Abstract
Background:
Initial research has shown a positive correlation between the severity of periodontal disease and matrix metalloproteinase-3 (MMP-3) concentrations in gingival crevicular fluid (GCF). However, there are no enough reports to correlate the MMP-3 concentrations in GCF in periodontal health, disease and after treatment. Hence, the present study is to estimate the levels of MMP-3 in GCF in periodontal health, disease and to evaluate the effect of periodontal therapy on MMP-3 concentrations in GCF.
Materials and Methods:
Periodontal examination and collection of GCF by extracrevicular method was performed in 30 subjects selected randomly and categorized into three groups. Group I (Healthy, n=10), group II (Chronic periodontitis, n=20) and group III (After treatment group, n=20). Scaling and root planing (SRP) was performed and GCF was collected after 8 weeks of treatment. MMP-3 levels were estimated in GCF samples using enzyme linked immunosorbent assay (ELISA).
Results:
MMP-3 was detected in all samples. Highest mean MMP-3 concentrations in GCF were obtained for group II (7.490 ng/ml), while the lowest concentrations were seen in group I (0.344 ng/ml) and group III (2.129 ng/ml). This suggests that MMP-3 levels in GCF increases proportionally with the progression of periodontal disease and decreases after treatment.
Conclusion:
There is a substantial increase in the concentrations of MMP-3 as periodontal disease progresses. Since MMP-3 levels in GCF are positively correlated with gingival index, probing pocket depth and clinical attachment level, MMP-3 may be considered as a “novel biomarker” in periodontal disease progression. However, controlled, longitudinal studies are needed to confirm this possibility.
Keywords: Gingival crevicular fluid, matrix metalloproteinase-3, periodontal disease, scaling and root planing
INTRODUCTION
Periodontitis is a chronic inflammatory disease causing destruction of the attachment apparatus of teeth. Progression of the periodontal lesion during the inflammatory process is the consequence of breakdown of the collagenous sharpey's fibers anchored in the root cementum. Degradation of the collagenous matrix involves the activity of a group of enzymes known as matrix metalloproteinases.[1]
These matrix metalloproteinases are important sub family of zinc-and calcium-dependent endopeptidases secreted or released by a variety of host cells such as polymorphonuclear leucocytes, macrophages, fibroblasts, bone, epithelial and endothelial cells that function at neutral pH and utilize the various constituents of the extracellular matrix as their substrates, and are responsible for their remodelling and degradation. These molecules degrade interstitial and basement membrane collagens, gelatins, fibronectin, elastin, laminin, vitronectin and the proteoglycan core protein etc.[2]
These proteinases are involved in a number of physiological events such as embryological development, tissue remodelling, wound healing, salivary gland morphogenesis and tooth eruption, in addition to various pathological processes such as periodontal disease, arthritis, cancer, atherosclerosis, pulmonary emphysema and osteoporosis.[3]
Stromelysin-1 (MMP-3) is a matrix metallopro-teinase with broad substrate specificity that has been linked to tissue destruction associated with chronic inflammatory diseases such as periodontitis. The extracellular matrix not only consists of collagen fibrils but also their associated proteoglycan and fibronectin which must be removed first in order for the collagenase to have access to the collagen substrate. Matrix metalloproteinase-3 is effective at degrading proteoglycans and fibronectin.[4,5]
This Clinico biochemical study is aimed to estimate the levels of MMP-3 in gingival crevicular fluid (GCF) in periodontal health, disease and to evaluate the effect of periodontal therapy on MMP-3 concentration in gingival crevicular fluid (GCF).
MATERIALS AND METHODS
This study was conducted in the division of periodontics, C.K.S Teja Institute of Dental Sciences and Research. Study population consisted of 30 subjects who attended our out-patient section; patients were included if they were in between 20-50 years of age group and had not received any periodontal treatment in previous 6 months and were excluded if suffering from any systemic diseases including diabetes mellitus, hypertension, psoriasis, tumors, having smoking habit, Sjogren's syndrome, delayed hypersensitivity, cardiac valvular diseases, subjects who have received anti-inflammatory drugs, antibiotics and neo vascularization inhibitors in the previous 6 months and rheumatoid arthritis. Ethical clearance for the study was obtained from the ethical committee of the institution. The patients were explained the study procedure and written informed consent was obtained from those who agreed to participate voluntarily in this study.
The subjects selected randomly were categorized into three groups. Group 1 consisted of 10 subjects with clinically healthy periodontium and with no evidence of disease. Group II consisted of 20 periodontitis subjects with clinical signs of inflammation, probing pocket depth (PPD) ≥5 mm and clinical attachment loss (CAL) ≥2 mm, with radiographic evidence of bone loss. Group III consisted of 20 subjects of group II treated by scaling and root planing (SRP) only.
Gingival index (GI) scores, probing pocket depth (PPD) and clinical attachment level (CAL) measurements were recorded for all the patients. Test site for GCF sample collection was selected based on the highest scored sites in the oral cavity, i.e., the site showing greatest amount of attachment loss (in chronic periodontitis cases), and the same test site for after treatment group was selected. GCF collection was done in the next appointment to avoid contamination of the sample.
Gingival crevicular fluid sampling and phase I therapy
After making the subjects sit comfortably in an upright position on the dental chair, the selected test site was air dried and isolated with cotton rolls. Without touching the marginal gingiva, supragingival plaque was removed to avoid contamination and blocking of the microcapillary pipette. GCF was collected by placing 1-3 μ1 calibrated volumetric microcapillary pipettes obtained from Sigma Aldrich Chemical Company, USA (Catalog No. p0549). By placing the tip of the pipette extracrevicularly (unstimulated) for 5-20 minutes, a standardized volume of 3 μl GCF was collected using the calibration on the micropipette from each test site . The test sites, which did not express standard volume (3 μ1) of GCF, and micropipette contaminated with blood and saliva, were excluded. Samples of GCF were collected at the initial visit in group II patients. Periodontal treatment (SRP) was performed for periodontitis patients at the same appointment after GCF collection. After 8 weeks, GCF was collected from the same site of these subjects were considered as group III. For this 8 week period subjects were called at one-week interval and plaque control measures were performed. The GCF collected was immediately transferred to aliquots and stored at –70°C till the time of the assay.
Gingival crevicular fluid enzyme-linked immunoabsorbent assay analysis for MMP-3
This assay employs the quantitative sandwich enzyme immunoassay technique with catalog number DMP300 from R and D systems. A polyclonal antibody specific for MMP-3 has been pre-coated onto a microplate. Standards and samples are pipetted into the wells and any MMP-3 present is bound by the immobilized antibody. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for MMP-3 is added to the wells. Following a wash to remove any unbound antibody-enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the concentrations of total MMP-3 (pro-and/or active) bound in the initial step. After the color development is stopped then the intensity of the color is measured.
Statistical analysis
All the data were analyzed using a software program; SPSS 17.1, IBM, Chicago, IL. To test the hypothesis of equality of means among the three groups non-parametric Kruskal-Wallis test was carried out. Further, multiple comparison using Mann-Whitney U test was carried on to find out which pair or pairs differ significantly. Group III (after treatment) and group II were compared using Wilcoxon signed Rank Test. Spearman's rank correlation test was done to observe for any correlation between the GCF MMP-3 concentration and clinical parameters.
RESULTS
The mean GI was significantly higher in group II, i.e., 1.666; SD 0.233, when compared with group I (i.e., 0.112, SD 0.018) and group III (i.e., 0.820, SD 0.047), which was statistically significant (P<0.001). The mean probing pocket depth was significantly higher in group II, i.e., 5.550, when compared with group I (i.e., 1.100, SD 0.316) and group III (i.e., 3.700, SD 0.571), which was statistically significant (P<0.001). The mean clinical attachment loss was significantly higher in group II, i.e., 3.450, when compared with group I (i.e., 0.00, SD 0.00) and group III (i.e., 1.800, SD 0.615), which was statistically significant (P<0.001) [Table 1].
Table 1.
Clinical characteristics and matrix metalloproteinase-3 levels in Groups I-III (mean±SD)
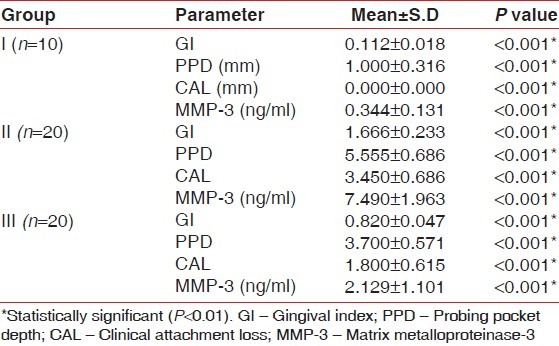
All the samples in each group tested positive for the presence of MMP-3. The mean concentration of MMP-3 concentration in group I was 0.344 ng/ml with SD 0.131. The mean MMP-3 concentration in group II was 7.490 ng/ml with SD 1.963. The mean MMP-3 concentration in group III was 2.129 ng/ml with SD 1.101. When we compare in between groups, the mean MMP-3 concentrations in GCF was observed to be highest in group II and lowest in group I. The mean MMP-3 concentration in group III fell between the highest and lowest values [Table 1].
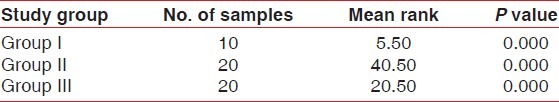
To test the hypothesis of equality of means among the three groups non-parametric Kruskal-Wallis test was carried on, the results of which are tabulated in Table 2. The mean ranks obtained for groups I-III are 5.50, 40.50, and 20.50 for GCF with P=0.00. Therefore, the hypothesis of equality of means is rejected at 5% level of significance (P<0.05), which indicates that the means differ significantly.
Table 2.
Results of Kruskal-Wallis test comparing mean matrix metalloproteinase-3 concentration in gingival crevicular fluid among groups
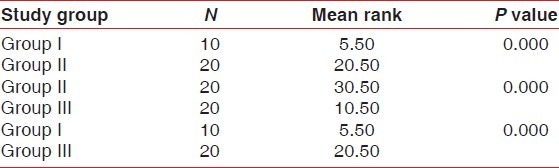
Further, multiple comparisons using Mann-Whitney U test was carried on to find out which pair or pairs differ significantly. When groups I and II, II and III and I and III were compared, the differences were statistically significant with mean ranks between group I and II, i.e., 5.50 and 20.50, between group II and III, i.e., 30.50 and 10.50 and between group I and III i.e., 5.50 and 20.50 with P=0.05 as shown in Table 3.
Table 3.
Mann-Whitney u test for pair wise comparison of matrix metalloproteinase-3 concentrations
When group III (after treatment) and group II were compared using Wilcoxon signed rank test, the difference in the concentration of MMP-3 was statistically significant (P<0.05), indicating that, after scaling and root planing , MMP-3 levels decreased considerably from 7.4092 ng/ml to 2.1298 ng/ml as shown in Table 4.
Table 4.
Results of Wilcoxon-signed rank test to compare matrix metalloproteinase-3 concentration in gingival crevicular fluid before and after treatment
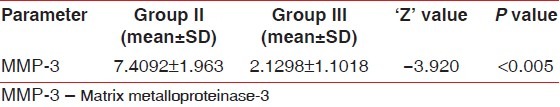
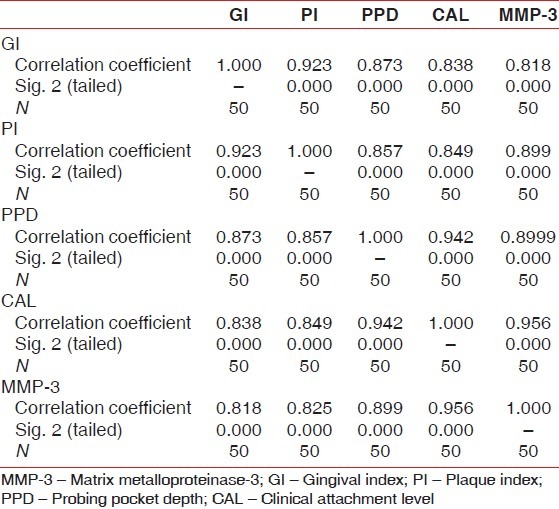
Spearman's rank correlation test was done to observe for any correlation between the GCF MMP-3 concentration and clinical parameters, i.e., GI, Plaque Index (PI), PPD, and CAL. The results of the test showed a positive correlation between GCF MMP3 and clinical parameters with ‘r’ value of 0.818 for GI, 0.899 for PPD, 0.956 for CAL in all the three groups as shown in Table 5.
Table 5.
Results of Spearman correlation test between matrix metalloproteinase-3 and clinical parameters
DISCUSSION
Periodontal diseases are characterized by a loss of collagen fibers in periodontal tissues. A possible mechanism for the degradation of periodontal fibers is the independent and/or co-operative action of both human and bacterial proteinases.[6,7] Periodontal tissue destruction is most likely mediated to a significant extent by the host cell-mediated MMPs.[8,9] The regulation of the enzymatic action of MMPs relies on conversion from the latent precursor to the active form.[8,10] MMP-3 is a broad-spectrum MMP and a pivotal activator of latent MMPs.[11] It has been showed that MMP-3 could activate procollagense including pro-MMP-1,-8,-9.[8] As the coordinated activity of the MMPs is critical in the collagenetic cascade, the regulatory effects of MMP-3 may be important in the overall regulation of connective tissue degradation in both physiologic and pathologic conditions.[12,13]
Therefore, in the present study, the levels of matrix metalloproteinase-3 (MMP-3) in gingival crevicular fluid in periodontal health, disease and after treatment were estimated and the objective is to evaluate the effect of phase I periodontal treatment on gingival crevicular fluid levels of MMP-3. In the present study, GCF collection was done using microcapillary pipettes, and MMP-3 concentrations analysed by ELISA. Whereas the earlier studies used filter paper strips and Periotron 8000 and 6000 which can result in non-specific attachment of the analyte to filter paper fibers ensuing in a false reduction in the detectable MMP-3 levels, which underestimates the correlation of MMP-3 levels to disease.
In the present study the mean concentrations of MMP-3 in GCF were found to increase progressively from healthy (i.e., 0.344 ng/ml) to periodontitis group (i.e., 7.490 ng/ml) with P=0.000. These results are in accordance with Tuter et al.[14] and Haerian et al.[15] According to Tuter et al.,[14] the mean MMP-3 concentrations were found to increase progressively from healthy (i.e., 0.15 ng/μl) to periodontitis group (i.e., 9.0 ng/μl) with P=0.001. As per the results of Haerian et al.,[16] the mean MMP-3 concentrations were found to increase progressively from healthy (i.e., 3.9 pg/30s) to periodontitis group (i.e., 9.4 pg/30s) with P=0.006.
In the present study, when groups I and II, II and III and I and III were compared, the differences were statistically significant with mean ranks between group I and II, i.e., 5.50 and 20.50, between group II and III, i.e., 30.50 and 10.50 and between group I and III, i.e., 5.50 and 20.50 with P=0.05.This clearly suggests that MMP-3 concentrations in GCF increases progressively from healthy to periodontitis.
In the present study chronic periodontitis subjects were treated by non-surgical periodontal therapy (SRP) and strict oral hygiene measures were instituted. The mean MMP-3 concentrations in GCF in chronic periodontitis group reduced from 7.490 ng/ml to an after treatment levels of 2.129 ng/ml, which were statistically significant with value P<0.001. The results are in accordance with Tuter et al.[14] with mean GCF MMP-3 levels in pre-treatment and post-treatment were 9.0 (4.12-33.55) ng/μl and 5.5 (1.65-10.87) ng/μl, respectively, with P=0.013. Haerian et al.[15] evaluated the effect of scaling and root planing on GCF levels of fibroblast collagenase, stromelysin and TIMP in a group of patients with advanced periodontal disease by a sandwich ELISA. As per the results of Haerian et al.[15] the mean GCF MMP-3 concentrations were found to be more in periodontitis group (i.e., 9.9 pg/30s) compared to after treatment group (i.e., 3.1 pg/30s) with P=0.029.
The results in the present study showed a positive correlation between GCF MMP3 and clinical parameters with ‘r’ value of 0.818 for GI, 0.899 for PPD, 0.956 for CAL in all the three groups. These results are in accordance with Tuter et al. and Haerian et al. According to Tuter et al.,[14] the positive correlations among all clinical parameters and MMP-3 levels in GCF were significant with ‘r’ value of 0.362 (P<0.001) for GI, 0.279 (P<0.05) for PPD and 0.477 (P<0.01) for CAL. According to Haerian et al.[16] the positive correlation between clinical parameters and SL levels in GCFweresignificant with ‘r’ value of 0.228 (P=0.012) and 0.256 (P=0.005) for GI and for PPD, respectively.
In the light of the present study results, it can be suggested that the mean concentrations of MMP-3 in diseased group were significantly higher than in healthy and after treatment groups. This data indicates that the high GCF levels of MMP-3 are at significantly greater risk for progression of periodontitis. Thus, this study is useful in assessing the health, disease status of periodontal tissues and efficacy of clinical treatment accompanied by reduction in MMP-3.
CONCLUSION
In conclusion, within the limits of our study, it can be postulated that, greater the extent of periodontal destruction, increase in the concentrations of MMP-3 in GCF was seen. In the present study when chronic periodontitis subjects were treated by non-surgical periodontal therapy (SRP), the mean GCF MMP-3 concentrations reduced significantly. However, further longitudinal studies are needed to evaluate the concentrations of MMP-3 in the periodontal disease tissues and GCF will be beneficial in clarifying the role in the pathogenesis of periodontitis and to validate MMP-3 as a “novel biomarker” of periodontal disease progression.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: Structures, evolution, and diversification. FASEB J. 1998;12:1075–95. [PubMed] [Google Scholar]
- 2.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function and biochemistry. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 3.Lemaitre V, D’Armiento J. Matrix metalloproteinases in development and disease. Birth Defects Res C Embryo Today. 2006;78:1–10. doi: 10.1002/bdrc.20065. [DOI] [PubMed] [Google Scholar]
- 4.Sorsa T, Tjaderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004;10:311–8. doi: 10.1111/j.1601-0825.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 5.Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993;64:474–84. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- 6.Sorsa T, Ding YL, Ingman T, Salo T, Westerlund U, Haapasalo M, et al. Cellular source, activation and inhibition of dental plaque collagenase. J Clin Periodontol. 1995;22:709–17. doi: 10.1111/j.1600-051x.1995.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 7.Heath JK, Gowen M, Meikle MC, Reynolds JJ. Human gingival tissues in culture synthesize three metalloproteinases and a metalloproteinase inhibitor. J Periodontal Res. 1982;17:183–90. doi: 10.1111/j.1600-0765.1982.tb01143.x. [DOI] [PubMed] [Google Scholar]
- 8.Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (Stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267:3581–4. [PubMed] [Google Scholar]
- 9.Naesse EP, Schreurs O, Helgeland K, Schenck K, Steinsvoll S. Matrix metalloproteinases and their inhibitors in gingival mast cells in persons with and without humanimmunodeficiency virus infection. J Periodontal Res. 2003;38:575–82. doi: 10.1034/j.1600-0765.2003.00687.x. [DOI] [PubMed] [Google Scholar]
- 10.Figueredo CM, Areas A, Mirinda LA, Fischer RG, Gustafsson A. The short term effectiveness of non-surgical treatment in reducing protease activity in gingival crevicular fluid from chronic periodontitis patients. J Clin Periodontol. 2004;31:615–9. doi: 10.1111/j.1600-051X.2004.00532.x. [DOI] [PubMed] [Google Scholar]
- 11.Kubota T, Nomura T, Takahashi T, Hara K. Expression of m RNA for matrix metalloproteinases and tissue inhibitors of metalloproteinases in periodontitis-affected human gingival tissue. Arch Oral Biol. 1996;41:253–62. doi: 10.1016/0003-9969(95)00126-3. [DOI] [PubMed] [Google Scholar]
- 12.Borghaei RC, Sullivan C, Mochan E. Identification of a cytokine induced repressor of Interleukin-1 stimulated expression of stromelysin-1 (MMP-3) J Biol Chem. 1999;274:2126–31. doi: 10.1074/jbc.274.4.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domeij H, Yucel–Lindberg T, Modeer T. Signal pathways involved in the production of MMP-1 and MMP-3 in human gingival fibroblasts. Eur J Oral Sci. 2002;110:302–6. doi: 10.1034/j.1600-0722.2002.21247.x. [DOI] [PubMed] [Google Scholar]
- 14.Tuter G, Kurtis B, Serdar M, Yucel A, Ayhan E, Karaduman B, et al. Effects of phase 1 periodontal treatment on gingival crevicular fluid levels of matrix metallo.loproteinase-3 and tissue inhibitor of metalloproteinase-1. J Clin Periodontol. 2005;32:1011–5. doi: 10.1111/j.1600-051X.2005.00816.x. [DOI] [PubMed] [Google Scholar]
- 15.Haerian A, Adonogianaki E, Mooney J, Docherty JP, Kinane DF. Gingival crevicularstromelysin, collagenase and tissue inhibition of metalloproteinase levels in healthy and diseased sites. J Clin Periodontol. 1995;22:505–9. doi: 10.1111/j.1600-051x.1995.tb00797.x. [DOI] [PubMed] [Google Scholar]
- 16.Haerian A, Adonogianaki E, Moneey J, Manos A, Kinane DF. Effects of treatment on gingival crevicular collagenase, stromelysin and tissue inhibitor of metalloproteinase and their ability to predict response to treatment. J Clin Periodontol. 1996;23:83–91. doi: 10.1111/j.1600-051x.1996.tb00539.x. [DOI] [PubMed] [Google Scholar]


