv Rev. 2010;62:1064–79. doi: 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei A, Leonov AP, Wei Q. Gold nanorods: multifunctional agents for cancer imaging and therapy. Methods Mol Biol. 2010;624:119–30. doi: 10.1007/978-1-60761-609-2_8. [DOI] [PubMed] [Google Scholar]
- 33.Murphy CJ, San TK, Gole AM. et al. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. J Phys Chem B. 2005;109:13857–70. doi: 10.1021/jp0516846. [DOI] [PubMed] [Google Scholar]
- 34.Jana NR, Gearheart L, Murphy CJ. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv Mater. 2001;13:1389–93. [Google Scholar]
- 35.Gole A, Murphy CJ. Seed-mediated synthesis of gold nanorods: Role of the size and nature of the seed. Chem of Mater. 2004;16:3633–40. [Google Scholar]
- 36.Qiu Y, Liu Y, Wang L. et al. Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods. Biomaterials. 2010;31:7606–19. doi: 10.1016/j.biomaterials.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 37.Alkilany AM, Shatanawi A, Kurtz T. et al. Toxicity and cellular uptake of gold nanorods in vascular endothelium and smooth muscles of isolated rat blood vessel: importance of surface modification. Small. 2012;8:1270–8. doi: 10.1002/smll.201101948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lankveld DP, Rayavarapu RG, Krystek P. et al. Blood clearance and tissue distribution of PEGylated and non-PEGylated gold nanorods after intravenous administration in rats. Nanomedicine (Lond) 2011;6:339–49. doi: 10.2217/nnm.10.122. [DOI] [PubMed] [Google Scholar]
- 39.Boca SC, Astilean S. Detoxification of gold nanorods by conjugation with thiolated poly(ethylene glycol) and their assessment as SERS-active carriers of Raman tags. Nanotechnology. 2010;21:235601. doi: 10.1088/0957-4484/21/23/235601. [DOI] [PubMed] [Google Scholar]
- 40.Xiao Y, Hong H, Matson VZ. et al. Gold nanorods conjugated with doxorubicin and cRGD for combined anticancer drug delivery and PET imaging. Theranostics. 2012;2:757–68. doi: 10.7150/thno.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Black KC, Kirkpatrick ND, Troutman TS. et al. Gold nanorods targeted to delta opioid receptor: plasmon-resonant contrast and photothermal agents. Mol Imaging. 2008;7:50–7. [PubMed] [Google Scholar]
- 42.Yamashita S, Fukushima H, Akiyama Y. et al. Controlled-release system of single-stranded DNA triggered by the photothermal effect of gold nanorods and its in vivo application. Bioorg Med Chem. 2011;19:2130–5. doi: 10.1016/j.bmc.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 43.Oyelere AK, Chen PC, Huang X. et al. Peptide-conjugated gold nanorods for nuclear targeting. Bioconjug Chem. 2007;18:1490–7. doi: 10.1021/bc070132i. [DOI] [PubMed] [Google Scholar]
- 44.Park H, Lee S, Chen L. et al. SERS imaging of HER2-overexpressed MCF7 cells using antibody-conjugated gold nanorods. Phys Chem Chem Phys. 2009;11:7444–9. doi: 10.1039/b904592a. [DOI] [PubMed] [Google Scholar]
- 45.Gorelikov I, Matsuura N. Single-step coating of mesoporous silica on cetyltrimethyl ammonium bromide-capped nanoparticles. Nano Lett. 2008;8:369–73. doi: 10.1021/nl0727415. [DOI] [PubMed] [Google Scholar]
- 46.Slowing II, Vivero-Escoto JL, Wu CW. et al. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev. 2008;60:1278–88. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Wu SH, Hung Y, Mou CY. Mesoporous silica nanoparticles as nanocarriers. Chem Commun (Camb) 2011;47:9972–85. doi: 10.1039/c1cc11760b. [DOI] [PubMed] [Google Scholar]
- 48.Xu L, Liu Y, Chen Z. et al. Surface-engineered gold nanorods: promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012;12:2003–12. doi: 10.1021/nl300027p. [DOI] [PubMed] [Google Scholar]
- 49.Bayer CL, Chen YS, Kim S, Mallidi S, Sokolov K, Emelianov S. Multiplex photoacoustic molecular imaging using targeted silica-coated gold nanorods. Biomed Opt Express. 2011;2:1828–35. doi: 10.1364/BOE.2.001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen YS, Frey W, Kim S. et al. Silica-coated gold nanorods as photoacoustic signal nanoamplifiers. Nano Lett. 2011;11:348–54. doi: 10.1021/nl1042006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang P, Bao L, Zhang C. et al. Folic acid-conjugated silica-modified gold nanorods for X-ray/CT imaging-guided dual-mode radiation and photo-thermal therapy. Biomaterials. 2011;32:9796–809. doi: 10.1016/j.biomaterials.2011.08.086. [DOI] [PubMed] [Google Scholar]
- 52.Luo T, Huang P, Gao G. et al. Mesoporous silica-coated gold nanorods with embedded indocyanine green for dual mode X-ray CT and NIR fluorescence imaging. Opt Express. 2011;19:17030–9. doi: 10.1364/OE.19.017030. [DOI] [PubMed] [Google Scholar]
- 53.Chen YS, Frey W, Kim S. et al. Enhanced thermal stability of silica-coated gold nanorods for photoacoustic imaging and image-guided therapy. Opt Express. 2010;18:8867–78. doi: 10.1364/OE.18.008867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang TT, Chai F, Wang CG. et al. Fluorescent hollow/rattle-type mesoporous Au@SiO2 nanocapsules for drug delivery and fluorescence imaging of cancer cells. J Colloid Interface Sci. 2011;358:109–15. doi: 10.1016/j.jcis.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Z, Wang L, Wang J. et al. Mesoporous silica-coated gold nanorods as a light-mediated multifunctional theranostic platform for cancer treatment. Adv Mater. 2012;24:1418–23. doi: 10.1002/adma.201104714. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Liu Y, Li W. et al. Selective targeting of gold nanorods at the mitochondria of cancer cells: implications for cancer therapy. Nano Lett. 2011;11:772–80. doi: 10.1021/nl103992v. [DOI] [PubMed] [Google Scholar]
- 57.Rayavarapu RG, Petersen W, Hartsuiker L. et al. In vitro toxicity studies of polymer-coated gold nanorods. Nanotechnology. 2010;21:145101–10. doi: 10.1088/0957-4484/21/14/145101. [DOI] [PubMed] [Google Scholar]
- 58.Hauck TS, Ghazani AA, Chan WC. Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small. 2008;4:153–9. doi: 10.1002/smll.200700217. [DOI] [PubMed] [Google Scholar]
- 59.Alkilany AM, Nagaria PK, Hexel CR. et al. Cellular uptake and cytotoxicity of gold nanorods: molecular origin of cytotoxicity and surface effects. Small. 2009;5:701–8. doi: 10.1002/smll.200801546. [DOI] [PubMed] [Google Scholar]
- 60.Puvanakrishnan P, Diagaradjane P, Kazmi SM. et al. Narrow band imaging of squamous cell carcinoma tumors using topically delivered anti-EGFR antibody conjugated gold nanorods. Lasers Surg Med. 2012;44:310–7. doi: 10.1002/lsm.22019. [DOI] [PubMed] [Google Scholar]
- 61.Yang S, Ye F, Xing D. Intracellular label-free gold nanorods imaging with photoacoustic microscopy. Opt Express. 2012;20:10370–5. doi: 10.1364/OE.20.010370. [DOI] [PubMed] [Google Scholar]
- 62.Kirui DK, Krishnan S, Strickland AD. et al. PAA-derived gold nanorods for cellular targeting and photothermal therapy. Macromol Biosci. 2011;11:779–88. doi: 10.1002/mabi.201100050. [DOI] [PubMed] [Google Scholar]
- 63.Rejiya CS, Kumar J, V R. et al. Laser immunotherapy with gold nanorods causes selective killing of tumour cells. Pharmacol Res. 2012;65:261–9. doi: 10.1016/j.phrs.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Li Z, Huang P, Zhang X. et al. RGD-conjugated dendrimer-modified gold nanorods for in vivo tumor targeting and photothermal therapy. Mol Pharm. 2010;7:94–104. doi: 10.1021/mp9001415. [DOI] [PubMed] [Google Scholar]
- 65.Zhou W, Shao J, Jin Q. et al. Zwitterionic phosphorylcholine as a better ligand for gold nanorods cell uptake and selective photothermal ablation of cancer cells. Chem Commun (Camb) 2010;46:1479–81. doi: 10.1039/b915125g. [DOI] [PubMed] [Google Scholar]
- 66.Torchilin V. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58:1532–55. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 67.Torchilin V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur J Pharm Biopharm. 2009;71:431–44. doi: 10.1016/j.ejpb.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Timko BP, Dvir T, Kohane DS. Remotely triggerable drug delivery systems. Adv Mater. 2010;22:4925–43. doi: 10.1002/adma.201002072. [DOI] [PubMed] [Google Scholar]
- 69.Mayer G, Heckel A. Biologically active molecules with a "light switch". Angew Chem Int Ed Engl. 2006;45:4900–21. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi H, Niidome Y, Yamada S. Controlled release of plasmid DNA from gold nanorods induced by pulsed near-infrared light. Chem Commun (Camb) 2005:2247–9. doi: 10.1039/b500337g. [DOI] [PubMed] [Google Scholar]
- 71.Shiotani A, Mori T, Niidome T. et al. Stable incorporation of gold nanorods into N-isopropylacrylamide hydrogels and their rapid shrinkage induced by near-infrared laser irradiation. Langmuir. 2007;23:4012–8. doi: 10.1021/la0627967. [DOI] [PubMed] [Google Scholar]
- 72.Yamashita S, Fukushima H, Niidome Y. et al. Controlled-release system mediated by a retro Diels-Alder reaction induced by the photothermal effect of gold nanorods. Langmuir. 2011;27:14621–6. doi: 10.1021/la2036746. [DOI] [PubMed] [Google Scholar]
- 73.Chakravarthy KV, Bonoiu AC, Davis WG. et al. Gold nanorod delivery of an ssRNA immune activator inhibits pandemic H1N1 influenza viral replication. Proc Natl Acad Sci USA. 2010;107:10172–7. doi: 10.1073/pnas.0914561107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Min Y, Mao C, Xu D. et al. Gold nanorods for platinum based prodrug delivery. Chem Commun (Camb) 2010;46:8424–6. doi: 10.1039/c0cc03108a. [DOI] [PubMed] [Google Scholar]
- 75.Huang X, El-Sayed IH, Qian W. et al. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128:2115–20. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 76.Huff TB, Tong L, Zhao Y. et al. Hyperthermic effects of gold nanorods on tumor cells. Nanomedicine (Lond) 2007;2:125–32. doi: 10.2217/17435889.2.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi J, Yang J, Bang D. et al. Targetable gold nanorods for epithelial cancer therapy guided by near-IR absorption imaging. Small. 2012;8:746–53. doi: 10.1002/smll.201101789. [DOI] [PubMed] [Google Scholar]
- 78.Yi DK, Sun IC, Ryu JH. et al. Matrix metalloproteinase sensitive gold nanorod for simultaneous bioimaging and photothermal therapy of cancer. Bioconjug Chem. 2010;21:2173–7. doi: 10.1021/bc100308p. [DOI] [PubMed] [Google Scholar]
- 79.Kuo WS, Chang CN, Chang YT. et al. Gold nanorods in photodynamic therapy, as hyperthermia agents, and in near-infrared optical imaging. Angew Chem Int Ed Engl. 2010;49:2711–5. doi: 10.1002/anie.200906927. [DOI] [PubMed] [Google Scholar]
- 80.Bonoiu AC, Bergey EJ, Ding H. et al. Gold nanorod-siRNA induces efficient in vivo gene silencing in the rat hippocampus. Nanomedicine. 2011;6:617–30. doi: 10.2217/nnm.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hauck TS, Jennings TL, Yatsenko T. et al. Enhancing the toxicity of cancer chemotherapeutics with gold nanorod hyperthermia. Adv Mater. 2008;20:3832–8. [Google Scholar]
- 82.Xiao Z, Ji S, Shi J. et al. DNA self-assembly of targeted near-infrared-responsive gold nanoparticles for cancer thermo-chemotherapy. Angew Chem Int Ed Engl. 2012;51:11853–7. doi: 10.1002/anie.201204018. [DOI] [PMC free article] [PubMed] [Google Scholar]