Abstract
Background:
Alkaline phosphatase (ALP) enzyme is involved in the destruction of the human periodontium. The present study was conducted to determine the presence and levels of ALP activity in gingival crevicular fluid (GCF) in periodontal health, gingivitis, and chronic periodontitis.
Materials and Methods:
GCF samples were collected from 45 sites which were divided into three equal groups of healthy samples and gingivitis and chronic periodontitis samples. Various clinical parameters were evaluated and the levels of ALP were estimated using a semi-autoanalyzer. Analysis of variance was employed to compare the ALP levels in different groups. Pearson's correlation coefficient was utilized to find the correlation between ALP levels and various clinical parameters.
Results:
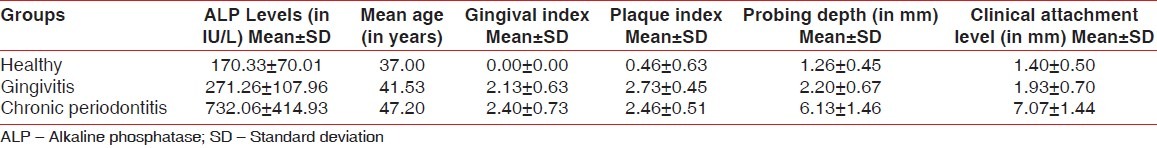
Difference in the mean ALP levels between healthy and gingivitis groups was found to be nonsignificant (P>0.05) and that between the chronic periodontitis group and healthy as well as gingivitis groups was found to be highly significant (P<0.001). Significant correlations existed between ALP levels and gingival index, probing depths, as well as clinical attachment levels.
Conclusion:
The finding of the present study confirms the relationship between ALP level and periodontal disease, thus indicating that GCF ALP levels can be used as potential biochemical markers for the detection and progression of periodontal disease.
Keywords: Alkaline phosphatase, chronic periodontitis, gingival crevicular fluid, gingivitis
INTRODUCTION
Periodontitis is a chronic inflammatory disease characterized by gingival inflammation, periodontal ligament destruction, and alveolar bone resorption.[1]
A diagnosis of periodontal disease is made after analyzing the information collected from a periodontal examination.[2] Traditional periodontal diagnostic parameters used clinically include probing depths, bleeding on probing, clinical attachment levels, Plaque index and radiographs assessing alveolar bone level.[3]
The goal of periodontal diagnostic procedures is to provide useful information to the clinician regarding the present periodontal disease type, location, and severity which serve as a basis for treatment planning and provide essential data during periodontal maintenance and disease-monitoring phases of treatment. Traditional diagnostic procedures are inherently limited, in that only disease history, not current disease status, can be assessed. Advances in diagnostic research in oral and periodontal disease are moving toward methods whereby periodontal risk can be identified and quantified by objective measures (e.g., biomarkers).[4]
The response of an organism to periodontal infection includes production of several enzymes released from stromal, epithelial, inflammatory, or bacterial cells. These intracellular enzymes are released increasingly from the damaged cells of periodontal tissues into the gingival crevicular fluid (GCF) and saliva. Several enzymes that are evaluated for the early diagnosis of periodontal disease are aspartate and alanine aminotransferases (AST and ALT), lactate dehydrogenase (LDH), gamma-glutamyl transferase (GGT), creatine kinase (CK), alkaline phosphatase (ALP), and acid phosphatase (ACP).[5]
ALP is a calcium- and phosphate-binding protein and a phosphor-hydrolytic enzyme.[6] It is a membrane-bound glycoprotein produced by many cells such as polymorphonuclear leukocytes (PMNLs), osteoblasts, macrophages, and fibroblasts within the area of the periodontium and gingival crevice.[7]
ALP is considered to be an important indicator of bone formation and is a phenotypic marker for osteoblast cells. ALP is detected in the parotid, submandibular, and minor salivary glands, as well as in desquamated epithelial cells, leukocytes, and bacteria from dental plaque. The presence of ALP in the saliva and GCF is usually indicative of inflammation and/or destruction of the periodontal tissues. The level of ALP is positively correlated with the severity of the periodontal disease.[6]
GCF is an inflammatory exudate that seeps into gingival crevices or periodontal pockets around teeth with inflamed gingiva.[8] It represents serum components overlaid with products from local physiologic phenomena, such as connective tissue destruction and bone loss, and has diagnostic value. It is a convenient, noninvasive, and efficient means to sample biomarkers of inflammation and bone resorption in the oral cavity. Although individual GCF samples have the possibility of describing the inflammatory events occurring at the site, pooled samples from a small number of sites may characterize the overall vulnerability to periodontitis and allow periodic assessment during periodontal treatment or maintenance.[9]
Thus the current study aimed at determining the ALP enzyme levels in GCF in periodontal health, gingivitis, and chronic periodontitis and estimating its possible cross-sectional relationship with various clinical parameters.
MATERIALS AND METHODS
Subject selection
The study was conducted at the Department of Periodontology, KLE VK Institute of Dental Sciences, KLE University, Belgaum, Karnataka, India. Ethical clearance was obtained from the ethical committee of the institute, and a written informed consent was obtained from all the participants prior to the study. The study sample consisted of 45 sites. The subjects included belonged to both sexes (male and female) and the age ranged from 20 to 65 years. The mesiofacial, distofacial, or midfacial surface of the tooth was considered as a site. The sites were divided into three groups of 15 each: Group 1 (control group), group 2 (gingivitis group), and group 3 (chronic periodontitis group) [Figure 1].
Figure 1.
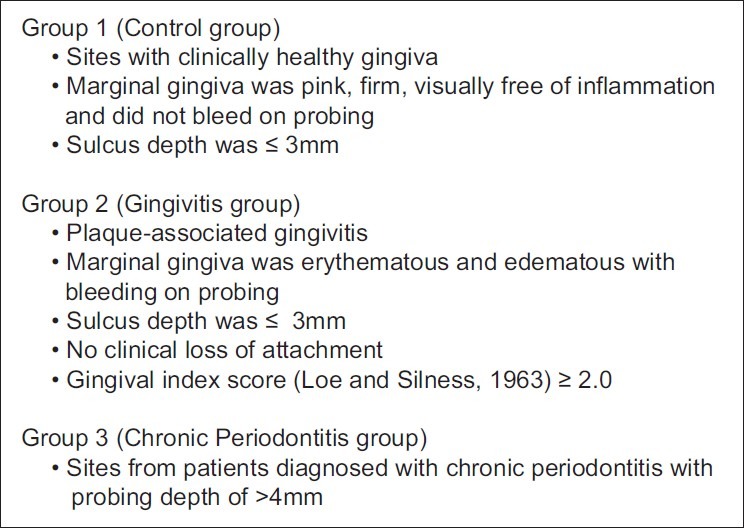
Criteria for group division
Group 1: Sites with clinically healthy gingiva or marginal gingiva was pink, firm, visually free of inflammation, and did not bleed on probing. Sulcus depth was ≤3 mm.
Group 2: Plaque-associated gingivitis or marginal gingiva was erythematous and edematous with bleeding on probing. Sulcus depth was ≤3 mm. There was no clinical loss of attachment and gingival index score (Loe and Silness, 1963) was ≥2.0.
Group 3: Sites from patients diagnosed with chronic periodontitis with a probing depth of >4 mm.
Exclusion criteria included patients with systemic disease/illness or with any oral mucosal inflammatory condition (e.g., aphthous ulcers, lichen planus, leukoplakia, oral cancer) and who had taken anti-inflammatory drugs, antibiotics, nutritional supplements, or any other drugs over the past six months. Patients with a history of smoking and/or alcoholism and those who had undergone any periodontal treatment over the past six months and women who were in menstruation period, had received hormonal treatment or oral contraceptives, or were pregnant or lactating were excluded from the study.
Clinical examination
Gingival index (Loe and Silness, 1963) and Plaque index (Silness and Loe, 1964) were recorded for each site by one examiner before the collection of GCF. Probing pocket depth (PD) and clinical attachment level were measured using a William's graduated periodontal probe.
GCF collection
The subjects were seated comfortably in an upright position in the dental chair, with adequate lighting condition. The sites were chosen from maxillary teeth to avoid contamination with saliva. Supra gingival plaque and calculus were removed with hand instrumentation prior to the collection of GCF. Care was taken not to touch the gingival margin to prevent stimulation. The calibrated microcapillary tubes (0–5 μL range) were placed extracrevicular at the mesiofacial, distofacial, or midfacial surface of the tooth. A standardized volume of 5 μL of GCF was collected. The entire volume was collected from one site only. Test sites which did not express adequate volume of fluid or microcapillary tubes contaminated with blood were discarded. The collected GCF was immediately transferred to the sterilized microcentrifuge (Eppendorff) tubes that contained 45 μL of normal saline. The tubes were then transported for analysis which was done within two hours of collection of the crevicular fluid.
Enzyme assay
A diagnostic kit was used for the estimation of ALP. The kit consisted of two reagents:
Reagent 1
p-Nitrophenyl phosphate
Magnesium ion (Mg[2]+)
Tris/carbonate buffer (pH 10.2±0.2 at 25°C)
Reagent 2 (Aqua-4)
Distilled water
The GCF (5 μL) was diluted 10 times with 45 μL of normal saline, to make 50 μL of sample. The diluted GCF was then centrifuged for 3–5 minutes at 3500–4500 rotations per minute (rpm) in a microcentrifuge; 10 μL of the clear supernatant was utilized for the analysis of ALP activity.
The working agent was prepared by adding 2.2 mL of distilled water to reagent 1.
Then, 500 μL of the prepared reagent was taken in a microcentrifuge (Eppendorff tube) and 10 μL of the sample was added to it. The enzyme activity was recorded using a semi-autoanalyzer and the result was obtained in the form of a digital readout.
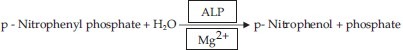
Principle of the reaction [Figure 2]
Figure 2.
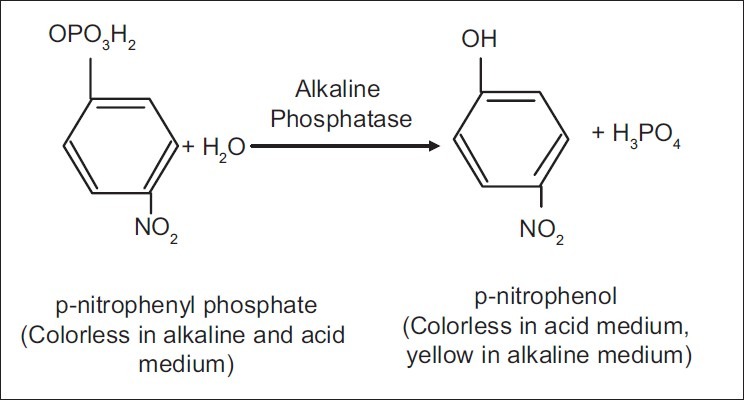
Principle of the reaction
Statistical analysis
The mean and the standard deviation with regard to the level of ALP, age, gingival index, Plaque index, pocket probing depth, and clinical attachment level were calculated. The mean values of the measured clinical parameters and ALP levels were assessed by one-way ANOVA. The pair-wise comparison was done using the posthoc Scheffe's test. Pearson's correlation coefficient was utilized to find the correlation between ALP levels and various clinical parameters.
RESULTS
The mean and the standard deviation with regard to level of ALP, age, gingival index, Plaque index, pocket probing depth, and clinical attachment level are demonstrated in Table 1.
Table 1.
Mean ALP levels, age, and the various clinical parameters of the three groups
The clinical parameters recorded in groups 1-3 were compared with each other [Figure 3]. The difference in gingival index and Plaque index was found to be highly significant (P<0.001) when groups 1 & 2 and groups 1 & 3 were compared. No statistically significant difference was found when groups 2 and 3 were compared. The probing pocket depth and attachment levels showed significant difference between groups 2 & 3 and 3 & 1 and no statistical difference between groups 1 and 2 [Table 2].
Figure 3.
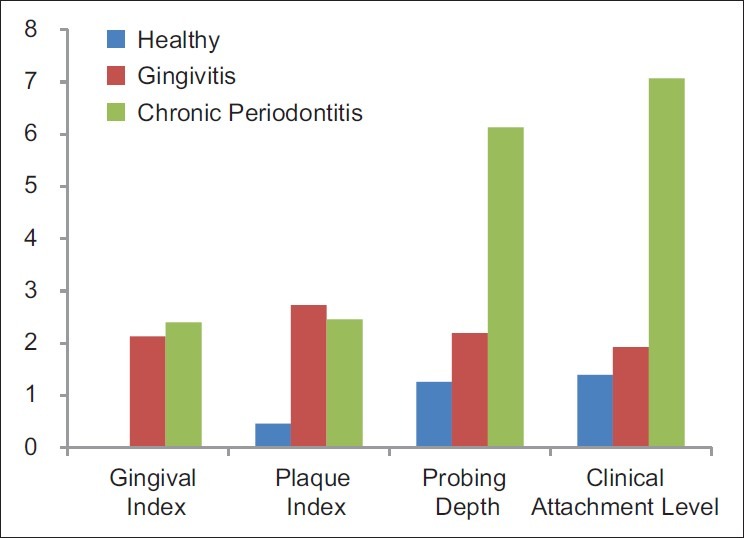
Comparison of various clinical parameters in the three groups
Table 2.
Comparison of the various clinical parameters and ALP levels between the three groups
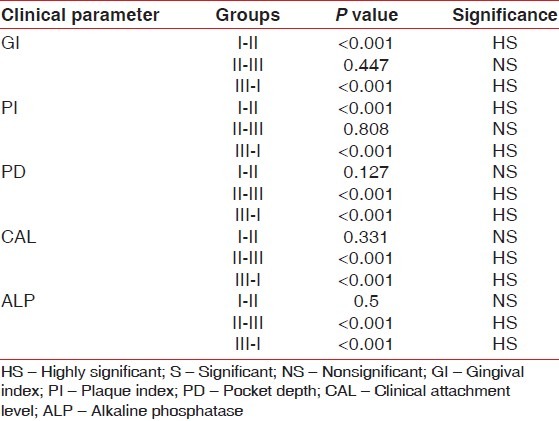
Difference in the mean ALP levels between group 1 and 2 was found to be nonsignificant (P>0.05) and that between the groups 2 and 3 as well as groups 3 and 1 was found to be highly significant (P<0.001) [Table 2].
DISCUSSION
The processes responsible for the destruction of the human periodontium are highly complex and the vast range of biological substances involved have their own relevance to specific aspects of different disease types.[10]
A biomarker or biologic marker is a substance that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention (Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework 2001).[4]
One of the most active areas in periodontal research has been the search for a biochemical marker of the progression of periodontitis in GCF.
The potential of ALP as an important biochemical marker of GCF was identified by Ishikawa and Cimasoni.[11] ALP has the peculiarity of being involved in inflammation[11,12] as well as regeneration.[13]
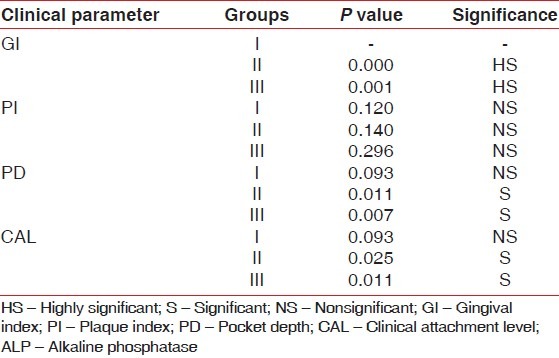
In this present study, the ALP levels in GCF in health, gingivitis, and chronic periodontitis were estimated. Moreover, the clinical parameters namely gingival index score, Plaque index, probing pocket depth, and clinical attachment level of the site correlated with the ALP levels in group 1 (healthy), group 2 (gingivitis), and group 3 (chronic periodontitis) [Table 3].
Table 3.
Correlation of the mean ALP levels with the various clinical parameters in the three groups
The sites were chosen from the maxillary arch, due to lesser chances of contamination from saliva. A single site was chosen for collection because of the known ‘site specificity’ of the periodontal diseases as shown by Chapple et al.[14]
The mean ALP levels of group 1 (healthy) were found to be lower than other two groups (gingivitis and chronic periodontitis) Figure 4. In support of this finding, Lilja, Lindskog, and Hammarstrom (1984) have shown through histochemical techniques that ALP is present in the normal periodontal membrane in rats.[15] Lindhe (1996) showed that even in healthy gingiva, certain leukocytes (neutrophils) can be seen in the junctional epithelium.[16]
Figure 4.
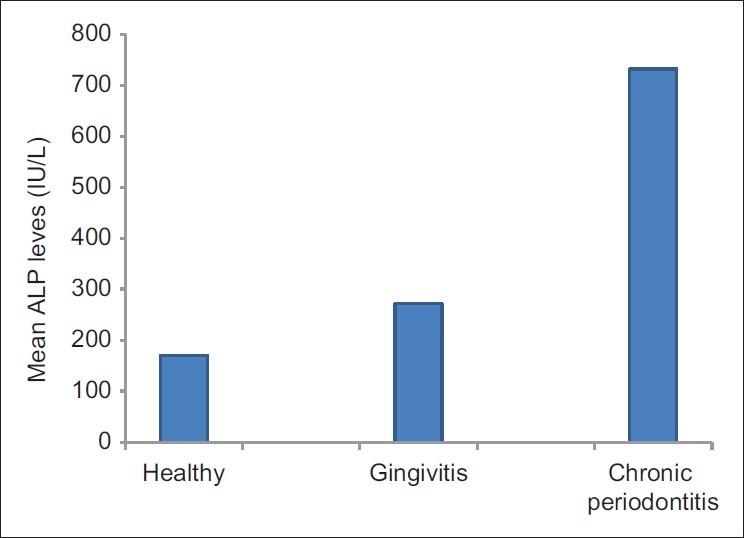
Comparison of mean GCF ALP levels (IU/L) in the three groups
Four out of 15 sites showed increased levels of ALP as compared to the remaining 11 sites reflecting the subclinical difference in inflammatory status despite the sites being clinically identical. This indicates the difference in the amount of enzyme produced locally within the periodontal tissues. It was noticed that GCF ALP levels were detectable before increase in gingival indices and thus it appears to be a better marker of gingival inflammation.
In group 2 (gingivitis), the increase in the mean level of ALP can be due to tissue alteration as a result of host-parasite reaction or host-bacterial interplay, as gingivitis is an inflammatory process. During progression of the disease, enzymes are released from dead and dying cells of the periodontium, PMNLs, inflammatory, epithelial, and connective tissue cells of the affected sites.[17] As PMNLs are the major source of ALP, they could have contributed to the increased levels of ALP in GCF through secondary granule release.[14] Micro-organisms like Prevotella intermedia and Streptococcus sanguis are thought to be important in the initiation and progression of gingivitis and are known to show high ALP activity.[18] Thus the increase in ALP level in gingivitis could be due to the increased number of crevicular PMLs (CPMNLs) and bacteria.
Among the three groups, the mean values of ALP levels were highest in group 3. Periodontal pockets are chronic inflammatory lesions and are constantly undergoing repair. The condition of the soft tissue wall of the periodontal pocket results from the interplay of destructive and constructive tissue changes. Complete healing does not occur because of the persistence of local irritants. These irritants continue to stimulate fluid and cellular exudates, which in turn causes degeneration of the new tissue elements found in the continuous effort at repair. At some period following the initial attack on the periodontal connective tissue, as a result of host defence mechanism or other changes in the local environment, the lesion begins to undergo remission.[19,20] There is an increase in the number of CPMNLs which play a vital role in pathogenesis of periodontal lesions (Genco et al. 1990) by migrating into the sulcus.[21] Among various periodontopathic bacteria, P. intermedia and Porphyromonas gingivalis are known to have high ALP activity and Aggregatibacter actinomycetomcomitans show very low ALP activity.[18] In periodontitis, one of the mechanisms of collagen loss is that the fibroblasts phagocytize collagen fibers. The increase in the fibroblast activity contributes to the total ALP level. Both histochemical and biochemical studies have shown that periodontal cells have intense ALP activity, unlike gingival fibroblasts.[17]
The increased ALP in the 11 sites out of 15 in group 3 could be due to increased CPMNLs, periodontopathic bacteria, and increased fibroblast activity. The remaining four sites had the highest ALP levels as compared to all sites in the sample size. This could be because these sites were undergoing remission. Remission means healing and there is increased osteoblastic activity which stimulates bone formation by mineralization. ALP is an enzyme of osteoblasts and serum levels of ALP are often elevated when bone formation is increased.[22,23] ALP plays a major role in bone mineralization.[17] Robinson's ALP theory formulated in 1923 suggested that in mineralization, a possible function of ALP is to raise the local concentration of inorganic phosphate ions and thus cause the precipitation of calcium phosphate. ALP is responsible for the liberation of free phosphate from phosphate esters. The ALP yields high concentrations of inorganic phosphate salts that get deposited locally to form bone.
ALP might also promote mineralization by hydrolyzing inorganic pyrophosphate, a potent inhibitor of hydroxyapatite crystal formation and dissolution, within the extracellular calcifying matrix vesicles.[24]
Significant correlation was found between ALP levels and gingival index which is in agreement with Chapple et al.[10] and Nakashima et al.[25] The increase in gingival index denotes inflammation as a result of which the number of PMNLs increases. Plaque scores and ALP levels were not significantly correlating which is in favor of studies done by Binder et al.[26] and Chapple et al.[14] Significant correlations existed between ALP levels and probing depths as well as clinical attachment levels except in the healthy group. These findings were consistent with the results of the study done by Ishikawa and Cimasoni[11] and Binder et al.[26] respectively.
Thus the findings of this study state that ALP levels in GCF could differentiate between health, gingivitis, and periodontitis appreciably. Various other studies have shown a remarkably increased activity of ALP in the acute phase of periodontal disease, and after the periodontal therapy, the activity of these enzymes were restored to values as found with healthy individuals.[27] Change in ALP in GCF, saliva, and serum has been used as an inflammatory marker of periodontium as well as of bone metabolism.[7,17,28,29]
Studies have shown that ALP is involved in gingivitis[14] and periodontitis[11,30] with an increase seen in GCF ALP activity. Increased GCF ALP activity has been shown to have predictive value in terms of attachment loss that is significantly more accurate than the use of clinical parameters.[26,17] GCF ALP is also shown to reflect the short-term periodontal healing/recurrent inflammation phases in patients with chronic periodontitis.[31]
This results of this study also showed that a significant difference exists in ALP levels in GCF between health and chronic periodontitis as well as gingivitis and chronic periodontitis. Thus ALP promises to be a potential biochemical marker for the detection and progression of periodontal disease. With the advent of novel technologies such as ‘lab-on-a-chip’, microfluidic devices, and rapid point-of-care oral diagnostics, the generation of a periodontal disease biomarker report appears promising for future application to diagnose periodontal diseases and to prognosticate treatment outcomes of periodontal disease.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Shu L, Guan SM, Fu SM, Guo T, Cao M, Ding Y. Estrogen Modulates Cytokine Expression in Human Periodontal Ligament Cells. J Dent Res. 2008;87:142–7. doi: 10.1177/154405910808700214. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Peridontology. Position Paper: Diagnosis of periodontal diseases. J Periodontol. 2003;74:1237–47. doi: 10.1902/jop.2003.74.8.1237. [DOI] [PubMed] [Google Scholar]
- 3.Armitage GC. The complete periodontal examination. Periodontol 2000. 2004;34:22–33. doi: 10.1046/j.0906-6713.2002.003422.x. [DOI] [PubMed] [Google Scholar]
- 4.Taba M, Kinney J, Kim AS, Giannobile WV. Diagnostic Biomarkers for Oral and Periodontal Diseases. Dent Clin N Am. 2005;49:551–71. doi: 10.1016/j.cden.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Todorovic T, Dozic I, Vicente-Barrero M, Ljuskovic B, Pejovic J, Marjanovic M, et al. Salivary enzymes and periodontal disease. Med Oral Patol Oral Cir Bucal. 2006;11:E115–9. [PubMed] [Google Scholar]
- 6.Bezerra AA, Pallos D, Cortelli JR, Saraceni CH, Queiroz C. Evaluation of organic and inorganic compounds in the saliva of patients with chronic periodontal disease. Rev Odonto Cienc. 2010;25:234–8. [Google Scholar]
- 7.Daltaban O, Saygun I, Bal B, Balo K, Serdar M. Gingival crevicular fluid alkaline phosphatase levels in postmenopausal women: Effects of phase I periodontal treatment. J Periodontol. 2006;77:67–72. doi: 10.1902/jop.2006.77.1.67. [DOI] [PubMed] [Google Scholar]
- 8.Armitage GC. Analysis of gingival crevicular fluid and risk of progression of periodontitis. Periodontol 2000. 2004;34:109–19. doi: 10.1046/j.0906-6713.2002.003427.x. [DOI] [PubMed] [Google Scholar]
- 9.Reinhardt RA, Stoner JA, Golub LM, Lee HM, Nummikoski PV, Sorsa T, et al. Association of gingival crevicular fluid biomarkers during periodontal maintenance with subsequent progressive periodontitis. J Periodontol. 2010;81:251–9. doi: 10.1902/jop.2009.090374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapple IL, Mathews JB, Thorpe GH, Glenwright HD, Smith JM. A new ultrasensitive chemiluminesent assay for the site- specific quantification of alkaline phosphatase in gingival crevicular fluid. J Periodontal Res. 1993;28:266–73. doi: 10.1111/j.1600-0765.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa I, Cimasoni G. Alkaline phosphatase in human gingival fluid and its relation to periodontitis. Arch Oral Biol. 1970;15:1401–4. doi: 10.1016/0003-9969(70)90032-4. [DOI] [PubMed] [Google Scholar]
- 12.McCulloch CA. Host enzymes in gingival crevicular fluid as diagnostic indicators of periodontitis. J Clin Periodontol. 1994;21:497–506. doi: 10.1111/j.1600-051x.1994.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 13.Groeneveld MC, Van den Bos T, Everts V, Beertsen W. Cell-bound and extracellular matrix-associated alkaline phosphatase activity in rat periodontal ligament. Experimental Oral Biology Group. J Periodontal Res. 1996;31:73–9. doi: 10.1111/j.1600-0765.1996.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 14.Chapple IL, Glenwright HD, Matthews JB, Thorpe GH, Lumley PJ. Site-specific alkaline phosphatase levels in gingival crevicular fluid in health and gingivitis: Cross-sectional studies. J Clin Periodontol. 1994;21:409–14. doi: 10.1111/j.1600-051x.1994.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 15.Lilja E, Lindskog S, Hammarstrom L. Alkaline phosphatase activity and tetracycline incorporation during initial orthodontic tooth movement in rats. Acta Odontol Scand. 1984;42:1–11. doi: 10.3109/00016358409041125. [DOI] [PubMed] [Google Scholar]
- 16.Lindhe J, Lang NP, Karring T. Clinical Periodontology and Implant Dentistry. 5th ed. New York: Blackwell Munksgaard; 2008. [Google Scholar]
- 17.Chapple IL, Garner I, Saxby MS, Moscrop H, Matthews JB. Prediction and diagnosis of attachment loss by enhanced chemiluminescent assay of crevicular fluid alkaline phosphatase levels. J Clin Periodontol. 1999;26:190–8. doi: 10.1034/j.1600-051x.1999.260310.x. [DOI] [PubMed] [Google Scholar]
- 18.Shibata Y, Yamashita Y, Miyazaki H, Ueno S, Takehara T. Effective method for discriminating between oral bacterial and human alkaline phosphatase activity. Oral Microbiol Immunol. 1994;9:35–9. doi: 10.1111/j.1399-302x.1994.tb00212.x. [DOI] [PubMed] [Google Scholar]
- 19.Goodson JM, Haffajee AD, Socransky SS. The relationship between attachment level loss and alveolar bone loss. J Clin Periodontol. 1984;11:348–59. doi: 10.1111/j.1600-051x.1984.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 20.Goodson JM, Haffajee AD, Socransky SS. Comparison of different data analysis for detecting changes in attachment level. J Clin Periodontol. 1983;10:298–310. doi: 10.1111/j.1600-051x.1983.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 21.Lamster IB. The host response in gingival crevicular fluid: Potential applications in periodontitis clinical trials. J Periodontal. 1992;63:1117–23. doi: 10.1902/jop.1992.63.12s.1117. [DOI] [PubMed] [Google Scholar]
- 22.Delmas PD. Biochemical markers of bone turnover: Methodology and clinical use in osteoporosis. Am J Med. 1991;91:59S–63. doi: 10.1016/0002-9343(91)90250-2. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan A, Jack R, Opheim KE, Toivola B, Lyon AW. Clinical Chemistry: Interpretation And Techniques. St. Louis: C.V. Mosby Company; 1995. [Google Scholar]
- 24.Garnero P, Delmas PD. Biochemical markers of bone turnover. Applications for osteoporosis. Endocrinol Metab Clin North Am. 1998;27:303–23. doi: 10.1016/s0889-8529(05)70007-4. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima K, Demeurisse C, Cimasoni G. The recovery efficiency of various materials for sampling enzymes and polymorphonuclear leukocytes from gingival crevices. J Clin Periodontol. 1994;21:479–83. doi: 10.1111/j.1600-051x.1994.tb00411.x. [DOI] [PubMed] [Google Scholar]
- 26.Binder TA, Goodson JM, Socransky SS. Gingival fluid levels of acid and alkaline phosphatase. J Periodontal Res. 1987;22:14–9. doi: 10.1111/j.1600-0765.1987.tb01534.x. [DOI] [PubMed] [Google Scholar]
- 27.Yan F, Cao C, Li X. Alkaline phosphatase levels in gingival crevicular fluid of periodontitis before and after periodontal treatment. Zhonghua Kou Qiang Yi Xue Za Zhi. 1995;30:204–6. 255-6. [PubMed] [Google Scholar]
- 28.Gibert P, Tramini P, Sieso V, Piva MT. Alkaline phosphatase isozyme activity in serum from patients with chronic periodontitis. J Periodontal Res. 2003;38:362–5. doi: 10.1034/j.1600-0765.2003.00388.x. [DOI] [PubMed] [Google Scholar]
- 29.Totan A, Greabu M, Totan C, Spinu T. Salivary aspartate amninotransferase, alanine aminotransferase and alkaline phosphatase: Possible markers in periodontal diseases. Clin Chem Lab Med. 2006;44:612–5. doi: 10.1515/CCLM.2006.096. [DOI] [PubMed] [Google Scholar]
- 30.Nakashima K, Giannopoulou C, Andersen E, Roehrich N, Brochut P, Dubrez B, et al. A longitudinal study of various crevicular fluid components as markers of periodontal disease activity. J Clin Periodontol. 1996;23:832–8. doi: 10.1111/j.1600-051x.1996.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 31.Perinetti G, Paolantonio M, Femminella B, Serra E, Spoto G. Gingival crevicular fluid alkaline phosphatase activity reflects periodontal healing/recurrent inflammation phases in chronic periodontitis patients. J Periodontol. 2008;79:1200–7. doi: 10.1902/jop.2008.070519. [DOI] [PubMed] [Google Scholar]


