Abstract
Aims:
The aim of this study was to estimate the pH of saliva, concentration of calcium and inorganic phosphate, and calculus formation before and after usage of Recaldent® (GC Tooth Mousse Plus™), Functionalized Tricalcium Phosphate (3M ESPE ClinPro™ Tooth Crème) and standard dentifrice (Colgate dental cream).
Settings and Design:
Randomized double-blind study.
Materials and Methods:
A total of 50 subjects were recruited, the subjects were assessed at their first visit, on the 21st day and on the 42nd day. At the first visit, scaling was carried out and oral hygiene instructions were given. After 21 days, the subjects were given coded dentifrices where the operator and the subjects both were unaware of the type of dentifrice. Clinical parameters assessed were Plaque index, Gingival index, and Calculus index. Salivary samples were obtained to measure calcium, phosphate levels, and pH at 21st day and 42nd day.
Statistical Analysis:
ANOVA test, t-test, Mann–Whitney test, Kruskal–Wallis test.
Results:
The mean salivary calcium level and mean salivary phosphate level were higher in Group III (functionalized tricalcium phosphate (3M ESPE ClinPro™ Tooth Creme) as compared to Group II (Recaldent® GC Tooth Mousse Plus™) and Group I (Colgate dental cream) on the 42nd day after using dentifrices, which was statistically significant. This showed that the usage of remineralizing dentifrices led to an increase in the salivary calcium, phosphate, and pH but it did not reach the level of super saturation of the ions caused by elevated pH which could lead to calculus formation.
Conclusions:
Thought here was a statistically significant increase in salivary calcium and phosphate level in all three groups from baseline to 42nd day, there was no calculus formation.
Keywords: Calcium, calculus formation, dentifrices, phosphate, remineralization
INTRODUCTION
Dentifrices with active ingredients of Recaldent® (GC Tooth Mousse Plus™) and Functionalized Tricalcium Phosphate (fTCP) (3M ESPE ClinPro™ Tooth Creme) have taken a surge for remineralization of enamel and dentine.[1]
Recaldent® is a casein phosphopeptide–amorphous calcium phosphate (CCP–ACP) and fTCP is an innovative calcium-based additive which increases the bioavailability of calcium and phosphate ions to facilitate remineralization.[2,3]
According to the mineral precipitation theory for calculus formation, calcificationwill occurwhen pH, calcium, and phosphate concentrations are high enough to allow the precipitation of a calcium phosphate salt.[4] As per mechanism of action of the these dentifrices, its usage may lead to an undesirable effect of calculus formation.[1,5–7]
MATERIALS AND METHODS
Source of data
This is randomized, double-blind clinical study where 50 subjects with gingivitis were recruited from the Department of Periodontology in Oxford Dental College, Bangalore, India.
Criteria for selection
Inclusion criteria
Age group between 18 and 50 years
Systemically healthy subjects
Patient with at least 28 teeth
Patients with clinically healthy gingiva and mild gingivitis.
Exclusion criteria
Smokers and chronic alcoholics
Subjects who are on long-term use of drug such as non steroidal anti inflammatory drugs (NSAIDS) corticosteroids, and hormone replacement therapy
Pregnant and lactating women
No history of scaling and root planing in past 6 months.
The parameters recorded for all the patients were Plaque index, Calculus index, Gingival index during the patient's first visit, baseline at 21st day, and end of study 42nd day.
Estimation of Calcium and inorganic phosphate in saliva was performed by standard colorimetric method and the pH of saliva was measured using a digital pH meter at the end of 21st day and 42nd day.
For all recruited subjects, scaling was completed. Oral hygiene instructions were given, and all clinical parameters and indices were brought to zero. All the subjects were instructed to use standard dentifrice and to follow oral hygiene instructions for next 21 days. After 21 days, the patients were given dentifrices which were coded by the operator 2, while the operator 1 who treated these subjects and the subjects themselves were unaware of this code. Also, Each of the coded dentifrices was dispensed in a soft-squeeze tube. Hence making this a double-blind approach.
At the baseline and the end of study, the subjects were refrained from food or drink for a minimum of 30 min prior to the collection of saliva samples to avoid carryover effect from previous saliva stimulation. All the samples were collected during morning hours only by Navazesh's cotton roll method.[8,9]
RESULTS
The results were statistically analyzed by ANOVA test, t-test, Mann–Whitney test, Kruskal–Wallis test.
No statistically significant results were found for Plaque index, gingival index, and calculus index using Pear coefficient correlation which is significant only if P<0.05. After using the dentifrices, no calculus formation was seen. However, it was found that there was a significant increase in calcium levelsin Group III followed by Group II and I at 42nd day as compared to Calcium levels at 21st Day which was highest in Group I followed by Group II and III [Tables 1 and 2]. Further, it was found that significant difference existed between Group I and III in relation to phosphate levels also. These data indicated that values of phosphate levels had a significant increase at 42nd day as compared to phosphate value at 21st day [Tables 3 and 4]. The difference in the mean pH between the groups was not statistically significant at 21st day and 42nd day [Tables 5 and 6].
Table 1.
Comparison of calcium between the three groups on 42nd day - ANOVA
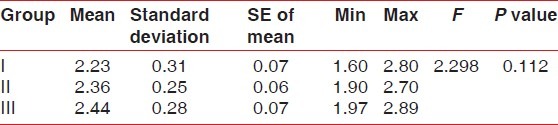
Table 2.
Comparison of calcium between the three groups on 21st day (baseline) - ANOVA followed by Bonferroni test
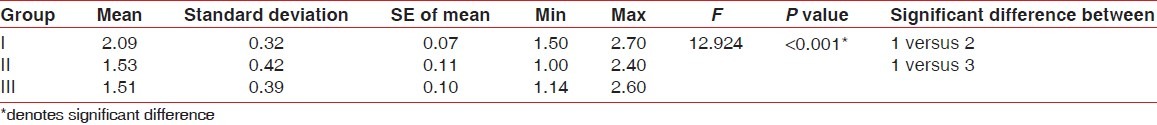
Table 3.
Comparison of phosphate between the three groups on 21st day (baseline) - (Kruskal-Wallis test)

Table 4.
Comparison of phosphate between the three groups on 42nd day - (Kruskal-Wallis test followed by Mann-Whitney test)

Table 5.
Comparison of pH between the three groups on 21st day (baseline) - (ANOVA)
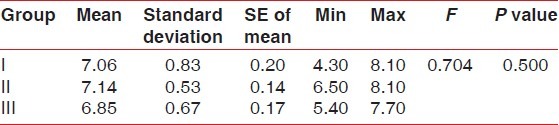
Table 6.
Comparison of pH between the three groups on 42nd day - (Kruskal-Wallis test)
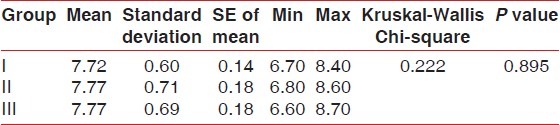
DISCUSSION
Over the last few decades, remineralization process has shown to reduce caries. Recently, novel approaches to enhance remineralization based on a milk derivative containing CPP-ACP, i.e., Recaldent® (GC Tooth Mousse Plus™ and fTCP (3M ESPE ClinPro™ Tooth crème have been suggested.
As per mechanism of action of the above-mentioned dentifrices, which coincide with mineral precipitation theory of calculus formation; their usage may lead to an undesirable effect of calculus formation.
According to the study design; which was randomized double-blind study, after the results were obtained, decoding was done and codes were given to the investigator[1] following which it was revealed that Group I was using (Colgate dental cream), Group II was using (Recaldent® GC Tooth Mousse Plus™) and Group III was using fTCP (3M ESPE ClinPro™ Tooth Creme).
The clinical parameters were evaluated at the first visit, 21st day (baseline), and 42nd day. Since the subjects presented with varying oral hygiene status, it was standardized by subjecting them to scaling followed by standardized oral hygiene instructions on the 1st day of visit, which was maintained for the 1st 21 days.
Analysis for calculus formation was carried out in the frame of 21 days, althoughthe soft plaque was shown to be hardened by precipitation of mineral salts, between the first and the 14th day of plaque formation itself. During calculus formation, calcifying plaques may become 50% mineralized within 2 days and 60-90% mineralized in 12 days.[10,11] In vivo production of calculus has also been reported by Jin et al.[5] using thin celluloid strips wired to the lingual and proximal surfaces oflower anterior teeth to collect deposits with a time interval of 1-29 days.
In the present study, no significant relation was found in relation to plaque, gingival index, and calculus index. No calculus formation was seen during the 21st day (baseline) and 42nd day during this study. Regular oral hygiene re-enforcement motivated the subjects positively.
In a randomized controlled trial,[12] a mouth rinse containing 2.0% CPP-ACP nanocomplexes plus 450 ppm fluoride significantly increased supragingival plaque content when compared to dry weight of plaque attained by use of a rinse containing the equivalent concentration of fluoride ionssuch as sodium fluoride. Although marked increases in the concentrations of plaque calcium, phosphate and fluoride were found, calculus was not observed in any of the subjects. It was found that plaque calcium fluoride phosphate remained stabilized at the tooth surface by CPP as bioavailable ions and did not transform in to a crystalline phase. Perhaps, this could be same reason that the patients in our study did not show calculus formation.
In this study, salivary calcium and phosphate levels were compared between the three groups at 21st day (baseline) and 42nd day that was before and after using the dentifrices. However, it was found that Calcium and phosphate levels was found to be the highest in Group III followed by Group II and I at 42nd day as compared to levels at 21st day which was highest in Group I followed by Group II and III which was statistically significant.
The data given by Walsh[1] assert that (Recaldent® GC Tooth Mousse Plus™) provides 325 mM of calcium and 187 mM of phosphate which is bioavailable as compared to fTCP (3M ESPE ClinPro™ Tooth Creme) which provides only 48 mM of calcium and 32 mM of phosphate, while with fTCP (3M ESPE ClinPro™ Tooth Creme), it is unclear as tohow much of calcium and phosphate contained the product is bioavailable. fTCP (3M ESPE ClinPro™ Tooth Creme) could only deliver a small amount of unbound, non-stabilized calcium in 2 min of brushing before being expectorated from the mouth, while Recaldent® GC Tooth Mousse Plus™ delivers large amounts of stabilized calcium over an extended period of time.
As in our study, calcium and phosphate levels were found to be high, which is in confirmation witha study conducted by Rahiotis et al.[6] on the characterization of oral biofilms formed on germanium crystals mounted in the custom-made retainers of removable orthodontic appliance insitu in the presence or absence of CCP–ACP agents. I was stated that the presence of CCP–ACP agents causes delays in biofilm formation, increase in Ca/O, Ca/P, and decrease in K/CL ratio and favored the nucleation and crystallization of calcium phosphates, possibly in apatitic form, in matured biofilms.
In spite of statistically significant increase in calcium and phosphate levels, no calculus was seen in any of the subjects which can be attributed to good oral hygiene maintenance.
Mean pH level at 21st day (baseline) was recorded to be higher in Group II followed by Group I and III. At the 42nd day, pH was higher in all three groups but equal mean pH was found in Group II and III followed by Group I which was not significant statistically. It was found in various clinical as well as laboratory evidence by Reynoldsfor the Recaldent® GC Tooth Mousse Plus™ that this material is pH-responsive, with increasing pH increasing the level of bound ACP and stabilizing free calcium and phosphate, so spontaneous precipitation of calcium phosphate does not occur. According to manufacturers of fTCP, it was thought that modified TCP technology will act best at neutral or slightly alkaline pH.[1] In this study too, there was increase in pH in Group II and III which was possibly enough for remineralization but not for calculus formation.
According to Dawes et al.[13] in a healthy oral environment, saliva is supersaturated with calcium and phosphate levels, without precipitation. However, when this equilibrium was disturbed, formation of dental calculus occurred by the elevated level of pH. In this study, though the pH was increased, it had not reached the level of super saturation with calcium and phosphate, thus explaining the absence of calculus formation in any of the subjects.
When the change in all clinical and salivary parameters were compared in each group individually, it was found that in all three groups there was anincrease in the mean salivary calcium level, inorganic phosphate level, and pH level from 21st to 42nd day was found to be statistically significant. Since all the patients in the three groups maintained their oral hygiene status from the first visit to the 42nd day, it could be speculated that the alteration in calcium and phosphate levels in saliva was only due to the use of dentifrices and not due to oral hygiene status.
Though this study obtained statistically significant results, one of the major limiting factors was that the duration of the study to evaluate the formation of calculus and also the pH of plaque was not assessed whichis known to playan important role in calculus formation. Hence, keeping this in mind, further studies with a longer duration and increased sample size are warranted to validate calculus formation.
CONCLUSION
It is known that remineralizing dentifrices used in Group III (fTCP [3M ESPE ClinPro™ Tooth crème] and Group II) (Recaldent® GC Tooth Mousse Plus™) work by providing calcium, fluoride, phosphate ions, and optimizing the pH of the oral environment.
As per mechanism of action of the dentifrices in Group III and II, its usage may lead to an undesirable effect of calculus formation. However, in this study, we did not find any calculus formation in Group III and II, despite an increase in salivary calcium and phosphate level, after the usage of the dentifrices for 21 days. So, from the observation in this study it can be stated that though the usage of remineralizing dentifrices led to an increase the level of salivary calcium, phosphate, and pH but did not reach the level of super saturation of the ions caused by elevated pH which can lead to calculus formation.
Although longitudinal studies will be required to see long-term influence of these dentifrices in the oral cavity, at this juncture, these remineralizing dentifrices can be safely recommended to treat early carious lesions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Walsh LJ. Contemporary technologies for remineralization therapies: A Review. Int Den SA. 2009;11:6–15. [Google Scholar]
- 2.Llena C, Forner L, Baca P. Anticariogenicity of casein phosphopeptide-amorphous calcium phosphate: A review of the literature. J Contemp Dent Pract. 2009;10:1–9. [PubMed] [Google Scholar]
- 3.Marchisio O, Esposito MR, Genovesi A. Salivary pH level and bacterial plaque evaluation in orthodontic patients treated with Recaldent products. Int J Dent Hyg. 2010;8:232–6. doi: 10.1111/j.1601-5037.2009.00374.x. [DOI] [PubMed] [Google Scholar]
- 4.Hazen SP. Supragingival dental calculus. Periodontol 2000. 1995;8:125–36. doi: 10.1111/j.1600-0757.1995.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 5.Jin Y, Yip HK. Supragingival calculus: Formation and control. Crit Rev Oral Biol Med. 2002;13:426–41. doi: 10.1177/154411130201300506. [DOI] [PubMed] [Google Scholar]
- 6.Rahiotis C, Vougiouklakis G, Eliades G. Characterization of oral films formed in the presence of a CPP-ACP agent: An in situ study. J Dent. 2008;36:272–80. doi: 10.1016/j.jdent.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Shetty S. Quantitative evaluation of salivary calcium, phosphorus, protein and pH in health and disease periodontium. Ann Essence Dent. 2010;2:21–4. [Google Scholar]
- 8.Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci. 1993;694:72–7. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- 9.Navazesh M, Christensen CM. A comparison of whole mouth resting and stimulated salivary measurement procedures. J Dent Res. 1982;61:1158–62. doi: 10.1177/00220345820610100901. [DOI] [PubMed] [Google Scholar]
- 10.Muehlemann HR, Schroeder HE. Dynamics of supragingival calculus formation. Adv Oral Biol. 1964;1:175–203. doi: 10.1016/b978-1-4832-3117-4.50012-7. [DOI] [PubMed] [Google Scholar]
- 11.Sharawy AM, Sabharwal K, Socransky SS, Lobene RR. A quantitative study of plaque and calculus formation in normal and periodontally involved mouths. J Periodontol. 1966;37:495–501. doi: 10.1902/jop.1966.37.6.495. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds EC, Cai F, Cochrane NJ, Shen P, Walker GD, Morgan MV, et al. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2008;87:344–8. doi: 10.1177/154405910808700420. [DOI] [PubMed] [Google Scholar]
- 13.Dawes C. Why does supragingival calculus form preferentially on the lingual surface of the 6 lower anterior teeth? J Can Dent Assoc. 2006;72:923–6. [PubMed] [Google Scholar]


