Abstract
Objective
To develop a model for identifying areas at high risk for sporadic measles outbreaks based on an analysis of factors associated with a national outbreak in South Africa between 2009 and 2011.
Methods
Data on cases occurring before and during the national outbreak were obtained from the South African measles surveillance programme, and data on measles immunization and population size, from the District Health Information System. A Bayesian hierarchical Poisson model was used to investigate the association between the risk of measles in infants in a district and first-dose vaccination coverage, population density, background prevalence of human immunodeficiency virus (HIV) infection and expected failure of seroconversion. Model projections were used to identify emerging high-risk areas in 2012.
Findings
A clear spatial pattern of high-risk areas was noted, with many interconnected (i.e. neighbouring) areas. An increased risk of measles outbreak was significantly associated with both the preceding build-up of a susceptible population and population density. The risk was also elevated when more than 20% of infants in a populous area had missed a first vaccine dose. The model was able to identify areas at high risk of experiencing a measles outbreak in 2012 and where additional preventive measures could be undertaken.
Conclusion
The South African measles outbreak was associated with the build-up of a susceptible population (owing to poor vaccine coverage), high prevalence of HIV infection and high population density. The predictive model developed could be applied to other settings susceptible to sporadic outbreaks of measles and other vaccine-preventable diseases.
Résumé
Objectif
Mettre au point un modèle permettant d'identifier les zones à haut risque d'épidémies sporadiques de rougeole, sur la base d'une analyse des facteurs associés à une épidémie nationale en Afrique du Sud, entre 2009 et 2011.
Méthodes
Les données sur les cas survenus avant et pendant l'épidémie nationale ont été obtenues auprès du programme sud-africain de surveillance de la rougeole, et les données sur la vaccination contre la rougeole et la taille de la population auprès du système d'informations sanitaires des districts. Un modèle hiérarchique bayésien de Poisson a été utilisé pour étudier l'association entre le risque de rougeole chez les nourrissons dans un district et la couverture vaccinale pour la première dose, la densité de population, la prévalence sous-jacente de l'infection par le virus de l'immunodéficience humaine (VIH) et l'échec attendu de la séroconversion. Les projections du modèle ont été utilisées pour identifier les nouvelles zones à haut risque en 2012.
Résultat
Un schéma spatial clair des zones à haut risque a été observé, avec de nombreuses zones interconnectées (c'est-à-dire voisines). Un risque accru d'épidémie de rougeole a été significativement associé à la constitution préalable d'une population vulnérable ainsi qu'à la densité de population. Le risque était aussi élevé lorsque plus de 20% des nourrissons dans une zone fortement peuplée n'avaient pas reçu une première dose du vaccin. Le modèle a été en mesure d'identifier les zones à haut risque d’épidémie de rougeole en 2012, ainsi que là où des mesures préventives supplémentaires pourraient être prises.
Conclusion
L'épidémie de rougeole en Afrique du Sud a été associée à la constitution d'une population vulnérable (en raison d'une couverture vaccinale médiocre), à la prévalence élevée de l'infection par le VIH et à une forte densité de population. Le modèle prédictif développé pourrait être appliqué à d'autres contextes sensibles aux épidémies sporadiques de rougeole et à d'autres maladies qui peuvent être prévenues par la vaccination.
Resumen
Objetivo
Desarrollar un modelo para identificar las áreas con riesgo alto de sufrir brotes esporádicos de sarampión en base a un análisis de los factores asociados a un brote nacional en Sudáfrica entre los años 2009 y 2011.
Métodos
Los datos de los casos ocurridos antes y durante el brote nacional se obtuvieron del programa de vigilancia del sarampión de Sudáfrica, y la información acerca de la vacunación contra el sarampión y el tamaño de la población, del sistema de información sanitaria por distritos. Se empleó un modelo de Poisson jerárquico bayesiano para investigar la relación entre el riesgo de sarampión en niños de un distrito con la cobertura de la primera dosis de la vacuna, la densidad de población, el historial de prevalencia de la infección por el virus de la inmunodeficiencia humana (VIH) y el fracaso previsto de la seroconversión. Las proyecciones del modelo se utilizaron para identificar áreas de alto riesgo nuevas en 2012.
Resultados
Se observó un patrón espacial claro de las áreas de alto riesgo con muchas zonas conectadas (esto es, colindantes). El riesgo superior de sufrir un brote de sarampión se asoció de modo significativo tanto con el crecimiento previo de un grupo de población vulnerable como con la densidad de población, y también fue elevado cuando más del 20% de los niños en un área populosa no había recibido la primera dosis de la vacuna. El modelo fue capaz de identificar las áreas con riesgo alto de sufrir un brote de sarampión en 2012 y donde podrían emprenderse medidas de prevención adicionales.
Conclusión
El brote de sarampión en Sudáfrica estuvo asociado con el aumento de un grupo de población vulnerable (debido a una cobertura de vacunación insuficiente), una prevalencia elevada de la infección por VIH y una densidad de población alta. El modelo predictivo desarrollado podría aplicarse a otros lugares susceptibles de sufrir brotes esporádicos de sarampión y otras enfermedades inmunoprevenibles.
ملخص
الغرض
وضع نموذج لتحديد المناطق شديدة التعرض لمخاطر فاشيات الحصبة الفرادية بالاستناد إلى تحليل للعوامل المرتبطة بفاشية وطنية في جنوب أفريقيا في الفترة من 2009 إلى 2011.
الطريقة
تم الحصول على بيانات حول الحالات الواقعة قبل الفاشية الوطنية وأثناءها من برنامج ترصد الحصبة في جنوب أفريقيا، وبيانات حول التمنيع ضد الحصبة وحجم السكان من نظام المعلومات الصحية للمناطق. وتم استخدام نموذج بواسون الهرمي وفق نظرية بايز لتحري الارتباط بين مخاطر الحصبة لدى الرضع في تغطية التطعيم بالجرعة الأولى من لقاح الحصبة على مستوى المقاطعة والكثافة السكانية ومدى الانتشار الأساسي لعدوى فيروس العوز المناعي البشري والفشل المتوقع في انقلاب تفاعلية المصل. وتم استخدام استقراءات النموذج لتحديد المناطق شديدة التعرض للمخاطر المستجدة في عام 2012.
النتائج
لوحظ وجود نمط مكاني واضح للمناطق شديدة التعرض للمخاطر مع وجود العديد من المناطق المتداخلة (أي المتجاورة). وارتبط ازدياد مخاطر فاشية الحصبة إلى حد كبير بكل من التراكم السابق للسكان سريعي التأثر والكثافة السكانية. وازدادت كذلك المخاطر عندما فوت 20 % من الرضع في منطقة مكتظة بالسكان الجرعة الأولى من اللقاح. واستطاع النموذج تحديد المناطق شديدة التعرض لمخاطر الإصابة بفاشية الحصبة في عام 2012 والمناطق التي يمكن اتخاذ تدابير وقائية إضافية فيها.
الاستنتاج
ارتبطت فاشية الحصبة في جنوب أفريقيا بتراكم السكان سريعي التأثر (نظراً لضعف التغطية باللقاح) وارتفاع نسبة انتشار عدوى فيروس العوز المناعي البشري وزيادة الكثافة السكانية. ويمكن تطبيق النموذج التنبؤي الموضوع على البيئات الأخرى الحساسة لفاشيات الحصبة الفرادية وغيرها من الأمراض التي يمكن توقيها باللقاح.
摘要
目的
基于2009 年和2011 年之间南非全国疫情相关的因素分析,建立一种用以识别麻疹疫情点状爆发高风险地区的模型。
方法
从南非麻疹监测项目获取全国疫情之前和期间的病例数据,从区域卫生信息系统获取有关麻疹免疫和人口规模的数据。使用贝叶斯分层泊松模型研究一个地区婴儿麻疹风险和第一剂疫苗接种覆盖率、人口密度、艾滋病毒(HIV)感染背景患病率和预期血清转化失败之间的关系。使用模型预测识别2012 年新出现的高风险地区。
结果
指出一个明确的高风险地区空间形态,其具有多个相互关联的(即相邻)地区。麻疹爆发的风险增加与先前易感人群累积和人口密度显著相关。如果一个人口稠密的地区有超过20%的婴儿错过第一剂疫苗接种,则风险还会提高。该模型能够识别2012 年爆发麻疹疫情的高风险地区以及可以采取额外预防措施的地区。
结论
南非麻疹爆发与易感人群(由于较差的疫苗覆盖率)累积、HIV感染的高发生率和高人口密度相关。所建立的预测模型可以应用于其他易受麻疹和其他疫苗可预防疾病点状爆发影响的背景。
Резюме
Цель
Разработать модель определения районов повышенного риска возникновения спорадических вспышек кори на основе анализа факторов, связанных со вспышкой кори национального масштаба в Южной Африке в период с 2009 по 2011 гг.
Методы
Были получены данные по случаям заболевания корью Южноафриканской программы эпидемиологического надзора за корью, зафиксированным до и во время национальной вспышки, а также данные по противокоревой иммунизации и численности населения Окружной медицинской информационной системы. Для исследования связи риска возникновения кори у детей в округе с охватом первой дозы вакцины, плотностью населения, сопутствующим преобладанием инфекции вируса иммунодефицита человека (ВИЧ) и ожидаемым отсутствием сероконверсии использовалась байесовская иерархическая модель Пуассона. Проекции модели использовались для определения новых районов повышенного риска в 2012 году.
Результаты
Отмечена четкая пространственная картина районов повышенного риска со многими взаимосвязанными (т.е. соседними) районами. Повышенный риск возникновения вспышек кори в значительной степени был связан как с предшествующим ростом уязвимых слоев населения, так и с его плотностью. Риск возникновения вспышки также повышался, когда более 20% детей в густонаселенном районе пропускали первую дозу вакцины. Данная модель позволила определить районы повышенного риска возникновения вспышки кори в 2012 году, и районы, где могли быть предприняты профилактические меры.
Вывод
Вспышка кори в Южной Африке была связана с ростом уязвимых слоев населения (по причине недостаточного охвата населения прививкой), высоким уровнем заболеваемости ВИЧ-инфекцией и высокой плотностью населения. Разработанная модель прогнозированиямогла бы применяться в других условиях, подверженных спорадическим вспышкам кори и другим инфекциям, контролируемым методами вакцинопрофилактики.
Introduction
Measles is highly contagious and the transmission intensity1 of the measles virus exceeds that of most human pathogens.2 Despite progress in controlling measles since 2000, the disease remains endemic in many countries and killed over 164 000 children worldwide in 2008.1 Deaths also occur during outbreaks in areas where the disease is no longer endemic.3
Measles vaccination is highly effective, safe and relatively cost-effective4 and has interrupted measles transmission in most parts of the world,5 although high-risk areas remain in Africa and southern Asia. Immunization coverage is a key indicator for monitoring health sector performance and progress towards Millennium Development Goal 4 (i.e. reducing child mortality).6 Measles is difficult to control and eliminate and more than 90% of the population must be immune to interrupt transmission and prevent outbreaks.7–11 Maintaining adequate vaccine coverage throughout countries is a global problem and, in 2010 and 2011, outbreaks occurred even in the developed world.4 Moreover, since the efficacy of the first vaccine dose is thought to be around 85%,12,13 non-responders will add to the pool of susceptible children. In addition, achieving a high level of population immunity may be difficult in areas such as sub-Saharan Africa where the prevalence of human immunodeficiency virus (HIV) infection is high because infected children are less likely to respond to vaccination or maintain a protective antibody level or may acquire lower antibody levels from an HIV-positive (HIV+) mother.14–16 Vaccination may need to be repeated more frequently in these areas.16
Since 1995, several African countries have launched initiatives to eliminate measles, as recommended by the World Health Organization (WHO).17,18 These initiatives aim to: (i) achieve a coverage of 90% or more for the first vaccine dose; (ii) identify areas with low coverage and high-risk areas for “mop-up” immunization of children who missed vaccination; and (iii) enhance case-based measles surveillance with laboratory support.17
In South Africa, first and second measles vaccine doses have been given free of charge to children aged 9 and 18 months since April 2009 as part of the WHO Expanded Program on Immunization. At present, measles vaccination is mandatory and provided in the public sector, whereas combination vaccination against measles, mumps and rubella is available in the private sector at a cost. The South African National Department of Health introduced case-based measles surveillance in 1998 with laboratory support from the National Institute for Communicable Diseases.
A large outbreak of measles involving around 1700 cases occurred in South Africa between 2003 and 2005 following its introduction from Mozambique. More recently, there was an outbreak between 2009 and 2011, with over 18 000 cases recorded.4 The transient trough in cases following a large outbreak, which increases herd immunity, affords planners an opportunity to investigate the epidemic and refine prevention strategies.4 Spatial analysis has become an important epidemiological tool for detecting and predicting patterns of disease spread.19 In situations in which population-wide interventions are too expensive or are inefficient because they dilute available resources, the effectiveness of vaccination is increased by targeting high-risk areas, such as those implicated in measles transmission and those where vaccination coverage varies.20,21
The objectives of this study were: (i) to investigate the pattern of measles spread in the South African outbreak between 2009 and 2011; (ii) to identify associations between the outbreak and population density, HIV prevalence and preceding lapses in measles vaccination; and (iii) to develop a model for identifying areas at a high risk for future large-scale sporadic outbreaks that can be used in prevention.
Methods
Administratively, South Africa is divided into 9 provinces and 52 districts, which include 6 metropolitan areas.3 Districts are further disaggregated into 248 local municipalities, with metropolitan areas being governed by metropolitan municipalities. Health service delivery is largely a municipal function.
We obtained data on individual cases observed between 2008 and 2011 from the measles surveillance programme at the National Department of Health and the National Institute for Communicable Diseases. Data on measles vaccination coverage among infants (i.e. children aged under 1 year), on the proportion of HIV+ infants aged under 2 months and on relevant denominators (e.g. the total number of infants in a given district, by year) were obtained from the District Health Information System. The main unit of analysis was the district.
We calculated the overall incidence of measles in each week between 2009 and 2011 to identify temporal peaks. In addition, we calculated the incidence in infants in each district by dividing the observed number of infant measles cases by the total infant population in the district. To identify districts in which the incidence was significantly above the average, either before or during the outbreak, we derived 95% confidence intervals (CIs) for each incidence proportion using the binomial distribution of the observed number of events.22 The incidence in a district was considered significantly above average in a particular year if the national incidence for that period was below the lower limit (i.e. α = 0.025) of the incidence proportion for the district.23
Modelling the risk of measles
Standardized risk ratios (RR) were calculated for each district as the ratio of the observed to the expected number of infant measles cases in each district. The expected number of cases was the national measles incidence multiplied by the infant population of the district.
The most widely used strategy for tackling problems posed by small-area analysis is Bayesian hierarchical modelling.24 The specific approach we adopted was developed by Besag, York and Molliè25 and includes two random-effects terms (i.e. the convolution approach) that account for: (i) structured district contiguity and (ii) unstructured district heterogeneity. It is the most widely used spatial Poisson model and the basic kriging formulation, which includes no covariates, is as follows:
| Oi ~ Poisson(Eiλi) |
| log(λi) = α + εi + ωi | (1) |
where Oi is the observed number of measles cases in district i, Ei is the expected number of cases, λi is the Poisson mean or expected value (and the variance), α is a constant, εi is the unstructured district heterogeneity random effect and ωi is the conditional autoregressive spatial random effect accounting for district contiguity. Further details are provided in Appendix A (available at: http://www.fileswap.com/dl/fbTMr0X3gU/Appendix1.doc). This basic spatial model can also be extended to a space–time formulation: the full formulations involving Bayesian inference using Gibbs sampling (BUGS) are provided in Appendix B (available at: http://www.fileswap.com/dl/vEAvea1JcR/Appendix2.doc). If a large number of areas have excess or repeat zero counts, which is often the case with rare outcomes, then a zero-inflated Poisson approach must be used.26–28
In anomaly detection, Bayesian exceedance probabilities were used to detect areas in which the risk was significantly increased.29 We plotted model estimates on district maps that depicted smoothed estimates of the RR and the posterior probability that the RR was greater than 1. We used a more stringent version of Richardson’s criterion,29 in which a district with a Bayesian exceedance probability (i.e. the probability that the RR would exceed 1) greater than 0.9 was deemed to be a high-risk area.
To investigate the influence of preceding lapses in vaccination, the prevalence of HIV infection among infants and vaccine failure on the observed risk of measles, we tested various determinants at the bivariate and multivariate level using the Bayesian approach described above with:
| log(λi) = α + βXi + εi + ωi | (2) |
where Xi represents the vector of the associated covariates and β represents the corresponding regression coefficients. Factors found to be significant at the bivariate level (i.e. P < 0.2) were included in the multivariate model. Districts were divided into tertiles according to population density: low, medium and high density. All metropolitan areas were classified as high density but there were many high-density areas that were not metropolitan areas. We created a dummy variable with three categories to reflect this: low- to medium-population-density area, high-population-density nonmetropolitan area and high-population-density metropolitan area.
The formula below was developed to use data from the preceding year to estimate the size of the infant population susceptible to measles in each district, which is possibly the most important predictor of future high-risk areas. In this study, the proportion of infants who were not covered by vaccination in a given year was defined as the proportion that did not receive the first measles vaccine dose in that year.30 The proportion that was not immunized was the combination of the proportion not covered by vaccination and the proportion that would fail to seroconvert following the first vaccine dose, taking into account the influence of HIV infection on the likelihood of seroconversion. A previous study showed that mortality in HIV-infected children without access to antiretroviral therapy can limit the effect of the HIV epidemic on increasing measles virus transmission.31 Consequently, we refined the formula by factoring in expected mortality rates among HIV-negative (HIV−) and HIV+ infants, with the proportion of HIV+ infants being defined as the proportion receiving antiretroviral therapy. The interaction between the proportion susceptible to measles and population density was also assessed.
The size of the infant population susceptible to measles in each district in year t (i.e. St) was derived using data from year t−1 for that district, by adding those not covered to those with vaccine failure among covered, as follows:
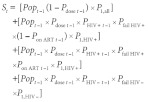 |
(3) |
where Popt−1 is the infant population of the district in year t−1, Pdose t−1 is the proportion of infants who receive their first measles dose in year t−1, P1,all is the proportion of all infants who survive to the age of 1 year, PHIV+ t−1 is the proportion of infants who are HIV+ in year t−1, Pfail HIV+ is the proportion of HIV+ infants who fail to seroconvert after the first measles vaccine dose, Pon ART t−1 is the proportion of HIV+ infants receiving antiretroviral therapy in year t−1, P1,HIV+ is the proportion of HIV+ infants who survive to the age of 1 year, PHIV– t−1 is the proportion of infants who are HIV− in year t−1, Pfail HIV– is the proportion of HIV– infants who fail to seroconvert after the first vaccine dose and P1,HIV– is the proportion of HIV– infants who survive to the age of 1 year.
The values of some of the parameters used in Equation 3 were based on data from the District Health Information System for 2006 to 2011.32 The proportion of infants who failed to seroconvert after the first vaccine dose was obtained from a recent meta-analysis33: 64% (95% CI: 49–78%) of HIV+ infants seroconverted by 9 months after measles vaccination, compared with between 62 and 100% (weighted average: 87%) of those who were exposed to HIV but did not become infected and between 76 and 92% (weighted average: 85%) of those who were not exposed to HIV. Hence, Pfail HIV+ was 0.36 (i.e. 1–0.64) and Pfail HIV− was 0.14 (i.e. [1–0.87] + [1–0.85] divided by 2). Values for the proportion of infants who survived to the age of 1 year were taken from a pooled analysis of data from sub-Saharan Africa, in which the estimate for P1,HIV+ was 0.648 and, for P1,HIV−, 0.951.34 The value for P1,all was derived from a weighted estimate of the proportion of infants who were HIV+ or HIV− and their respective expected survival proportions. The proportion of HIV+ infants receiving antiretroviral therapy was estimated to be 0.19 in 2008, 0.27 in 2009, 0.31 in 2010 and 0.40 in 2011.35,36 The analysis was carried out using Stata (StataCorp. LP, College Station, United States of America) and WinBUGS (MRC Biostatistics Unit, Cambridge, United Kingdom of Great Britain and Northern Ireland). Risk maps were constructed in MapInfo Professional (Pitney Bowes Inc., Stamford, USA).
Results
Demographic data showed that, during the 2009 to 2011 measles outbreak, the incidence of the disease was highest among infants: 61 per 10 000 (95% CI: 59.3–62.4). The second highest incidence was in children aged 1 to 4 years: 7.3 cases per 10 000 (95% CI: 7.0–7.6). The incidence in children aged 10 to 14 years and in those aged 15 to 19 years was higher than in those aged 5 to 9 years. The baseline incidence among infants before and after the outbreak was 1.0 per 10 000 (95% CI: 0.9–1.3).
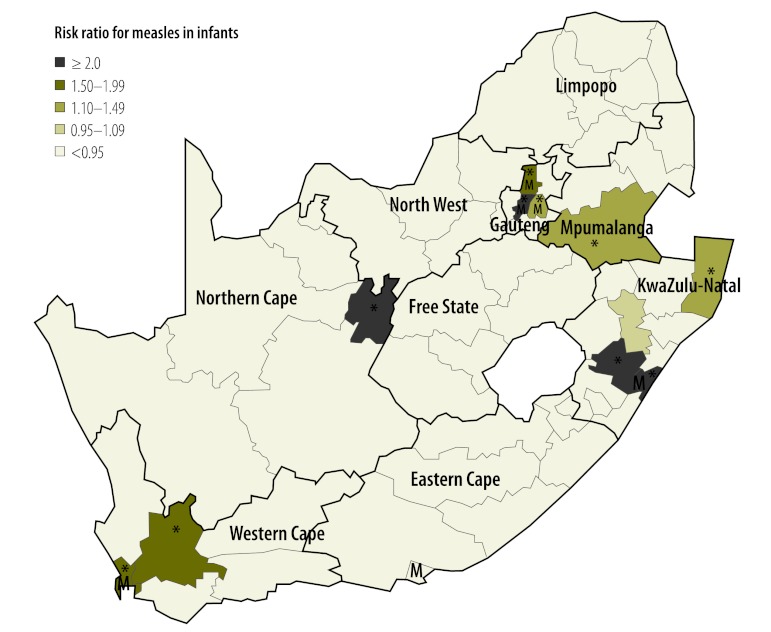
There were two peaks in measles incidence in infants during the outbreak: the smaller peak occurred in week 43 of 2009 and the slightly larger peak occurred approximately 20 weeks later, in week 10 of 2010 (Fig. 1). Spatial analysis identified districts in which the risk of measles was significantly increased over the course of the outbreak (Fig. 2 and Fig. 3). The distribution of disease incidence was distinctly nonrandom (Moran’s I global P < 0.001). Several neighbouring (i.e. connected) high-risk districts were observed: in Western Cape Province in the south-west of the country, in Gauteng and Mpumalanga Provinces in the north and in KwaZulu-Natal Province on the east coast (Fig. 2). In addition, the incidence was elevated in metropolitan areas. Fig. 3 shows the estimated incidence of measles in infants in all districts, the national baseline incidence in infants before the start of the outbreak and the national incidence during the outbreak. The two districts with the highest incidence were in Northern Cape and KwaZulu-Natal Provinces respectively, followed by the Johannesburg (Gauteng) and eThekwini (KwaZulu-Natal) metropolitan areas.
Fig. 1.
Incidence of measles in infants during an outbreak, South Africa, 2009–2011
Fig. 2.
Risk ratios for measles in infants in districts during an outbreak, South Africa, 2009–2011
Metropolitan areas are indicated by an M.
The * indicates districts in which the risk was significantly elevated as indicated by its Bayesian exceedance probability determined using a Bayesian convolution conditional autoregressive model.
Fig. 3.
Incidence of measles among infants in all districts during an outbreak, South Africa, 2009–2011
ECP, Eastern Cape Province; FSP, Free State Province; GAP, Gauteng Province; KZP, KwaZulu-Natal Province; LPP, Limpopo Province; MPP, Mpumalanga Province; NCP, Northern Cape Province; NWP, North West Province; WCP, Western Cape Province.
The length of the horizontal lines indicates the 95% confidence interval.
The grey dashed line indicates the baseline national incidence of around 1.0 per 10 000 before and after the outbreak.
The black dashed line indicates the national incidence of around 61 per 10 000 during the outbreak.
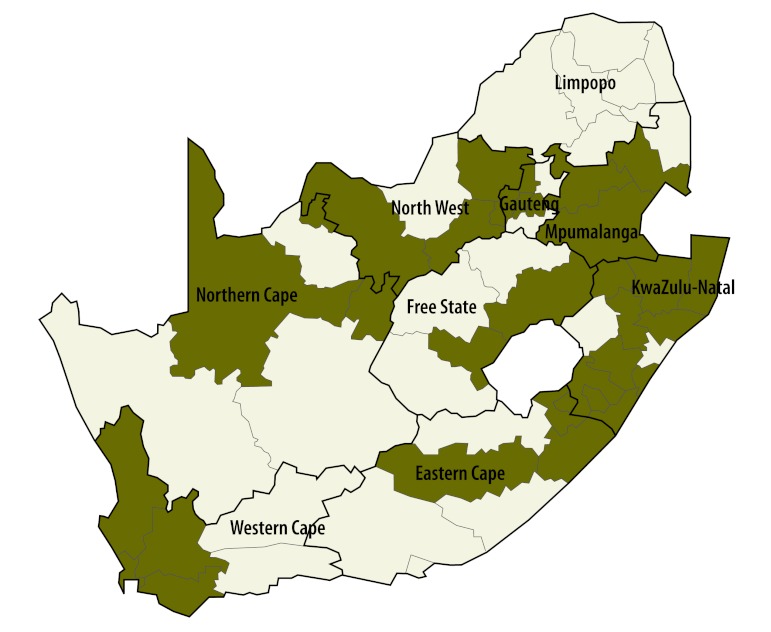
The first significant elevation in risk occurred in the Tshwane metropolitan area during weeks 35 and 36 of 2009, before the first peak in incidence. By week 37, the significant rise in risk had spread to the neighbouring metropolitan areas of Johannesburg and Ekuruleni. An analysis of the period leading up to the first peak suggested that the outbreak was concentrated in Gauteng province. Fig. 4 and Fig. 5 show the districts most affected by the outbreak at the two peaks in incidence. The districts with the highest incidence were similar at the two peaks, though the outbreak was more widespread at the first peak. There appeared to be no spread into surrounding districts.
Fig. 4.
Elevated risk of measles in infants in districts during the first outbreak peak,a South Africa, 2009
The shaded districts are those in which the risk was significantly elevated as indicated by their Bayesian exceedance probabilities determined using a Bayesian convolution conditional autoregressive model.
a The first outbreak peak occurred around week 43 in 2009.
Fig. 5.
Elevated risk of measles in infants in districts during the second outbreak peak,a South Africa, 2010
The shaded districts are those in which the risk was significantly elevated as indicated by their Bayesian exceedance probabilities determined using a Bayesian convolution conditional autoregressive model.
a The second outbreak peak occurred around week 10 in 2010.
Factors associated with measles outbreaks
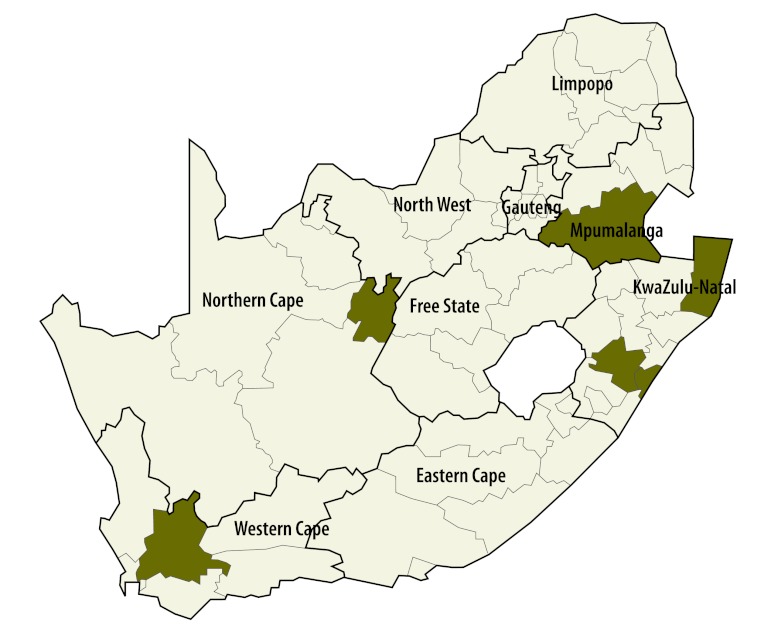
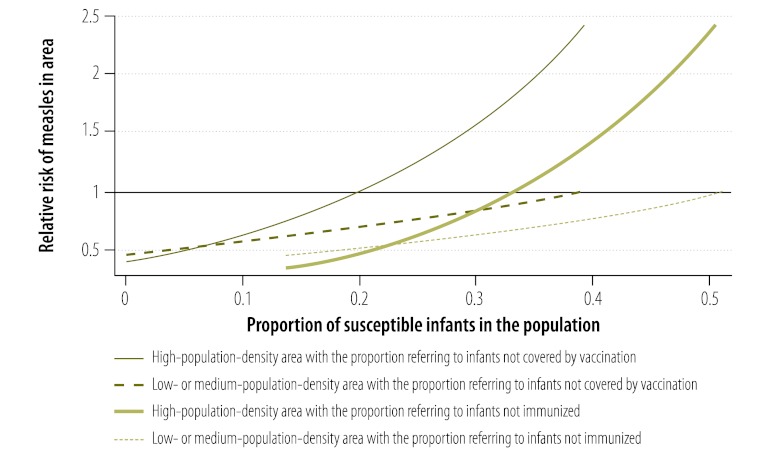
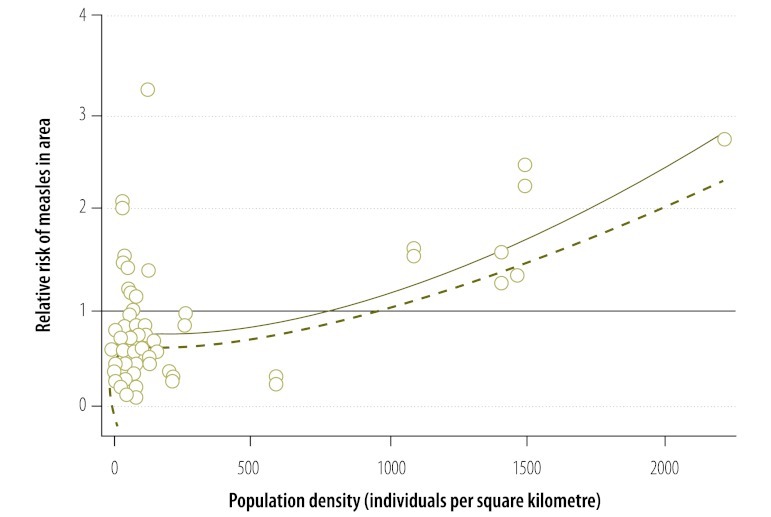
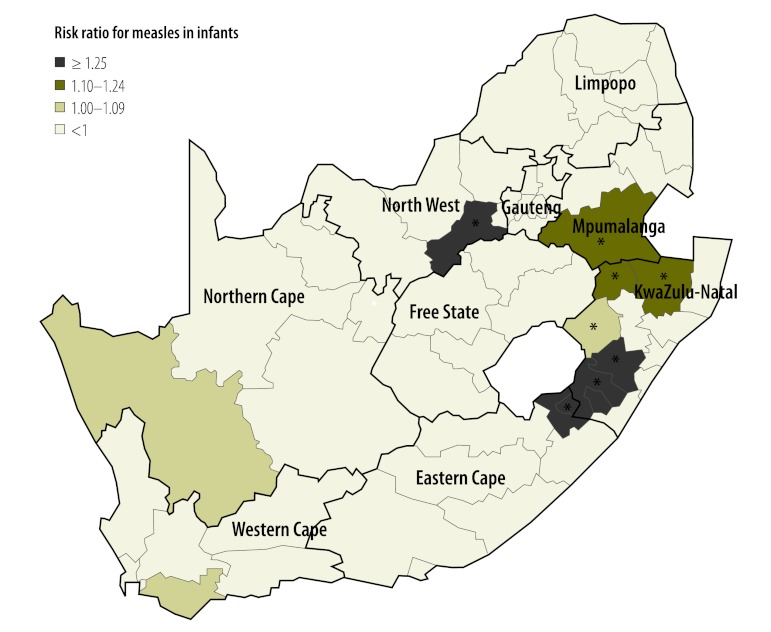
The average coverage of the first dose of measles vaccine in all districts was 88.1% (range: 85.4–90.7). Many districts repeatedly failed to meet the target of 90%: 56.9%, 49.0% and 64.5% of districts had coverage below this critical value in 2008, 2009 and 2010, respectively. The risk of measles in infants in a district showed a significant positive association with the proportion of unvaccinated infants and with the proportion of infants not immunized in the preceding year (Table 1). In addition, the risk of infection was increased in metropolitan districts, all of which had a high population density (P < 0.001). The model predicted that the critical cut-off proportion for coverage with the first vaccine dose is 0.2. Consequently, the risk would be elevated (i.e. RR > 1) in highly populated areas in which the percentage of susceptible infants exceeds 20% because of poor vaccination coverage in preceding years (Fig. 6). Bivariate interactions indicate that the threshold would be higher in less densely populated areas. Districts with a population density of more than 750 individuals per square kilometre also appear to be at an elevated risk (Fig. 7). Using data on the level of coverage of the first vaccine dose in districts in 2011, the model predicted that the high-risk areas in which the proportion of susceptible infants would exceed the critical cut-off level in 2012 lay mainly in KwaZulu-Natal (Fig. 8). Since these areas are highly connected (i.e. neighbouring), the outbreak could spread rapidly.
Table 1. Risk of measles in infants during an outbreak, South Africa, 2009–2011.
| Parameter | Multivariate analysis (coverage)a |
Multivariate analysis (immunization)b |
|||
|---|---|---|---|---|---|
| RRc (95% BCId) | RRe (95% CI) | RRc (95% BCId) | RRe (95% CI) | ||
| District parameter | |||||
| Low- or medium-population-density nonmetropolitan area | 1 (ref.) | NA | 1 (ref.) | NA | |
| High-population-density nonmetropolitan area | 1.23 (0.64–2.43) | NA | 1.24 (0.65–2.54) | NA | |
| High-population-density metropolitan area | 4.37 (2.27–8.56) | NA | 4.42 (1.60–9.54) | NA | |
| Proportion of infants not covered by vaccinationf in preceding yearg | 7.84 (5.19–13.2) | NA | NA | NA | |
| Proportion of infants not immunizedh in preceding yearg | NA | NA | 12.88 (6.52–32.43) | NA | |
| Interaction between infant population susceptible to measles and population densityi | |||||
| Proportion of infants not covered by vaccinationf in the preceding year × high population density | NA | 66.20 (40.49–108.25) | NA | NA | |
| Proportion of infants not covered by vaccinationf in the preceding year × low or medium population density | NA | 7.45 (4.72–11.77) | NA | NA | |
| Proportion of infants not immunizedh in the preceding year × high population density | NA | NA | NA | 89.35 (50.65–157.60) | |
| Proportion of infants not immunizedh in the preceding year × low or medium population density | NA | NA | NA | 8.89 (5.14–15.38) | |
BCI, Bayesian credibility interval; CI, confidence interval; NA, not applicable; RR, risk ratio.
a The analysis considered infants aged under 1 year who were not covered by vaccination in the preceding year (i.e. they did not receive their first measles vaccine dose).
b The analysis considered infants aged under 1 year who were not immunized in the preceding year (i.e. they were not covered by vaccination or failed to seroconvert following the first vaccine dose).
c The multivariate association between parameters and the incidence of measles among infants was evaluated using a Bayesian Poisson conditional autoregressive convolution model.
d The 95% Bayesian credibility interval is analogous to the conventional 95% confidence interval.
e The multivariate association between parameters and the incidence of measles among infants was evaluated using a conventional Poisson approach.
f The proportion of infants that did not receive the first measles vaccine dose.
g The risk ratio is for a 1% increase, on a continuous scale, in the proportion of infants who were covered by vaccination or immunized.
h The formula used to calculate this proportion is described in the methods.
i Since there was a strong, significant correlation between population density and metropolitan area (i.e. Spearman ρ = 0.64, P < 0.001), the interaction between metropolitan area and high population density was not included.
Fig. 6.
Predicteda relative risk of measles in infants in a populated area, by proportion of susceptible infantsb
Infants were regarded as not covered by vaccination if they did not receive their first measles vaccine dose.
Infants were regarded as not immunized if either they were not covered by vaccination or they failed to seroconvert following the first vaccine dose.
a Predicted by the model (see text).
b Infants were susceptible to measles because of poor vaccination coverage in preceding years or because they failed to seroconvert.
Fig. 7.
Relative risk of measles in infants, by district population density, South Africa, 2009–2011
The circles indicate the observed relative risk and population density in individual districts.
The solid line represents the fitted association between the observed relative risk and population density and the dashed line represents the lower bound of the 95% confidence interval for the association.
Fig. 8.
Predicteda high-risk areas for measles in infants in 2012, South Africa
The * indicates the districts in which the risk was significantly elevated.
a Predicted by the model (see text) by taking into account the proportion of infants not covered by the first vaccine dose in 2011.
Discussion
The study showed that HIV infection, preceding lapses in vaccination and high population density were associated with an increased risk of measles outbreak and the predictive model we developed provides a means of identifying areas at high risk of a future outbreak or where herd immunity does not meet elimination criteria. In resource-limited settings, it is essential to focus on high-risk areas where vaccination coverage is poor and where the build-up of a susceptible population could fuel future outbreaks. Our model, which takes into account the influence of HIV infection, could be applied to other settings where sporadic measles outbreaks remain a problem and can be modified easily for application to other vaccine-preventable diseases.
Infants were the group at highest risk in the measles outbreak we examined. This is not surprising given that their immune systems are undeveloped. Although maternal measles antibodies generally protect infants in the first 6 months of life, studies suggest that the infants of mothers with vaccine-induced immunity and of HIV+ mothers receive significantly fewer antibodies.37–39 In addition, HIV infection in infants in sub-Saharan Africa has been shown to reduce their ability to seroconvert following vaccination.33 These factors have implications for herd immunity: the build-up of a susceptible population in an area with low vaccine coverage and a high prevalence of HIV infection could lead to a large measles outbreak. In areas where measles and HIV infection are prevalent,40 both HIV-infected infants and infants exposed to HIV but not infected could benefit from earlier vaccination.38
We found that, overall, national coverage with the first measles vaccine dose in South Africa was high, at around 88%, but was variable, with the coverage in many districts routinely falling well below 90%. A previous study in the country concluded that the heterogeneity in measles vaccine coverage challenges the goal of eliminating measles and that supplemental immunization activities have a negative effect on routine immunization coverage and health system functioning.41,42 Areas with repeatedly low vaccine coverage should be targeted for improvement. It is also important to coordinate supplemental immunization activities between neighbouring countries during widespread outbreaks. It has been reported that the cases observed immediately following supplemental immunization tend to be due to immigration from nearby areas where it has not taken place.43
Our findings suggest that both the build-up of a susceptible population due to poor vaccine coverage and high population density contributed to the South African outbreak. Similarly, the large measles outbreak in Sierra Leone during 2009 and 2010 was probably caused by the accumulation of susceptible individuals, largely due to nonvaccination.44 The cumulative impact of nonvaccination is important and justifies using our predictive model to help identify and target areas with a large number of susceptible individuals. We observed that the measles outbreak in South Africa had a strong nonrandom spatial distribution with strong connectivity, as seen in other studies.11,45 Previous studies also indicate that measles outbreaks generally persist through epidemic troughs when the community is above a critical size. Below this threshold, measles tends towards extinction during the troughs.45,46 Our findings indicate that once the percentage of the population susceptible to measles in a densely populated district exceeds 20%, the risk of infection is increased. Districts with a smaller susceptible population appeared remarkably resilient to measles infiltration during the outbreak. Our observation that the risk of measles outbreak was significantly elevated in areas with a high population density and in metropolitan areas has also been reported in other settings.11,45 The highly connected nature of these areas may have contributed to the persistence of the outbreak.
Study limitations include possible underreporting of measles cases, fragmentation of vaccination services, a change in measles vaccination schedule in 2009 and repeated changes in reporting forms during the past 5 years. Underreporting in particular districts may have affected the spatial pattern observed and weakened associations with key indicators. In addition, we cannot discount the possibility of ecological bias given that aggregated measles vaccination and HIV infection data were used.
Our study has important implications for the elimination of measles in South Africa and sub-Saharan Africa. Lapses in immunization, a high burden of HIV infection and the build-up of a susceptible population appear to be important for driving sporadic outbreaks, especially in populous areas. Supplemental immunization is by definition a catch-up strategy. It is also costly, may not be implemented quickly enough to limit outbreaks and has a negative effect on routine immunization services.41,47 Identifying and targeting emerging high-risk areas in resource-limited settings where vaccine coverage is low or waning appears a more viable strategy for preventing outbreaks in sub-Saharan Africa than mass supplemental immunization. Improved vaccine coverage of small areas through highly targeted campaigns and by strengthening routine services are possible solutions.
Acknowledgements
We thank Professor John Pettifor. Cheryl Cohen is also affiliated with the School of Public Health, University of the Witwatersrand.
Competing interests:
None declared.
References
- 1.Centers for Disease Control and Prevention Global measles mortality, 2000–2008. MMWR Morb Mortal Wkly Rep. 2009;58:1321–6. [PubMed] [Google Scholar]
- 2.Moss WJ, Griffin DE. Global measles elimination. Nat Rev Microbiol. 2006;4:900–8. doi: 10.1038/nrmicro1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairns KL, Perry RT, Ryman TK, Nandy RK, Grais RF. Should outbreak response immunization be recommended for measles outbreaks in middle- and low-income countries? An update. J Infect Dis. 2011;204:S35–46. doi: 10.1093/infdis/jir072. [DOI] [PubMed] [Google Scholar]
- 4.Schoub BD. Lessons from the 2009 measles epidemic in South Africa. S Afr Med J. 2011;101:519. [PubMed] [Google Scholar]
- 5.Cliff A, Haggett P, Smallman-Raynor M. Measles: an historical geography of a major human viral disease from global expansion to local retreat, 1840–1990 Oxford: Blackwell; 1993. [Google Scholar]
- 6.United Nations Development Group. Indicators for monitoring the Millennium Development Goals: definitions, rationale, concepts and sources New York: United Nations; 2003. [Google Scholar]
- 7.Fine PE. Herd immunity: history, theory, practice. Epidemiol Rev. 1993;15:265–302. doi: 10.1093/oxfordjournals.epirev.a036121. [DOI] [PubMed] [Google Scholar]
- 8.Gay NJ. The theory of measles elimination: implications for the design of elimination strategies. J Infect Dis. 2004;189(Suppl 1):S27–35. doi: 10.1086/381592. [DOI] [PubMed] [Google Scholar]
- 9.Kelly H, Riddell M, Heywood A, Lambert S. WHO criteria for measles elimination: a critique with reference to criteria for polio elimination. Euro Surveill. 2009;14 [PubMed] [Google Scholar]
- 10.van Boven M, Kretzschmar M, Wallinga J, O’Neill PD, Wichmann O, Hahné S. Estimation of measles vaccine efficacy and critical vaccination coverage in a highly vaccinated population. J R Soc Interface. 2010;7:1537–44. doi: 10.1098/rsif.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari MJ, Grais RF, Bharti N, Conlan AJ, Bjørnstad ON, Wolfson LJ, et al. The dynamics of measles in sub-Saharan Africa. Nature. 2008;451:679–84. doi: 10.1038/nature06509. [DOI] [PubMed] [Google Scholar]
- 12.Hull HF, Williams PJ, Oldfield F. Measles mortality and vaccine efficacy in rural West Africa. Lancet. 1983;1:972–5. doi: 10.1016/S0140-6736(83)92091-3. [DOI] [PubMed] [Google Scholar]
- 13.Killewo J, Makwaya C, Munubhi E, Mpembeni R. The protective effect of measles vaccine under routine vaccination conditions in Dar es Salaam, Tanzania: a case-control study. Int J Epidemiol. 1991;20:508–14. doi: 10.1093/ije/20.2.508. [DOI] [PubMed] [Google Scholar]
- 14.Moss WJ, Cutts F, Griffin DE. Implications of the human immunodeficiency virus epidemic for control and eradication of measles. Clin Infect Dis. 1999;29:106–12. doi: 10.1086/520136. [DOI] [PubMed] [Google Scholar]
- 15.Permar SR, Moss WJ, Ryon JJ, Monze M, Cutts F, Quinn TC, et al. Prolonged measles virus shedding in human immunodeficiency virus-infected children, detected by reverse transcriptase-polymerase chain reaction. J Infect Dis. 2001;183:532–8. doi: 10.1086/318533. [DOI] [PubMed] [Google Scholar]
- 16.Moss WJ, Scott S, Mugala N, Ndhlovu Z, Beeler JA, Audet SA, et al. Immunogenicity of standard-titer measles vaccine in HIV-1-infected and uninfected Zambian children: an observational study. J Infect Dis. 2007;196:347–55. doi: 10.1086/519169. [DOI] [PubMed] [Google Scholar]
- 17.Measles mortality reduction and regional elimination strategic plan 2001–2005 Geneva: World Health Organization & United Nations Children’s Fund, New York; 2001. [Google Scholar]
- 18.Centers for Disease Control and Prevention Progress toward measles elimination–Southern Africa, 1996–1998. MMWR Morb Mortal Wkly Rep. 1999;48:585–9. [PubMed] [Google Scholar]
- 19.Lawson A. Statistical methods in spatial epidemiology Hoboken: Wiley; 2001. [Google Scholar]
- 20.Benzler J, Sauerborn R. Rapid risk household screening by neonatal arm circumference: results from a cohort study in rural Burkina Faso. Trop Med Int Health. 1998;3:962–74. doi: 10.1046/j.1365-3156.1998.00340.x. [DOI] [PubMed] [Google Scholar]
- 21.Cutts FT, Markowitz LE. Successes and failures in measles control. J Infect Dis. 1994;170:S32–41. doi: 10.1093/infdis/170.Supplement_1.S32. [DOI] [PubMed] [Google Scholar]
- 22.Este`ve J, Benhamou E, Raymond L. Statistical methods in cancer research: vol. IV, descriptive epidemiology Lyon: International Agency for Research on Cancer; 1994. [PubMed] [Google Scholar]
- 23.Pickle L, Mason T, Howard N, Hoover R, Fraumeni J. Atlas of US cancer mortality among whites: 1950-1980 Washington: United States Government Printing Office; 1987 (DHHS Publication No (NIH) 87-2900). [Google Scholar]
- 24.Gilks W, Richardson S, Spiegelhalter D. Markov chain Mont Carlo in practice London: Chapman and Hall; 1996. [Google Scholar]
- 25.Besag J, York J, Molliè A. Bayesian image restoration, with applications in spatial statistics. Ann Inst Stat Math. 1991;43:1–20. doi: 10.1007/BF00116466. [DOI] [Google Scholar]
- 26.Ghosh SK, Mukhopadhyay P, Lu JC. Bayesian analysis of zero-inflated regression models. J Statist Plann Inference. 2006;136:1360–75. doi: 10.1016/j.jspi.2004.10.008. [DOI] [Google Scholar]
- 27.Ramis Prieto R, García-Pérez J, Pollán M, Aragonés N, Pérez-Gómez B, López-Abente G. Modelling of municipal mortality due to haematological neoplasias in Spain. J Epidemiol Community Health. 2007;61:165–71. doi: 10.1136/jech.2005.041491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sartorius BK, Sartorius K, Chirwa TF, Fonn S. Infant mortality in South Africa–distribution, associations and policy implications, 2007: an ecological spatial analysis. Int J Health Geogr. 2011;10:61. doi: 10.1186/1476-072X-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson S, Thomson A, Best N, Elliott P. Interpreting posterior relative risk estimates in disease-mapping studies. Environ Health Perspect. 2004;112:1016–25. doi: 10.1289/ehp.6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO-recommended standards for surveillance of selected vaccine-preventable diseases Geneva: World Health Organization; 2003. [Google Scholar]
- 31.Scott S, Mossong J, Moss WJ, Cutts FT, Cousens S. Predicted impact of the HIV-1 epidemic on measles in developing countries: results from a dynamic age-structured model. Int J Epidemiol. 2008;37:356–67. doi: 10.1093/ije/dyn007. [DOI] [PubMed] [Google Scholar]
- 32.Day C, Barron P, Monticelli F, Sello E. The district health barometer 2007/2008 Durban: Health Systems Trust; 2009. [Google Scholar]
- 33.Scott P, Moss WJ, Gilani Z, Low N. Measles vaccination in HIV-infected children: systematic review and meta-analysis of safety and immunogenicity. J Infect Dis. 2011;204:S164–78. doi: 10.1093/infdis/jir071. [DOI] [PubMed] [Google Scholar]
- 34.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F, Ghent International AIDS Society Working Group on HIV Infection in Women and Children Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 35.Johnson LF, Davies MA, Moultrie H, Sherman GG, Bland RM, Rehle TM, et al. The effect of early initiation of antiretroviral treatment in infants on pediatric AIDS mortality in South Africa: a model-based analysis. Pediatr Infect Dis J. 2012;31:474–80. doi: 10.1097/INF.0b013e3182456ba2. [DOI] [PubMed] [Google Scholar]
- 36.Mid-year population estimates, 2011 Pretoria: Statistics South Africa: 2011. [Google Scholar]
- 37.Leuridan E, Hens N, Hutse V, Ieven M, Aerts M, Van Damme P. Early waning of maternal measles antibodies in era of measles elimination: longitudinal study. BMJ. 2010;340:c1626. doi: 10.1136/bmj.c1626. [DOI] [PubMed] [Google Scholar]
- 38.Scott S, Moss WJ, Cousens S, Beeler JA, Audet SA, Mugala N, et al. The influence of HIV-1 exposure and infection on levels of passively acquired antibodies to measles virus in Zambian infants. Clin Infect Dis. 2007;45:1417–24. doi: 10.1086/522989. [DOI] [PubMed] [Google Scholar]
- 39.Scott P, Moss WJ, Gilani Z, Low N. Measles vaccination in HIV-infected children: systematic review and meta-analysis of safety and immunogenicity. J Infect Dis. 2011;204:S164–78. doi: 10.1093/infdis/jir071. [DOI] [PubMed] [Google Scholar]
- 40.Global reductions in measles mortality 2000–2008 and the risk of measles resurgence. Wkly Epidemiol Rec. 2009;84:505-8. [PubMed] [Google Scholar]
- 41.Verguet S, Jassat W, Hedberg C, Tollman S, Jamison DT, Hofman KJ. Measles control in sub-Saharan Africa: South Africa as a case study. Vaccine. 2012;30:1594–600. doi: 10.1016/j.vaccine.2011.12.123. [DOI] [PubMed] [Google Scholar]
- 42.Dietz V, Cutts F. The use of mass campaigns in the expanded program on immunization: a review of reported advantages and disadvantages. Int J Health Serv. 1997;27:767–90. doi: 10.2190/QPCQ-FBF8-6ABX-2TB5. [DOI] [PubMed] [Google Scholar]
- 43.Yaméogo KR, Perry RT, Yaméogo A, Kambiré C, Kondé MK, Nshimirimana D, et al. Migration as a risk factor for measles after a mass vaccination campaign, Burkina Faso, 2002. Int J Epidemiol. 2005;34:556–64. doi: 10.1093/ije/dyi001. [DOI] [PubMed] [Google Scholar]
- 44.Sugerman DE, Fall A, Guigui MT, N’dolie M, Balogun T, Wurie A, et al. Preplanned national measles vaccination campaign at the beginning of a measles outbreak–Sierra Leone, 2009–2010. J Infect Dis. 2011;204:S260–9. doi: 10.1093/infdis/jir110. [DOI] [PubMed] [Google Scholar]
- 45.Bharti N, Djibo A, Ferrari MJ, Grais RF, Tatem AJ, McCabe CA, et al. Measles hotspots and epidemiological connectivity. Epidemiol Infect. 2010;138:1308–16. doi: 10.1017/S0950268809991385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartlett MS. Measles periodicity and community size. J R Stat Soc. 1957;120:48–70. doi: 10.2307/2342553. [DOI] [Google Scholar]
- 47.Heymann DL, Fine PE, Griffiths UK, Hall AJ, Mounier-Jack S. Measles eradication: past is prologue. Lancet. 2010;376:1719–20. doi: 10.1016/S0140-6736(10)61934-4. [DOI] [PubMed] [Google Scholar]