Abstract
Background:
Deficiency of Co-Q10 has been found to be responsible for periodontal destruction; therefore, this study was undertaken to evaluate the anti-gingivitis effect of Co-Q10 on plaque induced gingivitis.
Materials and Methods:
Thirty subjects with plaque induced gingivitis were enrolled in a split mouth randomized controlled trial. For each subject, scaling was randomly performed for any two quadrants, followed by the topical application of Co-Q10 randomly in a previously scaled and as an unscaled quadrant for a period of 28 days. Four treatment options were planned: option A: scaling only; option B: Co-Q10 along with scaling; option C: Co-Q10.
Results:
Marked reduction in gingival, bleeding, and plaque scores were recorded at the sites where C0-Q10 was applied. Mean±S.D of aforementioned periodontal parameters at 28th day showed significant reduction for option A, B, and C when compared with baseline.
Conclusion:
Promising results were obtained after the solitary application of Co-Q10 as well as when it was used as an adjunct to scaling and root planing for treatment of plaque induced gingivitis.
Keywords: Coenzyme-Q10, Plaque induced gingivitis, anti-gingivitis
INTRODUCTION
Coenzyme Q10 (Co-Q10; Ubiquinone) is a compound which is naturally found in every cell of the human body. It derives its name from the ubiquitous presence in nature and quinone structure, which is similar to that of vitamin K.[1] It is a fat-soluble compound which forms an important link in the electron transport system of mitochondria.
It is known to play a crucial role in the generation of adenosine triphosphate (ATP) and cellular respiration. It exists in two molecular forms, ubiquinone, the oxidized form, and ubiquinole, the reduced form, which are the basis for its antioxidant properties. Hence, Co-Q10 also functions as an intercellular antioxidant.[1]
True deficiency states are rare but often present with severe health consequences. Numerous disease processes which are linked to low levels of Co-Q10 can benefit from Co-Q10 supplementation including cardiovascular disease, Parkinson's disease, muscular dystrophy, breast and other cancers, diabetes mellitus, male infertility, acquired immunodeficiency syndrome (AIDS), asthma, thyroid disorders, and periodontal disease.
Co-Q10 has been the topic of research interest since 1970s, which experienced a series of trails depicting its anti-inflammatory, anti-oxidant, and immune modulatory activities. But there is scarcity of literature regarding the anti gingivitis effects of Co-Q10. Hence, the present study was designed with the aim to evaluate anti-gingivitis effect of Coenzyme Q-10 on plaque induced gingivitis.
MATERIALS AND METHODS
Thirty patients (16 female and 14 male) were recruited from the Outpatient Department of Periodontics and Implantology; Institute of Dental Sciences, Bareilly, after obtaining ethical clearance from the ethical committee. Systemically, healthy individuals with a minimum of 20 teeth present were enrolled in the study. The enrolled subjects had bleeding index (Mullerman and son), Plaque index (Silness and loe), and gingival index (loe and silness) scores of more than 2.0. Pregnant or lactating women, subjects with any systemic disease, and those who were using anti inflammatory drugs or antibiotics for the past six months were excluded from the clinical trial. Patients were informed about the study and written consent was obtained.
Coenzyme Q – 10 preparation* [Figure 1f] which was utilized had an active ingredient of 20% Co-Q10 Cyclodextrin Complex (Hydro – Q – Sorb). The split mouth study design was randomized with four treatment options. Option A: scaling only; Option B: Co–Q10 along with scaling; Option C: Co–Q10 application only; Option D: no treatment. Subjects were given a demonstration for the topical application of Co–Q10, and were then instructed for night application with a cotton roll stick. Eating, spitting, and drinking were restricted for 45 min, after application. Subjects were followed for a period of 28 days. Plaque, gingival and bleeding index was recorded at 0, 7th, 14th, 21st, and 28th day. Any oral itching, dryness, diarrhea, and taste alteration was enquired and recorded.
Figure 1.
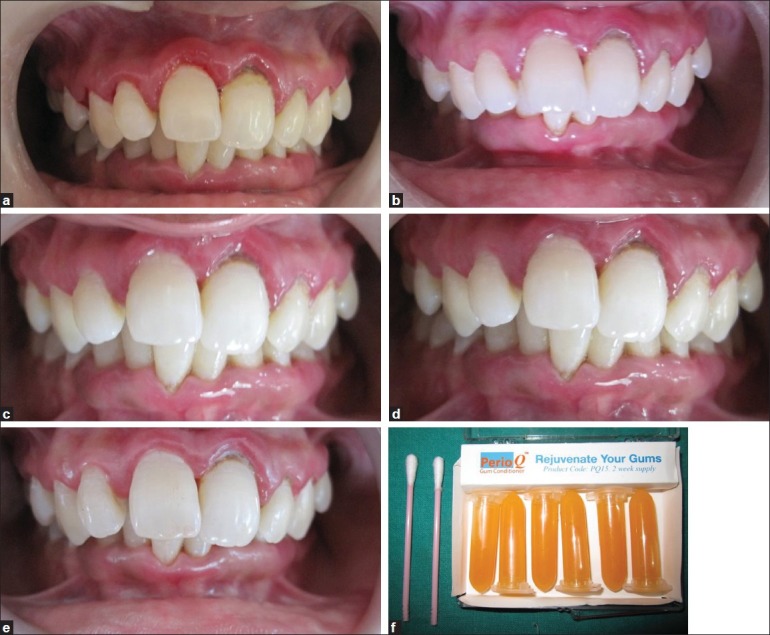
(a) Marked inflammation seen in all quadrants at baseline after employing treatment options; (b) 7th day recall; (c) 14th day recall; (d) 21sh day recall; (e) 28th day recall; (f) Co-Q10 material and application kit
The clinical trial was statistically analyzed by two way analysis of variation, followed by post hoc tukey test for inter group comparisons at 5% level of significance and 95% confidence limit. P value≤0.05 was considered statistically significant.
RESULTS
Two way ANOVA table was employed for plaque, gingival and bleeding index depicting a significant difference due to treatment options A, B, and C at all recall visits [Figure 2–4]. The results obtained for a particular case is depicted in Figure 1a through Figure 1e.
Figure 2.
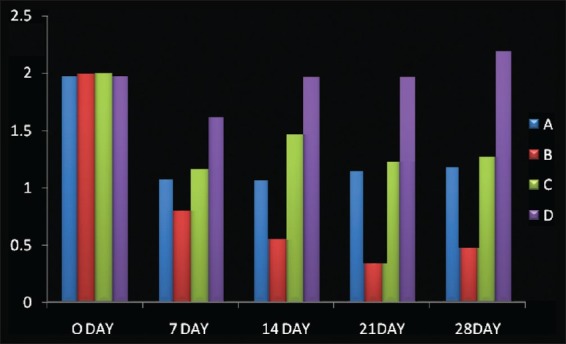
Mean±S.D (Plaque Index), A: scaling; B: CoQ 10+ scaling; C: CoQ 10; D: no treatment
Figure 4.
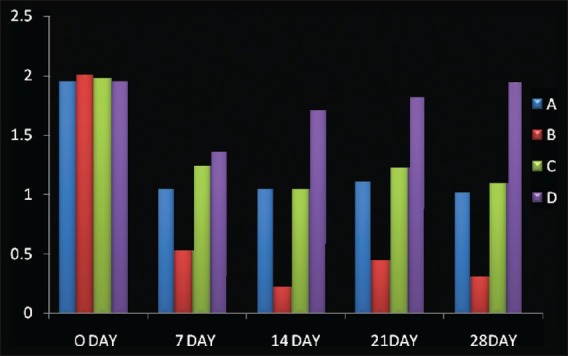
Mean±S.D (Bleeding Index), A: scaling; B: CoQ 10+ scaling; C: CoQ 10; D: no treatment
Figure 3.
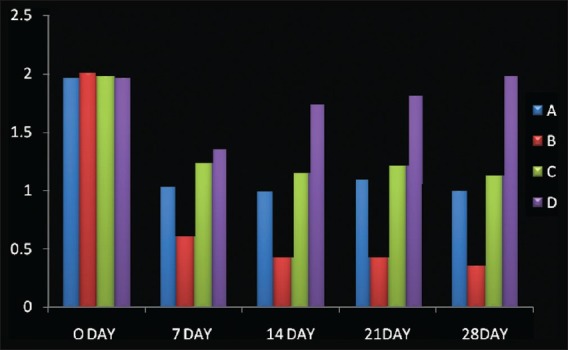
Mean±S.D (Gingival Index), A: scaling; B: CoQ 10+ scaling; C: CoQ 10; D: no treatment
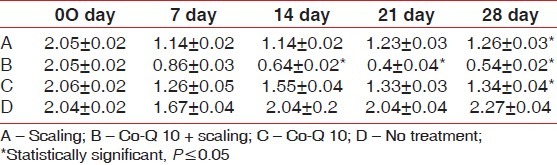
When mean ± S.D for Plaque index was considered significantly improved from baseline visit to 28th day recall was seen. Such as 2.05±0.02 reduced to 1.26±0.03 (option A); 2.05±0.02 to 0.54±0.02 (Option B) 2.06±0.02 to 1.34±0.04 (Option C), while no significant difference was seen in option D [Table 1].
Table 1.
Mean±S.D (Plaque Index)
Also, gingival index scores significantly improved from baseline to 28th day recall; 2.06±0.02 reduced to 1.08±0.03 (Option A); 2.08±0.02 reduced to 0.44±0.03 (Option B); and 2.06±0.02 reduced to. 1.23±0.05 (Option C), while no significant difference was seen in option D [Table 2]. Similarly, bleeding index scores significantly improved from baseline to 28th day recall; 2.04±0.02 reduced to 1.11±0.04 (option A); 2.08±0.02 reduced to 0.39±0.05 (Option B); and 2.07±0.02 reduced to 1.19±0.02 (Option C), while no significant difference was seen in option D [Table 3]. Later post HOC Tukey test was used for inter group comparison and following inferences were made. [Table 4]. When scaling alone was compared with Co-Q10 alone, application i.e (A versus C); there was no significant difference between the two treatment options depicting equal efficacy of two treatments at all recall visits. Also, when scaling alone (A) and Co –Q10 alone (C) were compared with no treatment group (D). There was a significant difference between them on all recall visits. Further, when scaling along with Co–Q10 (B) was compared with only scaling (A) and only Co–Q10 (C), a more significant difference was observed at all recall visits.
Table 2.
Mean±S.D (Gingival Index)
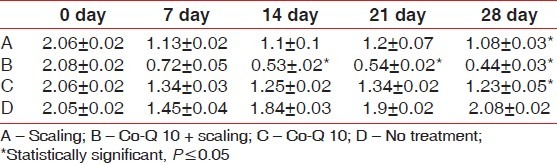
Table 3.
Mean±S.D (Bleeding Index)
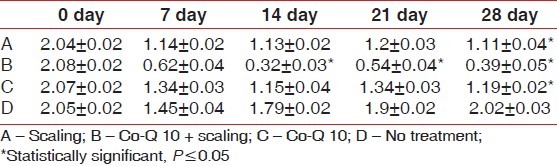
Table 4.
Post HOC tukey test
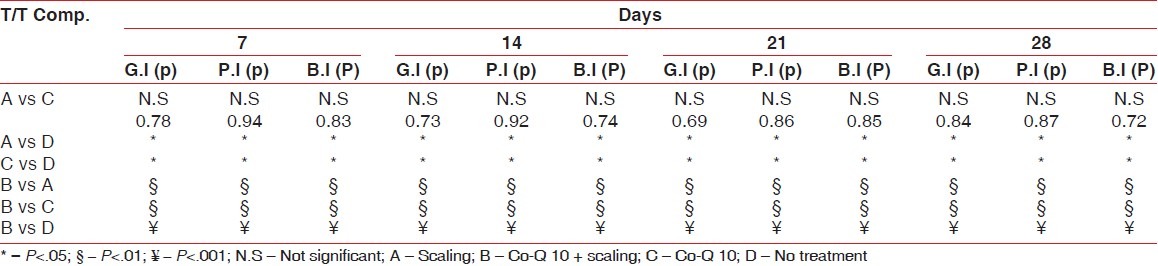
Finally, when scaling along with Co–Q10 (B) was compared with no treatment group (D). A highly significant difference was observed at all recall visits.
After randomization, the patient depicted in the picture [Figure 1a–e] received following treatment options. First quadrant received scaling along with Co-Q10 application; second quadrant received no treatment; third quadrant was treated only with Co-Q10 application; and fourth quadrant received only scaling. The clinical features improved significantly at 7th, 14th, 21st, and 28th day recalls, which were depicted by improved scores of aforementioned indices.
DISCUSSION
The mechanism of Co-Q10 had not been known until Littaru and Kamamura reported its deficiency in patients with periodontal disease.[2] They did a series of trials on succinate dehydrogenase – Co-Q10 reductase enzyme, which is found in Mitochondrial complex II of the cell. This was latter supported by the works Nakamura[3] and Matsumura[4]
The effect of Co-Q10 on the NFkB1-dependent pro-inflammatory cytokine TNF-α was studied and was suggested that Co-Q10 exerts anti-inflammatory properties via NFκB1-dependent gene expression.[5] Aforementioned is a common pathway for periodontal inflammation, suggesting that this could be the possible mechanism for gingival inflammation.
Patients with decreased level of serum Co-Q10 were at increased risk of rapid progression of gingivitis and periodontitis as compared to controls.[6,7] Application of Co-Q10 would improve the clinical parameters. Our study has shown significant decrease in plaque, bleeding, and gingival scores, which is consistent with the earlier studies done by Wilkinson et al[6,8] and Mcree et al,[9] Similarly, a split mouth trail conducted by Hanioka demonstrated improved periodontal scores along with gingival scores when Co-Q10 was applied alone or as an adjunct to scaling and root planing .[10] Clinical trials on human have revealed a relation between oral administration of Co-Q10 and improved gingival health and immune response. Our study showed similar results when Co-Q10 was solitary used or when used as an adjunct to scaling and root planing , although the present study was restricted only to plaque induced gingivitis and bleeding. The slight reduction or persistence of gingival inflammation of the teeth adjacent to the various treatment options could be credited to the carry cross effect of split mouth design. A study was conducted by Figuero (2006) to evaluate potential oxidant/antioxidant interactions of nicotine with antioxidant Coenzyme Q10 in subjects with periodontitis suggesting that the catabolic effects of nicotine could be reversed by the addition of antioxidants such as CoQ10.[11]
In a clinical trial of Co-Q10, systemic administration on patients of AIDS related complex, T4/T8 ratio was increased, and they were symptom free for duration of four years. Also when Co- Q10 was given to healthy individuals, a significant increase in T4/T8 ratio was observed.[12] The improved clinical parameters in our study could also possibly be credited by improvement in immunity in combating periodontal insult.
CONCLUSION
The present study shows promising results with the application of Coenzyme-Q10 solitary used and also as an adjunct to scaling and root planing for the treatment of plaque induced gingivitis; thus, opening new treatment options by strengthening the host side of the disease interaction. Further, long term trials are desired to comment on the application of Co-Q10 in periodontal diseases, in routine supportive periodontal therapy.
ACKNOWLEDGMENT
Special thanks to Dr. Subhash Gupta, Pennsylvania (U.S.A), for providing the sample of Coenzyme-Q10.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
Shows the clinical product avaliable
REFERENCES
- 1.Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 2.Littarru GP, Nakamura R, Ho L, Folkers K, Kuzell WC. Deficiency of coenzyme Q10 in gingival tissue from patients with periodontal disease. Proc Natl Acad Sci U S A. 1971;68:2332–5. doi: 10.1073/pnas.68.10.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura R, Littarru GP, Folkers K, Wilkinson EG. Study of CoQ1o-Enzymes in Gingiva from Patients with Periodontal Disease and Evidence for a Deficiency of Coenzyme Q10. Proc Natl Acad Sci. 1974;71:1456–60. doi: 10.1073/pnas.71.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumura T, Saji S, Nakamura R, Folkers K. Evidence for enhanced treatment of periodontal disease by therapy with coenzyme Q. Int J Vitam Nutr Res. 1973;43:537–48. [PubMed] [Google Scholar]
- 5.Schmelzer C, Lindner I, Rimbach G, Niklowitz P, Menke T, Döring F. Functions of coenzyme Q 10 in inflammation and gene expression. Biofactors. 2008;32:179–83. doi: 10.1002/biof.5520320121. [DOI] [PubMed] [Google Scholar]
- 6.Wilkinson EG, Arnold RM, Folkers K. “Bioenergetics in clinical medicine. VI. adjunctive treatment of periodontal disease with coenzyme Q”. Res Commun Chem Pathol Pharmacol. 1976;14:715–9. [PubMed] [Google Scholar]
- 7.Hansen IL, Iwamoto Y, Kishi T, Folkers K, Thompson LE. Bioenergetics in clinical medicine. IX. Gingival and leucocytic deficiencies of coenzyme Q in patients with periodontal disease. Res Commun Chem Pathol Pharmacol. 1976;14:729–38. [PubMed] [Google Scholar]
- 8.Wilkinson EG, Arnold RM, Folkers K, Hansen I, Kishi H. Bioenergetics in Clinical Medicine II. Adjunctive Treatment with Coenzyme Q in Periodontal Therapy. Res Commun Chem Pathol Pharmacol. 1975;12:111–23. [PubMed] [Google Scholar]
- 9.McRee JT, Hanioka T, Shizukuishi S, Folkers K. Therapy with coenzyme Q10 for patients with periodontal disease. J Dent Health. 1993;43:659–66. [Google Scholar]
- 10.Hanioka T, Tanaka M, Ojima M, Shizukuishi S, Folkers K. Effect of topical application of coenzyme Q10 on adult periodontitis. Mol Aspects Med. 1994;15:241–8. doi: 10.1016/0098-2997(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 11.Figuero E, Soory M, Cerero R, Bascones A. Oxidant/antioxidant Interactions of Nicotine, Coenzyme Q10, Pycnogenol and Phytoestrogens in Oral Periosteal Fibroblasts and MG63 Osteoblasts. Steroids. 2006;71:1062–72. doi: 10.1016/j.steroids.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Folkers K, Hanioka T, Xia LJ, McRee JT, Langsjoen P. Coenzyme Q10 increases T4/T8 ratios of lymphocytes in ordinary subjects and relevance to patients having the AIDS related complex. Biochem Biophys Res Commun. 1991;176:786–91. doi: 10.1016/s0006-291x(05)80254-2. [DOI] [PubMed] [Google Scholar]


