Abstract
Background:
Adverse drug reactions (ADR) are ranked as some of the major causes of patient morbidity and mortality. Spontaneous reporting of ADRs has remained the cornerstone of pharmacovigilance and is important in maintaining patient safety. This study was conducted to assess the nurses’ knowledge and attitude towards pharmacovigilance, reasons for not reporting ADRs, and their pharmacovigilance practice.
Materials and Methods:
A questionnaire was prepared to investigate knowledge, attitude and practice (KAP) of nurses regarding ADR reporting. In November 2009, the questionnaires were given to 500 nurses of a teaching hospital in Tehran.
Findings:
Knowledge and practice of participants were not satisfying; however, their attitude towards pharmacovigilance was at a high level. About 91% of the nurses had never reported an ADR. Most nurses liked to report the ADRs to the physicians (87.1%) and pharmacists in hospital's ADR center (1.8%) rather than the ADR National Center. The main cause of under-reporting of the suspected ADRs was unawareness about the existence of such a national center. Among nurses who had reported ADR for at least once, the majority preferred using phone (10 out of 50) or Yellow Cards (7 out of 50). Only 1 person out of 50 preferred using internet for submitting the reports
Conclusions:
Since the nurses in this study had little knowledge and poor practice regarding the pharmacovigilance and spontaneous reporting system, interventions such as holding pharmacovigilance workshops in the hospitals focusing on the aims of pharmacovigilance, completing the Yellow Card and clarifying the reporting criteria are strongly recommended.
Keywords: Knowledge, attitude, practice, nurse, adverse drug reaction, pharmacovigilance, Iran
INTRODUCTION
Adverse drug reactions (ADRs) are negative consequences of drug therapy.[1] They are one of the leading causes of morbidity and mortality. It has been estimated that around 2.9-5.6% of all hospital admissions are due to ADRs and as many as 35% of hospitalized patients experience an ADR during their hospitalization.[2] Fatal ADRs rank among the most common causes of death in the United States.[3,4] The economic burden of ADRs is also considerable; for example in the United States, annual total cost of $47.4 billion for 8.7 million drug related admissions were reported.[5] ADR spontaneous reporting systems are the basic components for the comprehensive post-marketing surveillance of drug-induced risks. It may detect previously unrecognized adverse reactions and identify risk factors that predispose to drug toxicity and investigate causality.[3,6] Spontaneous reporting of ADRs has remained the cornerstone of pharmacovigilance and is important in maintaining patient safety. However, reporting of serious ADRs rarely exceeds 10%.[7] Under-reporting of ADRs is a common problem in pharmacovigilance programs.[8,9]
The initiative of an international reporting system for ADRs came in the wake of the thalidomide tragedy in the early 1960s. Although the Food and Drug Administration had been established some years previously in the United States, this disaster was the catalyst for the initiation of a systematic collection of data on ADRs primarily through the hospital reporting program.[10] Voluntary ADR reporting schemes have operated since the early 1960s in many western countries, first one started in the United Kingdom in 1964. The Iranian Pharmacovigilance Center (IPC) was established in 1998. According to the World Health Organization standards, countries with the best reporting rates generate over 200 reports per 1,000,000 inhabitants per year. Therefore, in Iran with a population over 60 million, it is expected to receive at least 12000 reports per year. Unfortunately, only 2330 reports were sent to the IPC in 2006.[3] The reporting of ADRs in hospitals is very important because severe ADRs are most likely to be seen in hospitals, ADRs can be detected early and spontaneous reports can be more accurate.[6]
Nurses are known to have an important role in ADR reporting and constitute a potentially valuable source for spontaneous ADR reports in hospitals.[11] Thus, the opinions and attitudes of hospital nurses on the difficulties of spontaneous reporting of ADRs and the ways to solve them are very important. This survey was conducted to assess the knowledge, attitude and practice (KAP) of an educational hospital's nurses in Tehran, towards Iran's national ADR reporting schemes with the aim of identifying reasons for under-reporting and to determine the steps that could be adopted to increase reporting rates.
MATERIALS AND METHODS
In an observational-descriptive study, a questionnaire was prepared to investigate KAP of nurses regarding ADR reporting. The questionnaire included issues addressed in previous studies examining the KAP of medical practitioners to ADR reporting.[3,12] It was modified to take into account the national basis of the current investigation.[3] For the purpose of the study, the KAP questionnaire was primarily designed by pharmacovigilance researchers in pharmaceutical care department through searching in related internet websites. This KAP questionnaire consisted of a total of 17 Questions. Among these questions, 6 items were related to the knowledge, 7 to the attitude and the remaining 4 items were related to the practice aspects.
Knowledge questions mainly centered on general concept of pharmacovigilance, adverse drug reaction reporting system and yellow card details. Attitude questions focused mostly on nurses’ general point of view regarding different aspects of ADR reporting. Knowledge and practice related questions were designed as multiple choices. Knowledge related questions consisted of easy, moderate and difficult ones (in equal portions). Attitude related questions were developed in 5-point Likert scale. Formal and content validity of the questionnaire was evaluated by expert pharmacists. The initial draft was circulated to the members of the research team and modifications were carried out. Upon receiving the responses from health care professionals, internal consistency (reliability) of questionnaire was assessed by Cronbach's alpha coefficient using a sample consisted of 20 randomly selected hospital nurses. Test-related reliability was tested using intra-cluster correlation on the same sample after a week. After this modification, the finalized questionnaire was employed to collect data from the main sample.
In November 2009, we invited all nurses working in different wards in Dr. Shariati Hospital (a teaching hospital in Tehran) to participate in the survey. Nurses who were fulfilling their training program in the hospital were excluded from the survey. Thereupon, the sampling in this survey was not random.
Statistical Analysis
The filled KAP questionnaires were analyzed by producing descriptive statistics using SPSS version 17. Score 1 was assigned to the correct answers to knowledge and practice questions, and zero was assigned to wrong answers. The numerical variables (age, number of years from graduation, number of working hours per month and length of practice) were described numerically. The answers to attitude questions were ranked 1 to 5 so that score 5 represented the best attitude. In order to determine the effective factors on knowledge (the summary variable of knowledge), the regression model was employed using age, sex, number of working hours per month and length of practice as the independent variables. Accordingly, in order to determine the effective factors on attitude, the summary variable of knowledge was added to the series of independent variables, and to determine the effective factors on practice, both knowledge and attitude variables were added to the series of independent variables. The numerical values were reported as mean (standard deviation). The statistical significance level was considered as p-value less than 0.05.
FINDINGS
Internal reliability for knowledge, attitude and practice was calculated as 0.74, 0.51 and 0.75, respectively (Cronbach's alpha coefficient). Omission of none of the questions could increase the Cronbach's alpha coefficient.
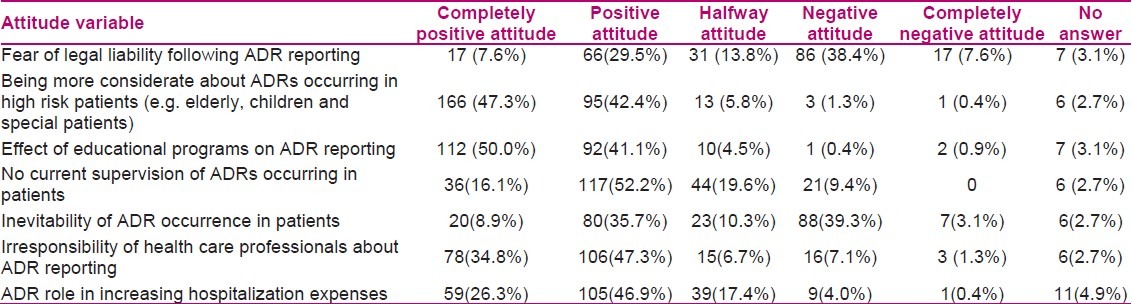
Two hundred twenty four (44.8%) out of 500 nurses who had received the questionnaires completed the questionnaires. Among these respondents, 211 (95%) were females and 11 (5%) were males. Ninety six percent of all respondents were graduated with BS degree in nursing, 34% from universities in Tehran and 66% from other universities. The average age of participants was 31.84(7.59) ranged from 20 to 50 and 8.93(7.43) years were passed from their graduation (ranged 0-32). Practice duration length was 8.6(7.41) years (ranged 0.25-29.5). Working duration per month was 202(53) hour (ranged 13-465). Nurses’ knowledge towards the ADR reporting was evaluated using 6 pharmacovigilance related questions. The results are shown in Table 1. To explore nurses’ attitudes to pharmacovigilance, 7 questions were designed. The descriptive results are presented in Table 2.
Table 1.
Nurses’ knowledge about the ADR reporting

Table 2.
Nurses’ attitudes toward the ADR reporting
Nearly 9% of the nurses (20 out of 224) had experienced ADR reporting. Majority of the nurses preferred using phone (10 out of 50) or Yellow Cards (7 out of 50) for reporting the ADRs. Only 1 person out of 50 preferred using internet for submitting the reports (Figure1). Almost 74% of the nurses (29 out of 39) had sent their reports to the hospital's ADR center.
Figure 1.
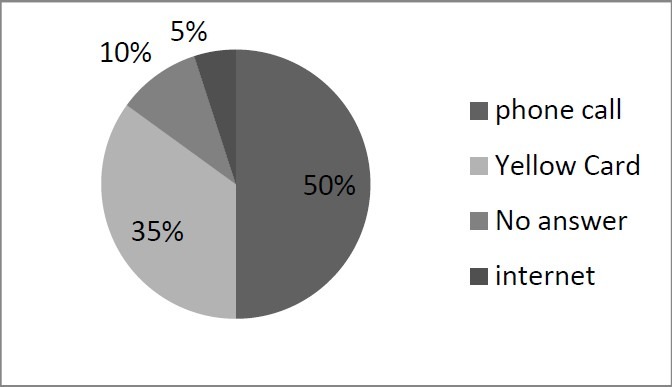
Different methods of reporting ADRs by nurses
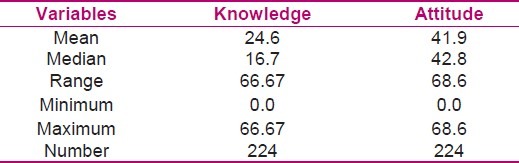
A question asked about the nurses’ reactions while facing an ADR. More than 87% of participants reported that they would prefer to announce the ADRs to the physicians in the ward. Figure 2 shows the distribution of their answers to this question. Distribution of knowledge and attitude variables and the mean and other statistical factors are shown in Table 3.
Figure 2.
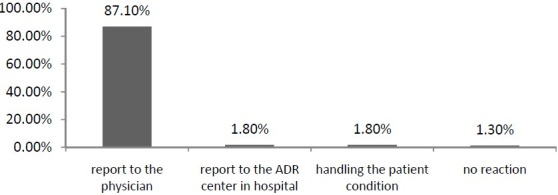
Distribution of the nurses’ reactions while facing an ADR
Table 3.
Distribution of knowledge and attitude variables
DISCUSSION
Hospital nurses could play an important role in ADRs reporting, because they are close to the patient and have good knowledge of health criteria, symptoms, drugs and ADRs. Given their unique position in drug administration and recording side effects, nurses are well-placed to monitor the patients’ response to drugs. They are often the source in alerting the responsible physician about possible ADRs. There is thus a logical reason to involve nurses and encourage them to contribute in ADR reporting system.[11,13] The most important finding in the present study was the nurses’ low knowledge and practice level about pharmacovigilance, while their attitude towards this subject was at a high level. The internal consistency of the questions investigating knowledge was low in this study which might be due to the low number of questions evaluating it. Therefore, considering the number of questions evaluating the attitude and their little internal consistency, we concluded that the knowledge level seems to be underestimated. However, the nurses’ attitude towards the subject was at a very high level. Although, the highest knowledge was about ADR reports characteristics, correct definition of the term ‘pharmacovigilance’ and the medicine which had the most fatality following ADRs, knowledge about the most important way of collecting ADR related information and ADRs which should be reported to ADR center, were at the lowest level. The attitude level towards giving more consideration to ADRs occurring in high risk patients (e.g. elderly, children and special patients) and effect of educational programs on ADR reporting was the highest, but the attitude level towards the responsibility of health care professionals about ADR reporting was at the lowest level compared to the other attitude questions.
There are several reports which have emphasized the problem of the ADR under-reporting among health care professionals[7,12,14] According to the results of this study, about 68% of the nurses did not even know the correct definition of the term “pharmacovigilance”. Similarly, only 17% of the pharmacists in a study by Toklu and Uysal could define ‘pharmacovigilance’ correctly.[12] In addition, minority of the nurses in our study were aware of the Iranian Pharmacovigilance Center (IPC). In fact, only 2.2% (5 out of 224) had sent the reports to this center. This shows that although ten years has passed since the establishment of the ADR center in Iran, there has not been enough publicity regarding its existence. This was similar to a study performed in Mazandaran province in Iran where only 2.3% of the nurses sent their reports to the national pharmacovigilance center,[15] and also a survey conducted in Italy which showed that minority of the health care practitioners stated that they had sent the reports to the National Health Service.[16] Unlike these surveys, among the hospital doctors in a study by Belton et al., 63% had sent in an ADR report either to the Committee on safety of medicines or to a pharmaceutical manufacturer.[17]
The majority of nurses in our study (91.1%) had never reported an ADR, a pattern differed from another study which 25.6% of the respondents (hospital pharmacists) had submitted Yellow Cards.[7] On the other hand, most of the nurses in our study (79.0%) were not aware of what kind of ADR should be reported. The fact that over 82% of our nurses believed that ADR reporting is not a professional responsibility clearly shows the need for appropriate education regarding this issue that will probably make a significant difference in the number of our reports. Dissimilarly, 86.1% of the hospital pharmacists in the study conducted in United Kingdom felt that ADR reporting was a professional obligation.[7]
According to the results, only 8.9% of the respondents had experienced ADR reporting. However, 61.3% of the physicians in the survey conducted in Germany said that had reported at least one case in their life.[18] Interestingly, none of the nurses who responded to the questionnaire in a study by Backstrom et al. had reported an ADR prior to the study[19]
The main limitation of this study was the poor response rate (44.8%). This low response rate was similar to some other studies used for comparison in this paper that involved medical practitioners.[3,7,12,16–18] We may speculate that those who did not participate in the study had even fewer knowledge regarding pharmacovigilance, and the results regarding knowledge and attitude would have been even less impressive if they had participated in the study.
This highlights the importance of ADR training in our population. Granas et al. showed that an educational program can significantly modify pharmacists’ reporting-related attitudes and influence the ADR reporting behavior in a positive manner.[14]
Another limitation of the study may have been the fact that our study did not claim to represent all the nurses in Iran. However, considering the fact that Tehran is one of the cities in Iran with the most submitted reports to the IPC, we can infer that most other cities in this country have even less knowledge about the pharmacovigilance.
CONCLUSION
The results of the present study demonstrated that nurses who participated in this study had insufficient knowledge about the operation, purposes and usefulness of ADR reporting system. Regarding the high level of attitude, we expected better practice in terms of pharmacovigilance. This indicates that ADR training would be a useful step in improving nurses’ ADR reporting in Iran. Some effective measures to improve the situation could be inclusion of pharmacovigilance into pre- and post-graduate continuing education programs, provision of guidelines for ADR spontaneous reporting and giving feed-back information to the reporters, establishment of regional pharmacovigilance units which could efficiently stimulate ADR reporting. In our future studies, we plan to prepare a training program for the nurses who participated in this study to be able to evaluate the influence of the education on all the measured parameters.
ACKNOWLEDGMENT
We wish to thank the nursing staff of Dr.Shariati Hospital who kindly participated in this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rao PG, Archana B, Jose J. Implementation and results of an adverse drug reaction reporting programme at an Indian teaching hospital. Indian J Pharmacol. 2006;38(4):293–4. [Google Scholar]
- 2.Baniasadi S, Fahimi F, Shalviri G. Developing an adverse drug reaction reporting system at a teaching hospital. Basic Clin Pharmacol Toxicol. 2008;102(4):408–11. doi: 10.1111/j.1742-7843.2008.00217.x. [DOI] [PubMed] [Google Scholar]
- 3.Vessal G, Mardani Z, Mollai M. Knowledge, attitudes, and perceptions of pharmacists to adverse drug reaction reporting in Iran. Pharm World Sci. 2009;31(2):183–7. doi: 10.1007/s11096-008-9276-6. [DOI] [PubMed] [Google Scholar]
- 4.Johnson JA, Bootman JL. Drug-related morbidity and mortality.A cost-of-illness model. Arch Intern Med. 2011;155(18):1949–56. [PubMed] [Google Scholar]
- 5.Millar JS. Consultations owing to adverse drug reactions in a single practice. Br J Gen Pract. 2001;51(463):130–1. [PMC free article] [PubMed] [Google Scholar]
- 6.Vallano A, Cereza G, Pedrds C, Agusti A, Danes I, Aguilera C, et al. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol. 2005;60(6):653–8. doi: 10.1111/j.1365-2125.2005.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green CF, Mottram DR, Rowe PH, Pirmohamed M. Attitudes and knowledge of hospital pharmacists to adverse drug reaction reporting. Br J Clin Pharmacol. 2001;51(1):81–6. doi: 10.1046/j.1365-2125.2001.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subish P, Izham MM, Mishra P. Evaluation of the knowledge, attitude and practices on adverse drug reactions and pharmacovigilance among healthcare professionals in a Nepalese hospital: a preliminary study. The Internet Journal of Pharmacology. 2008;6(1) [Google Scholar]
- 9.Ulfvarson J, Mejyr S, Bergman U. Nurses are increasingly involved in pharmacovigilance in Sweden. Pharmacoepidemiol Drug Saf. 2007;16(5):532–7. doi: 10.1002/pds.1336. [DOI] [PubMed] [Google Scholar]
- 10.Van Grootheest K, Olsson S, Couper M, De Jong-van den Berg L. Pharmacists’ role in reporting adverse drug reactions in an international perspective. Pharmacoepidemiol Drug Saf. 2004;13(7):457–64. doi: 10.1002/pds.897. [DOI] [PubMed] [Google Scholar]
- 11.Hall M, McCormack P, Arthurs N, Feely J. The spontaneous reporting of adverse drug reactions by nurses. Br J Clin Pharmacol. 1995;40(2):173–5. doi: 10.1111/j.1365-2125.1995.tb05774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toklu HZ, Uysal MK. The knowledge and attitude of the Turkish community pharmacists toward pharmacovigilance in the Kadikoy district of Istanbul. Pharm World Sci. 2008;30(5):556–62. doi: 10.1007/s11096-008-9209-4. [DOI] [PubMed] [Google Scholar]
- 13.Backstrom M, Ekman E, Mjorndal T. Adverse drug reaction reporting by nurses in Sweden. Eur J Clin Pharmacol. 2007;63(6):613–8. doi: 10.1007/s00228-007-0274-8. [DOI] [PubMed] [Google Scholar]
- 14.Granas AG, Buajordet M, Stenberg-Nilsen H, Harg P, Horn AM. Pharmacists’ attitudes towards the reporting of suspected adverse drug reactions in Norway. Pharmacoepidemiol Drug Saf. 2007;16(4):429–34. doi: 10.1002/pds.1298. [DOI] [PubMed] [Google Scholar]
- 15.Salehifar E, Ala SH, Gholami KH. Knowledge, attitude and performance of pharmacists and nurses in Mazandaran province, Iran regarding adverse drug reaction and its reporting, 2005. Journal of Mazandaran University of Medical Sciences. 2007;16(56):115–25. [Google Scholar]
- 16.Cosentino M, Leoni O, Banfi F, Lecchini S, Frigo G. Attitudes to adverse drug reaction reporting by medical practitioners in a Northern Italian district. Pharmacol Res. 1997;35(2):85–8. doi: 10.1006/phrs.1996.0138. [DOI] [PubMed] [Google Scholar]
- 17.Belton KJ, Lewis SC, Payne S, Rawlins MD, Wood SM. Attitudinal survey of adverse drug reaction reporting by medical practitioners in the United Kingdom. Br J Clin Pharmacol. 1995;39(3):223–6. doi: 10.1111/j.1365-2125.1995.tb04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasford J, Goettler M, Munter KH, Muller-Oerlinghausen B. Physicians’ knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. J Clin Epidemiol. 2002;55(9):945–50. doi: 10.1016/s0895-4356(02)00450-x. [DOI] [PubMed] [Google Scholar]
- 19.Backstrom M, Mjorndal T, Dahlqvist R. Spontaneous reporting of adverse drug reactions by nurses. Pharmacoepidemiol Drug Saf. 2002;11(8):647–50. doi: 10.1002/pds.753. [DOI] [PubMed] [Google Scholar]


