Abstract
Objective
To examine rates of paediatric hospitalization for empyema and pneumonia in Australia before and after the introduction of the seven-valent pneumococcal conjugate vaccine (PCV7).
Methods
Rates of paediatric hospitalization for empyema and pneumonia (bacterial, viral and all types) were calculated following the codes of the International Classification of Diseases, tenth revision (ICD-10) as a principal diagnosis. The expected number of hospitalizations after the PCV7 was introduced was estimated on the basis of the observed number of hospitalizations before the introduction of the PCV7. Incidence rate differences (IRDs) and incidence rate ratios (IRRs) were calculated. Hospitalization incidence in each study period was expressed as the number of hospitalizations per million (106) person–years. The population of children aged 0–19 years in Australia from 1998 to 2004 and from 2005 to 2010, as reported by the Australian Bureau of Statistics, was used to calculate the number of person–years in each period.
Findings
In the 5 years following the introduction of the PCV7, hospitalizations for pneumonia were fewer than expected (15 304 fewer; 95% confidence interval, CI: 14 646–15 960; IRD: −552 per 106 person–years; 95% CI: −576 to −529 per 106 person–years; IRR: 0.78; 95% CI: 0.77–0.78). Hospitalizations for empyema, on the other hand, were more than expected (83 more; 95% CI: 37–128; IRD: 3 per 106 person–years; 95% CI: 1–5 per 106 person–years; IRR: 1.35; 95% CI: 1.14–1.59). Reductions in hospitalizations were observed for all ICD-10 pneumonia codes across all age groups. The increase in empyema hospitalizations was only significant among children aged 1 to 4 years.
Conclusion
The introduction of the PCV7 in Australia was associated with a substantial decrease in hospitalizations for childhood pneumonia and a small increase in hospitalizations for empyema.
Résumé
Objectif
Examiner les taux d'hospitalisation pédiatrique due à un empyème et à une pneumonie en Australie, avant et après l'introduction du vaccin pneumococcique heptavalent conjugué (PCV7).
Méthodes
Les taux d'hospitalisation pédiatrique due à un empyème et à une pneumonie (infection bactérienne, virale et de tous types) ont été calculés selon les codes de la Classification internationale des maladies (CIM-10), dixième révision (CIM-10), en tant que diagnostic principal. Le nombre attendu d'hospitalisations après l'introduction du PCV7 a été estimé sur la base du nombre d'hospitalisations observées avant l'introduction du PCV7. Les différences de taux d'incidence (DTI) et les ratios de taux d'incidence (RTI) ont été calculés. L'incidence d'hospitalisation durant chaque période de l'étude a été exprimée en nombre d'hospitalisations par million (106) d'années-personnes. Les populations d'enfants âgés de 0 à 19 ans en Australie, de 1998 à 2004 et de 2005 à 2010, telles que rapportées par l'Australian Bureau of Statistics, ont été utilisées pour calculer le nombre d'années-personnes au cours de chaque période.
Résultats
Dans les 5 années suivant l'introduction du PCV7, les hospitalisations dues à une pneumonie ont été moins nombreuses que prévu (15 304 en moins; intervalle de confiance IC de 95%: 14 646 à 15 960; IRD: −552 par 106 d'années-personnes; IC de 95%: −576 à −529 par 106 d’années-personnes; RTI: 0,78, IC de 95%: 0,77 à 0,78). Les hospitalisations dues à un empyème, par contre, ont été plus nombreuses que prévu (83 de plus, IC de 95%: 37 à 128; DTI: 3 par 106 d’années-personnes, IC de 95%: 1 à 5 par 106 d’années-personnes; RTI: 1,35; IC de 95%: 1,14 à 1,59). Des réductions du nombre d'hospitalisations ont été observées pour tous les codes de pneumonie CIM-10 dans tous les groupes d'âge. L'augmentation du nombre d'hospitalisations dues à un empyème n'a été significative que chez les enfants âgés de 1 à 4 ans.
Conclusion
L'introduction du PCV7 en Australie a été associée à une diminution substantielle du nombre d'hospitalisations d'enfants dues à une pneumonie et à une légère augmentation du nombre d'hospitalisations dues à un empyème.
Resumen
Objetivo
Examinar las tasas de hospitalización pediátrica por empiema y neumonía en Australia antes y después de la introducción de la vacuna conjugada antineumocócica heptavalente (PCV7).
Métodos
Se calcularon las tasas de hospitalización pediátrica por empiema y neumonía (bacteriana, vírica y todos los tipos) siguiendo los códigos de la Clasificación internacional de enfermedades, Décima Revisión (CIE-10) como forma de diagnóstico principal. El número esperado de hospitalizaciones tras la PCV7 se calculó en base al número de hospitalizaciones observado antes de la introducción de la vacuna. Se calcularon las diferencias en la tasa de incidencia (DTI) y las proporciones de la tasa de incidencia (PTI). La frecuencia de las hospitalizaciones en cada periodo de estudio se expresó como el número de hospitalizaciones por millón (106) de años-persona. Para calcular el número de años-persona en cada periodo, se utilizó la población de niños entre 0 y 19 años en Australia desde 1998 hasta 2004 y desde 2005 hasta 2010, de acuerdo con las informaciones de la Oficina de Estadística Australiana.
Resultados
En los cinco años siguientes a la introducción de la PCV7, las hospitalizaciones por neumonía fueron inferiores a lo esperado (15 304 menos; intervalo de confianza del 95%, IC: 14 646–15 960; DTI: −552 por 106 años–persona; IC del 95%: −576 hasta −529 por 106 años–persona; PTI: 0,78; IC del 95%: 0,77–0,78). Por el contrario, las hospitalizaciones por empiema fueron más numerosas de lo esperado (83 más; IC del 95%: 37–128; DTI: 3 por 106 años–persona; IC del 95%: 1–5 por 106 años–persona; PTI: 1,35; IC del 95%: 1,14-1,59). Se observó una disminución de las hospitalizaciones para todos los códigos CIE-10 de neumonía en todos los grupos de edades. El aumento de las hospitalizaciones por empiema fue significativo únicamente entre los niños con edades comprendidas entre uno y cuatro años.
Conclusión
La introducción de la PCV7 en Australia estuvo asociada con un descenso notable en las hospitalizaciones por neumonía infantil, así como con un pequeño aumento de las hospitalizaciones por empiema.
ملخص
الغرض
دراسة معدلات إدخال الأطفال إلى المستشفيات إثر إصابتهم بالدبيلة والالتهاب الرئوي في أستراليا قبل إدخال اللقاح المتقارن المضاد لالتهاب المكورات الرئوية السباعي وبعده (PCV7).
الطريقة
تم حساب معدلات إدخال الأطفال إلى المستشفيات إثر إصابتهم بالدبيلة والالتهاب الرئوي (الجرثومي والفيروسي وجميع فئاته) وفق رموز التصنيف الدولي للأمراض، المراجعة العاشرة (ICD-10) باعتبارها تشخيصاً رئيسياً. وتم تقدير العدد المتوقع للحالات التي يتم إدخالها إلى المستشفيات بعد استخدام اللقاح المتقارن المضاد لالتهاب المكورات الرئوية السباعي على أساس العدد الذي تمت ملاحظته للحالات التي يتم إدخالها إلى المستشفيات بعد استخدام اللقاح المتقارن المضاد لالتهاب المكورات الرئوية السباعي. وتم حساب الفروق بين معدلات الإصابة (IRDs) ونسب معدلات الإصابة (IRRs). وتم التعبير عن حالات الإدخال إلى المستشفيات في كل فترة من فترات الدراسة في شكل عدد حالات الإدخال إلى المستشفيات لكل مليون (10 6 ) شخص-عام. وتم استخدام الفئة السكانية من الأطفال الذين يبلغون من العمر 0 - 19 سنة في أستراليا في الفترة من 1998 إلى 2004 وفي الفترة من 2005 إلى 2010، وفق تقرير المكتب الأسترالي للإحصاءات، لحساب عدد الأشخاص إلى عدد السنوات في كل فترة.
النتائج
خلال الخمس سنوات التي أعقبت استخدام اللقاح المتقارن المضاد لالتهاب المكورات الرئوية السباعي، انخفضت حالات الإدخال إلى المستشفيات نتيجة الإصابة بالالتهاب الرئوي عن المتوقع (انخفض عدد الحالات إلى 15304حالة؛ فاصل الثقة 95 %، فاصل الثقة: من 14646 إلى 15960؛ الفرق بين معدلات الإصابة: -552 لكل مليون شخص-عام؛ فاصل الثقة 95 %، فاصل الثقة: من -576 إلى -529 لكل مليون شخص-عام؛ نسبة معدلات الإصابة: 0.78؛ فاصل الثقة 95 %، فاصل الثقة: من 0.77 إلى 0.78). وعلى الجانب الآخر، زادت حالات الإدخال إلى المستشفيات إثر الإصابة بالدبيلة عن المتوقع (زادت الحالات بمقدار 83؛ فاصل الثقة 95 %، فاصل الثقة: من 37 إلى 128؛ الفرق بين معدلات الإصابة: 3لكل مليون شخص-عام؛ فاصل الثقة 95 %، فاصل الثقة: من 1 إلى 5 لكل مليون شخص-عام؛ نسبة معدلات الإصابة: 1.35؛ فاصل الثقة 95 %: من 1.14 إلى 1.59). ولوحظت انخفاضات في حالات الإدخال إلى المستشفيات بخصوص المراجعة العاشرة لجميع رموز التصنيف الدولي للأمراض المعنية بالالتهاب الرئوي بين جميع الفئات العمرية. ولم تكن الزيادة في حالات الإدخال إلى المستشفيات نتيجة الإصابة بالدبيلة كبيرة إلا بين الأطفال الذين تتراوح أعمارهم من سنة إلى 4 سنوات.
الاستنتاج
ارتبط إدخال اللقاح المتقارن المضاد لالتهاب المكورات الرئوية السباعي في أستراليا بانخفاض كبير في حالات إدخال الأطفال إلى المستشفيات إثر الإصابة بالالتهاب الرئوي وبزيادة صغيرة في حالات إدخال الأطفال إلى المستشفيات إثر الإصابة بالدبيلة.
摘要
目的
调查在引进七价肺炎球菌结合疫苗(PCV7)之前和之后澳大利亚脓胸症和肺炎儿科住院率。
方法
作为主要诊断,按照国际疾病分类第十次修订本(ICD-10)编码,计算脓胸症和肺炎(细菌、病毒和所有类型)的儿科住院率。根据PCV7 引进之前观察到的住院数据估计引进PCV7 之后的预期住院数量。计算发病率差异(IRD)和发病率比(IRR)。每个研究期间的住院发病率以每百万 (106) 人年的住院数量表示。使用澳大利亚统计局报告的 1998 年至 2004 年和 2005 年至 2010 年之间的澳大利亚 0-19 岁儿童人口来计算每个期间的人年。
结果
在引进PCV7后的 5 年内,肺炎住院量少于预期(少 15304;95%置信区间,CI:14646-15960;IRD:每百万人年−552 例;95% CI:每百万人年−576 至−529 例;IRR:0.78;95%CI:0.77-0.78)。另一方面,脓胸症住院量超过预期(多83 例,95% CI:37-128;IRD: 每百万人年3 例;95% CI:每百万人年1-5 例;IRR:1.35;95% CI:1.14-1.59)。观察到所有年龄组的所有ICD-10 肺炎编码住院率减少。仅1 至4 岁儿童脓胸症住院率显著增加。
结论
澳大利亚引进PCV7 与儿童肺炎住院率显著减少以及脓胸症住院率小幅增加有关联性。
Резюме
Цель
Изучить показатели госпитализации детей с эмпиемой и пневмонией в Австралии до и после введения семивалентной пневмококковой конъюгированной вакцины (PCV7).
Методы
Показатели госпитализации детей с эмпиемой и пневмонией (бактериальной, вирусной и всех типов) рассчитывались по кодам Международной классификации болезней 10-го пересмотра (МКБ-10) в качестве сопутствующего диагноза. Ожидаемое число госпитализаций после введения PCV7 определялось на основе наблюдаемого числа госпитализаций до введения PCV7. Был произведен расчет разницы в показателях заболеваемости (IRD) и коэффициентов частоты заболеваний (IRR). Частота случаев госпитализации в каждом периоде исследования выражалась в виде числа госпитализаций на миллион (106) человеко-лет. По сообщениям Австралийского бюро статистики, число человеко-лет в каждом периоде рассчитывалось на основе совокупного числа детей в возрасте от нуля до девятнадцати лет в Австралии с 1998 по 2004 гг. и с 2005 по 2010 гг.
Результаты
На протяжении пяти лет после введения PCV7 показатели госпитализации вследствие пневмонии были ниже ожидаемых (меньше на 15 304; 95% доверительный интервал, ДИ: 14 646–15 960; IRD: −552 на 106 человеко-лет; 95% ДИ: от −576 до −529 на 106 человеко-лет; IRR: 0,78; 95% ДИ: 0,77-0,78). Показатели госпитализации вследствие эмпиемы, напротив, оказались выше ожидаемых (больше на 83; 95% ДИ: 37–128; IRD: 3 на 106 человеко-лет; 95% ДИ: 1–5 на 106 человеко-лет; IRR: 1,35; 95% ДИ: 1,14-1,59). Уменьшение показателей госпитализации наблюдалось для всех кодов пневмонии по МКБ-10 во всех группах. Увеличение числа случаев госпитализации вследствие эмпиемы было значительным только среди детей в возрасте от одного года до четырех лет.
Вывод
Введение PCV7 в Австралии сопровождалось существенным уменьшением показателей госпитализации вследствие детской пневмонии и незначительным увеличением показателей госпитализации вследствие эмпиемы.
Introduction
Streptococcus pneumoniae is the leading cause of bacterial infection in children worldwide and the most common cause of bacterial pneumonia in Australia, where it accounts for approximately one third of all cases.1 In Australia, about 0.7% of all cases of pneumonia in children are complicated by empyema, which requires hospitalization for treatment and possible drainage.2 S. pneumoniae is also the most common pathogen causing empyema.3 The 7-valent pneumococcal conjugate vaccine (PCV7) was introduced in Australia's national immunization programme in two phases: in 2001 for indigenous and immunocompromised children less than 2 years of age, and in January 2005 for all children in this age group. In July 2011 the PCV7 was replaced by a 13-valent conjugate vaccine. The PCV7 was administered as a three-dose series at ages 2, 4 and 6 months, without a booster.
Although pneumococcal conjugate vaccines have reduced invasive pneumococcal disease rates in children and adults throughout the world, 4–9 several studies have reported a concomitant increase in empyema cases, both in the vaccinated and the non-vaccinated population.10–17 Because up to 90% of these cases have been caused by bacterial serotypes not included in the PCV7,10–12 some fear that empyema may have emerged as a “replacement disease”, i.e. disease produced by non-vaccine-related serotypes that have become predominant. Although we have reported the rates of childhood empyema in Australia elsewhere,2 no previous studies have examined how the introduction of PCV7 has affected childhood empyema rates in this country. The objective of this study is to examine the incidence of paediatric hospitalizations for pneumonia and empyema in Australia before and after the introduction of the PCV7.
Methods
We reviewed the annual incidence in Australia of paediatric hospitalizations for empyema and pneumonia (viral and bacterial) before and after the introduction of PCV7 among children in the following age groups: < 1 year, 1–4 years, 5–9 years, 10–14 years and 15–19 years. The pre-vaccine period extended from July 1998 to June 2004; the post-vaccine period, from July 2005 to June 2010. We excluded all hospitalizations from July 2004 to June 2005 because the PCV7 was introduced midway through this period. In Australia, hospital discharges are coded for the principal diagnosis (primary cause) in accordance with the coding system of the International Classification of Diseases, tenth revision. The Australian Institute of Health and Welfare collated the national hospital discharge codes supplied by state and territory authorities per financial year, from July to June, and introduced them into the National Hospital Morbidity Database, which is publicly available. From this database we obtained data on the hospitalizations in the age groups already indicated. Although a “child” is generally defined as a person under the age of 18 years, data cubes were only available for these age groups. We included codes for bacterial pneumonia (J13–J15.9, J16.8, J18.0, J18.1, J18.8–J20.0) and viral pneumonia (J10.0, J11.0, J12.0–J12.2, J12.8, J12.9), as well as codes for empyema (J86–J86.9). We expressed hospitalization incidence as hospitalizations per 106 person–years and used census data for the population less than 20 years of age from the Australian Bureau of Statistics to calculate the number of person–years in each study period. There were 31 846 901 and 27 713 068 person–years in the pre- and post-vaccine periods, respectively.
We compared the incidence of hospitalization for all pneumonias, as well as for bacterial and viral pneumonia separately and for empyema, in the pre- and post-vaccine periods. We estimated the expected number of hospitalizations in the post-vaccine period by multiplying the hospitalization incidence rate in the pre-vaccine period by the number of person–years in the post-vaccine period. We also calculated incidence rate differences (IRD = incidence rate in pre-vaccine period minus incidence rate in post-vaccine period) and incidence rate ratios (IRR = incidence rate in post-vaccine period divided by incidence rate in pre-vaccine period) with their exact 95% confidence intervals (CIs), assuming a Poisson distribution.
Results
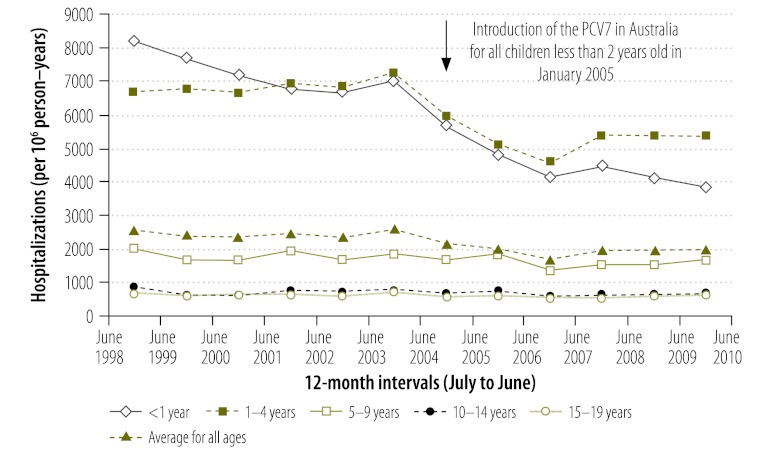
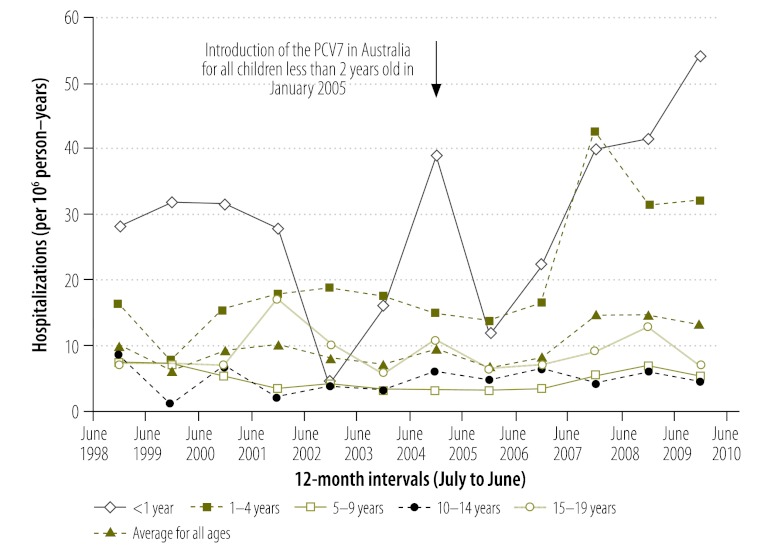
Overall, 78 552 and 53 052 pneumonia-coded hospitalizations took place in the periods before and after the introduction of the PCV7, respectively. The incidence of hospitalizations was 22% lower in the post-vaccine period than in the pre-vaccine period (IRR: 0.78; 95% CI: 0.77–0.78) (Fig. 1, Table 1). This reduction in hospitalization incidence was greatest among children whose age made them eligible for vaccination (i.e. < 1 year and 1 to 4 years in age) but was observed in all age groups. Most of the decrease in the incidence of hospitalizations was noted among children hospitalized for pneumonia with a bacteria-specific code (Table 1). In contrast, the incidence of hospitalizations coded as being for empyema was 35% higher (IRR: 1.35; 95% CI: 1.14–1.59) in the post-vaccine period than in the pre-vaccine period (Fig. 2, Table 1); the incidence of hospitalizations coded for viral pneumonia was 31% higher (IRR: 1.31; 95% CI: 1.27–1.35) (Table 1). However, the absolute increase in the incidence of hospitalizations for empyema and for viral pneumonia (3 and 70 hospitalizations more per 106 person–years, respectively) was much smaller than the absolute decrease in the incidence of hospitalization for bacterial pneumonia (623 hospitalizations fewer per 106 person–years). On subgroup analysis, the increase in empyema was only significant among children aged 1 to 4 years, which was the age group with the largest number of hospitalizations. There was little evidence of an increase in empyema among children born before the PCV7 became routinely available in Australia (i.e. those aged 5–9, 10–14 and 15–19 years).
Fig. 1.
Incidencea of hospital admissions for pneumonia, Australia, 1998–2004 and 2005–2010
a Number of hospitalizations per million (106) person–years. There were 31 846 901 person–years in the period from July 1998 to June 2004 and 27 713 068 person–years in the period from July 2005 to June 2010.
Table 1. Incidence rate differences (IRDs) and incidence rate ratios (IRRs) for paediatric hospitalizations for pneumonia and empyema before and after the introduction of the 7-valent pneumococcal conjugate vaccine, Australia.
| Age group (years), by disease category | July 1998 to June 2004 |
July 2005 to June 2010 |
IRDc (95% CI) | IRRd (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PY | Hospitalizations | Incidencea | PY | Hospitalizations (no.) |
Incidencea | |||||
| Expectedb | Observed | |||||||||
| All pneumonias | ||||||||||
| 0–19 | 31 846 901 | 78 552 | 2467 | 27 713 068 | 68 356 | 53 052 | 1914 | −552 (−576 to −529) | 0.78 (0.77 to 0.78) | |
| < 1 | 1 499 975 | 10 894 | 7263 | 1 388 287 | 10 083 | 5938 | 4277 | −2986 (−3160 to −2811) | 0.59 (0.57 to 0.61) | |
| 1–4 | 6 186 032 | 42 258 | 6831 | 5 346 009 | 36 520 | 27 534 | 5150 | −1681 (−1770 to −1592) | 0.75 (0.74 to 0.78) | |
| 5–9 | 8 064 185 | 14 333 | 1777 | 6 726 493 | 11 955 | 10 667 | 1586 | −192 (−233 to −150) | 0.89 (0.87 to 0.91) | |
| 10–14 | 8 084 575 | 5785 | 716 | 7 011 457 | 5017 | 4510 | 643 | −72 (−99 to −46) | 0.90 (0.86 to 0.93) | |
| 15–19 | 8 012 134 | 5291 | 660 | 7 240 822 | 4782 | 4403 | 608 | −52 (−78 to −27) | 0.92 (0.88 to 0.96) | |
| Bacterial pneumonia | ||||||||||
| 0–19 | 31 846 901 | 71 338 | 2240 | 27 713 068 | 62 078 | 44 822 | 1617 | −623 (−645 to −600) | 0.72 (0.71 to 0.73) | |
| < 1 | 1 499 975 | 9364 | 6243 | 1 388 287 | 8667 | 4761 | 3429 | −2813 (−2973 to −2654) | 0.55 (0.53 to 0.57) | |
| 1–4 | 6 186 032 | 37 493 | 6061 | 5 346 009 | 32 402 | 21 716 | 4062 | −1999 (−2081 to −1917) | 0.67 (0.66 to 0.68) | |
| 5–9 | 8 064 185 | 13 707 | 1700 | 6 726 493 | 11 433 | 9819 | 1460 | −240 (−281 to −199) | 0.86 (0.84 to 0.88) | |
| 10–14 | 8 084 575 | 5614 | 694 | 7 011 457 | 4869 | 4279 | 610 | −84 (−110 to −58) | 0.88 (0.84 to 0.91) | |
| 15–19 | 8 012 134 | 5160 | 644 | 7 240 822 | 4663 | 4247 | 587 | −58 (−82 to −33) | 0.91 (0.87 to 0.95) | |
| Viral pneumonia | ||||||||||
| 0–19 | 31 846 901 | 7223 | 227 | 27 713 068 | 6285 | 8230 | 297 | 70 (62 to 78) | 1.31 (1.27 to 1.35) | |
| < 1 | 1 499 975 | 1530 | 1020 | 1 388 287 | 1416 | 1177 | 848 | −172 (−243 to −102) | 0.83 (0.77 to 0.90) | |
| 1–4 | 6 186 032 | 4765 | 591 | 5 346 009 | 3159 | 5818 | 1088 | 318 (283 to 354) | 1.41 (1.36 to 1.47) | |
| 5–9 | 8 064 185 | 626 | 78 | 6 726 493 | 522 | 848 | 126 | 48 (38 to 59) | 1.62 (1.46 to 1.80) | |
| 10–14 | 8 084 575 | 171 | 21 | 7 011 457 | 150 | 231 | 33 | 12 (6 to 17) | 1.56 (1.27 to 1.91) | |
| 15–19 | 8 012 134 | 131 | 16 | 7 240 822 | 118 | 156 | 22 | 5 (1 to 10) | 1.32 (1.04 to 1.68) | |
| Empyema | ||||||||||
| 0–19 | 31 846 901 | 274 | 9 | 27 713 068 | 238 | 321 | 12 | 3 (1 to 5) | 1.35 (1.14 to 1.59) | |
| < 1 | 1 499 975 | 35 | 23 | 1 388 287 | 32 | 48 | 35 | 11 (−1 to 24) | 1.48 (0.94 to 2.36) | |
| 1–4 | 6 186 032 | 96 | 16 | 5 346 009 | 83 | 146 | 27 | 12 (6 to 17) | 1.76 (1.35 to 2.30) | |
| 5–9 | 8 064 185 | 39 | 5 | 6 726 493 | 33 | 31 | 5 | 0 (−2 to 2) | 0.95 (0.57 to 1.57) | |
| 10–14 | 8 084 575 | 29 | 4 | 7 011 457 | 25 | 35 | 5 | 1 (−1 to 4) | 1.39 (0.83 to 2.36) | |
| 15–19 | 8 012 134 | 71 | 9 | 7 240 822 | 64 | 61 | 8 | 0 (−3 to 3) | 0.95 (0.66 to 1.36) | |
CI, confidence interval; PY, person–years.
a Hospitalizations per million (106) person–years.
b The number of hospitalizations expected in the post-vaccine period was derived by multiplying the hospitalization incidence rate in the pre-vaccine period by the number of person–years in the post-vaccine period.
c IRD is the incidence rate in pre-vaccine period minus incidence rate in post-vaccine period
d IRR is the incidence rate in post-vaccine period divided by incidence rate in pre-vaccine period
Fig. 2.
Incidencea of hospital admissions for empyema, Australia, 1998–2004 and 2005–2010
a Number of hospitalizations per million (106) person–years. There were 31 846 901 person–years in the period from July 1998 to June 2004 and 27 713 068 person–years in the period from July 2005 to June 2010.
Discussion
Hospitalizations for childhood empyema in Australia appear to have increased after the introduction of the PCV7, despite a significant decrease in hospitalizations for pneumonia as a whole over the same period. The reduction in the incidence of hospitalizations for pneumonia was greatest among children whose age made them eligible for vaccination (i.e. < 1 year and 1–4 years in age), but more modest reductions were also observed among older children. Although the increase in the incidence of hospitalizations for empyema (which is present in fewer than 1% of pneumonia hospitalizations) was greatly outweighed by the reduction in the incidence of hospitalizations for pneumonia, empyema accounts for an important fraction of the cases of severe, complicated pneumonia. We assume that the IRDs we report here are primarily the result of vaccine-attributable changes in the epidemiology of childhood pneumonia in Australia, although they could also be reflecting other factors, including underlying secular trends. Our method is analogous to the method used in studies in England and Scotland on trends in childhood pneumonia and empyema.18,19
We based this analysis on publicly available hospitalization and population data. A limitation of the study is that we used hospital discharge data coded for the primary diagnosis. Bacterial pneumonias in children are difficult to distinguish from viral pneumonias because bacterial culture has a low positive yield. Furthermore, as many as 33% of the children with pneumonia are infected by mixed pathogens.20 Hence, the coding data may be inaccurate. To avoid this problem, we chose to compare the hospitalization rates for pneumonia as a whole rather than for bacteria-specific pneumonias. The reduction in hospitalizations for bacterial pneumonia was accompanied by a smaller absolute increase in pneumonias coded as viral (Table 1). This might reflect changes in coding practices or perhaps improvements in molecular diagnostics and a greater availability of viral antigens over time. Pneumonias coded as viral decreased among infants. This was also seen in a field trial of conjugate vaccine that demonstrated a reduction in viral pneumonia, presumably because viral pneumonia in young children is often complicated by co-infection with pneumococcus.21
Another limitation of our study is that we only included hospitalizations for which pneumonia and empyema were the primary diagnosis. Hence, our findings probably represent minimum estimates of disease incidence. Furthermore, we were unable to perform subgroup analysis by ethnic group and place of residence because these data are not publicly available.
For convenience, we examined the period preceding and the period following the introduction of the PCV7. Since the vaccine was introduced in January 2005, we excluded from the analysis all data for the period from July 2004 to June 2005 (Table 1). Nonetheless, the post-vaccine period included some children whose age would not have made them eligible for vaccination, and this could have resulted in an underestimation of the vaccine’s effect except in the youngest age group (< 1 year). In addition, targeted vaccination of indigenous children occurred during part of the pre-vaccine period. However, this probably affected our estimates only minimally since indigenous people account for only 3% of the Australian population.22
Although the PCV7 has substantially reduced the rates of invasive pneumococcal disease, reports from around the world suggest that the incidence of empyema in children has increased following the introduction of the PCV7.10,11 The reasons for this are not entirely clear, but virulent serotypes not covered by the PCV7 may have emerged as a result of the selective pressure applied by the vaccine. Most cases of empyema among Australian children are caused by serotypes 1, 3 and 19A,23 which are believed to be particularly virulent and more likely to invade the pleural cavity.24,25 The reported increase in serotypes 1, 3 and 19A among invasive isolates might explain the increase in empyema but not in pneumonia per se.10–12 On the other hand, an increase in the incidence of empyema was observed in some studies before the introduction of the PCV7.2,26–28 This suggests that the observed association between the introduction of the PCV7 and the rise in empyema incidence may be coincidental.
Irrespective of its cause, the observed increase in empyema adds to health system costs. In a clinical study in the United Kingdom of Great Britain and Northern Ireland, we calculated that the cost per admission for a child with empyema was between 7600 and 11 700 United States dollars (US$).29 A study in the United States yielded similar estimates.30 Thus, the 35% increase in empyema admissions observed in Australia following the introduction of the PCV7 has brought an additional expenditure of US$ 126 160–194 220 per year to the Australian health-care system. On the other hand, the reduction in pneumonia admissions has saved the health system US$ 3.8 million–6.1 million annually (based on United Kingdom cost-analysis data for pneumonia admissions).31 Although modest, the additional costs owing to the increase in cases of empyema among children would be reduced by the use of a vaccine with broader pneumococcal serotype coverage.3
In July 2011 (October 2011 in Australia’s Northern Territory), Australia’s 13-valent pneumococcal conjugate vaccine was introduced into the national immunization programme. This vaccine includes six serotypes that the PCV7 does not contain, including serotypes 1, 3 and 19A. Despite this, the large number of pneumococcal serotypes that have been identified makes it likely that other virulent pneumococcal strains will emerge. This underscores the need for on-going, enhanced molecular surveillance of invasive pneumococcal strains, including those that cause empyema in children. The results of such surveillance, which is already being practised in the United Kingdom, will directly affect future policy decisions regarding the adoption of newer vaccines as part of the national immunization schedule in Australia.
Competing interests:
AJ previously received an unrestricted grant from GlaxoSmithKline, Belgium.
References
- 1.Burgner D, Richmond P. The burden of pneumonia in children: an Australian perspective. Paediatr Respir Rev. 2005;6:94–100. doi: 10.1016/j.prrv.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strachan R, Jaffé A, Australian Research Network in Empyema Assessment of the burden of paediatric empyema in Australia. J Paediatr Child Health. 2009;45:431–6. doi: 10.1111/j.1440-1754.2009.01533.x. [DOI] [PubMed] [Google Scholar]
- 3.Strachan RE, Cornelius A, Gilbert GL, Gulliver T, Martin A, McDonald T, et al. Australian Research Network in Empyema Bacterial causes of empyema in children, Australia, 2007–2009. Emerg Infect Dis. 2011;17:1839–45. doi: 10.3201/eid1710.101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitney CG, Farley MM, Hadler JL, Harrison LH, Bennett NM, Lynfield R, et al. Active Bacterial Core Surveillance of the Emerging Infections Program Network Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction – eight states, 1998–2005. MMWR Morb Mortal Wkly Rep. 2008;57:144–8. [PubMed] [Google Scholar]
- 6.Chapman KE, Wilson D, Gorton R. Serotype dynamics of invasive pneumococcal disease post-PCV7 and pre-PCV13 introduction in North East England. Epidemiol Infect. 2012;5:1–9. doi: 10.1017/S0950268812000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson DR, D’Onise K, Holland RA, Raupach JC, Koehler AP. Pneumococcal disease in South Australia: vaccine success but no time for complacency. Vaccine. 2012;30:2206–11. doi: 10.1016/j.vaccine.2011.12.119. [DOI] [PubMed] [Google Scholar]
- 8.Kellner JD, Vanderkooi OG, MacDonald J, Church DL, Tyrrell GJ, Scheifele DW. Changing epidemiology of invasive pneumococcal disease in Canada, 1998–2007: update from the Calgary-area Streptococcus pneumoniae research (CASPER) study. Clin Infect Dis. 2009;49:205–12. doi: 10.1086/599827. [DOI] [PubMed] [Google Scholar]
- 9.Lacapa R, Bliss SJ, Larzelere-Hinton F, Eagle KJ, McGinty DJ, Parkinson AJ, et al. Changing epidemiology of invasive pneumococcal disease among White Mountain Apache persons in the era of the pneumococcal conjugate vaccine. Clin Infect Dis. 2008;47:476–84. doi: 10.1086/590001. [DOI] [PubMed] [Google Scholar]
- 10.Calbo E, Díaz A, Cañadell E, Fábrega J, Uriz S, Xercavins M, et al. Spanish Pneumococcal Infection Study Network Invasive pneumococcal disease among children in a health district of Barcelona: early impact of pneumococcal conjugate vaccine. Clin Microbiol Infect. 2006;12:867–72. doi: 10.1111/j.1469-0691.2006.1502_1.x. [DOI] [PubMed] [Google Scholar]
- 11.Hendrickson DJ, Blumberg DA, Joad JP, Jhawar S, McDonald RJ. Five-fold increase in pediatric parapneumonic empyema since introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2008;27:1030–2. doi: 10.1097/INF.0b013e31817e5188. [DOI] [PubMed] [Google Scholar]
- 12.Byington CL, Korgenski K, Daly J, Ampofo K, Pavia A, Mason EO. Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema. Pediatr Infect Dis J. 2006;25:250–4. doi: 10.1097/01.inf.0000202137.37642.ab. [DOI] [PubMed] [Google Scholar]
- 13.Bekri H, Cohen R, Varon E, Madhi F, Gire R, Guillot F, et al. Streptococcus pneumoniae serotypes involved in children with pleural empyemas in France. Arch Pediatr. 2007;14:239–43. doi: 10.1016/j.arcped.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Byington CL, Spencer LY, Johnson TA, Pavia AT, Allen D, Mason EO, et al. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: Risk factors and microbiological associations. Clin Infect Dis. 2002;34:434–40. doi: 10.1086/338460. [DOI] [PubMed] [Google Scholar]
- 15.Calbo E, Garau J. Invasive pneumococcal disease in children: changing serotypes and clinical expression of disease. Clin Infect Dis. 2005;41:1821–2. doi: 10.1086/498316. [DOI] [PubMed] [Google Scholar]
- 16.Obando I, Arroyo LA, Sanchez-Tatay D, Moreno D, Hausdorff WP, Brueggemann AB. Molecular typing of pneumococci causing parapneumonic empyema in Spanish children using multilocus sequence typing directly on pleural fluid samples. Pediatr Infect Dis J. 2006;25:962–3. doi: 10.1097/01.inf.0000235684.89728.38. [DOI] [PubMed] [Google Scholar]
- 17.Obando I, Arroyo LA, Sanchez-Tatay D, Tarrago D, Moreno D, Hausdorff WP, et al. Molecular epidemiology of paediatric invasive pneumococcal disease in southern Spain after the introduction of heptavalent pneumococcal conjugate vaccine. Clin Microbiol Infect. 2007;13:347–8. doi: 10.1111/j.1469-0691.2006.01646.x. [DOI] [PubMed] [Google Scholar]
- 18.Roxburgh CS, Youngson GG, Townend JA, Turner SW. Trends in pneumonia and empyema in Scottish children in the past 25 years. Arch Dis Child. 2008;93:316–8. doi: 10.1136/adc.2007.126540. [DOI] [PubMed] [Google Scholar]
- 19.Koshy E, Murray J, Bottle A, Sharland M, Saxena S. Impact of the seven-valent pneumococcal conjugate vaccination (PCV7) programme on childhood hospital admissions for bacterial pneumonia and empyema in England: national time-trends study, 1997–2008. Thorax. 2010;65:770–4. doi: 10.1136/thx.2010.137802. [DOI] [PubMed] [Google Scholar]
- 20.Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, et al. British Thoracic Society Standards of Care Committee British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(Suppl 2):ii1–23. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- 21.Madhi SA, Klugman KP, Vaccine Trialist Group A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–3. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Australian Bureau of Statistics [Internet]. Aboriginal and Torres Strait Islander population estimates, 2011 – preliminary. Camberra: ABS; 2012. Available from: http://www.abs.gov.au/ausstats/abs@.nsf/Latestproducts/3101.0Feature%20Article1Mar%202012?opendocument&tabname=Summary&prodno=3101.0&issue=Mar%202012&num=&view= [accessed 10 December 2012].
- 23.Strachan RE, Cornelius A, Gilbert GL, Gulliver T, Martin A, McDonald T, et al. Pleural fluid nucleic acid testing enhances pneumococcal surveillance in children. Respirology. 2012;17:114–9. doi: 10.1111/j.1440-1843.2011.02035.x. [DOI] [PubMed] [Google Scholar]
- 24.Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis. 2005;5:83–93. doi: 10.1016/S1473-3099(05)01280-6. [DOI] [PubMed] [Google Scholar]
- 25.Luján M, Gallego M, Belmonte Y, Fontanals D, Vallès J, Lisboa T, et al. Influence of pneumococcal serotype group on outcome in adults with bacteraemic pneumonia. Eur Respir J. 2010;36:1073–9. doi: 10.1183/09031936.00176309. [DOI] [PubMed] [Google Scholar]
- 26.Eastham KM, Freeman R, Kearns AM, Eltringham G, Clark J, Leeming J, et al. Clinical features, aetiology and outcome of empyema in children in the north east of England. Thorax. 2004;59:522–5. doi: 10.1136/thx.2003.016105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Playfor SD, Smyth AR, Stewart RJ. Increase in incidence of childhood empyema. Thorax. 1997;52:932. [PubMed] [Google Scholar]
- 28.Singleton RJ, Hennessy TW, Bulkow LR, Hammitt LL, Zulz T, Hurlburt DA, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297:1784–92. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 29.Sonnappa S, Cohen G, Owens CM, van Doorn C, Cairns J, Stanojevic S, et al. Comparison of urokinase and video-assisted thoracoscopic surgery for treatment of childhood empyema. Am J Respir Crit Care Med. 2006;174:221–7. doi: 10.1164/rccm.200601-027OC. [DOI] [PubMed] [Google Scholar]
- 30.St Peter SD, Tsao K, Harrison C, Jackson MA, Spilde TL, Keckler SJ, et al. Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: a prospective, randomized trial. J Pediatr Surg. 2009;44:106–11. doi: 10.1016/j.jpedsurg.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorgelly PK, Atkinson M, Lakhanpaul M, Smyth AR, Vyas H, Weston V, et al. Oral versus i.v. antibiotics for community-acquired pneumonia in children: a cost-minimisation analysis. Eur Respir J. 2010;35:858–64. doi: 10.1183/09031936.00087209. [DOI] [PubMed] [Google Scholar]