Abstract
Aims and objectives:
The aim of this study is to evaluate the effectiveness of a controlled-release chlorhexidine chip as an adjunctive therapy to scaling and root planing when compared with scaling and root planing alone in the treatment of chronic periodontitis.
Materials and Methods:
20 patients with a total number of 40 posterior sites were selected. These sites were divided into two groups in a split mouth design,: Group A (control site) had 20 sites treated with scaling and root planing alone and Group B (test site) had 20 sites treated with scaling and root planing and PerioCol™-CG. The clinical parameters (Plaque index, bleeding on probing, probing pocket depth, clinical attachment level) were recorded at baseline, 90th and 180th day for both the groups.
Results:
When both groups were compared the change in Plaque index was significantly higher in Group B when compared to Group A on the 90th day and 180th day. However, there was no statistically significant difference in the mean percentage of gingival bleeding sites between the two groups on the 90th day, though Group B showed a statistically higher reduction in the mean percentage of gingival bleeding sites at the end of 180th day. There was no statistically significant difference in probing pocket depth between the two groups on both 90th and 180th day. Gain in clinical attachment level was significantly higher in Group B when compared to Group A on the 90th and 180th day.
Conclusion:
From the results observed in this study, it can be concluded that the adjunctive use of PerioCol™-CG was safe and provided significant improvement in both Plaque index and gingival bleeding index. It was also more favorable than scaling and root planing alone for gain in clinical attachment level.
Keywords: Chronic periodontitis, chlorhexidine, scaling and root planing, probing pocket depth and clinical attachment level
INTRODUCTION
The main therapeutic approach for the management of periodontal disease includes mechanical scaling and root planing, thereby removing the bacterial deposits from tooth surface. Because of the complex anatomy of the roots and contours of the lesion, mechanical periodontal treatment alone may not be effective, and sufficient in the reduction of the bacterial load to make the tooth surface biologi cally acceptable. Moreover, the success of mechanical periodontal treatment is closely related to the patient's oral hygiene performance. Recurrent periodontal tissue destruction is almost inevitable in patients who fail to achieve an acceptable plaque control during treatment or maintenance phase of periodontal therapy.[1]
Adjunctive administration of systemic antimicrobials has been useful in treating periodontal pockets. However, the dose necessary to achieve sufficient local concentrations of antimicrobials in the periodontal environment might be associated with undesirable side effects. Local administration therefore may be considered as an alternative to overcome these problems. The local delivery device consists of a drug reservoir and a limiting element that controls the rate of the medicament release. The goal is to maintain effective concentrations of chemotherapeutic agents at the site of action for longer periods.[1]
Local delivery devices can be divided into two classes according to the duration of the medicament release:
sustained release devices
controlled delivery devices
Sustained release formulations are designed to provide drug delivery for less than 24 h. On the other hand, controlled delivery systems should have duration of drug release that exceeds 1 day.[2,3]
Chlorhexidine has long been used as an effective antimicrobial agent. Its mechanism of action relates to reduction in pellicle formation, alteration of bacterial adherence to teeth and an alteration of bacterial cell wall causing lysis. Chlorhexidine is effective against subgingival plaque bacteria when delivered via a sustained-release device for 9 days. The antimicrobial effects were evident up to 11 weeks after treatment, and clinical efficacy in terms of reduced probing depth, clinical attachment levels, and reduction of bleeding on probing was evident. A biodegradable chip for sustained and direct delivery of chlorhexidine to the periodontal pocket has been developed.[4]
The aim of this study is to evaluate the effectiveness of a controlled-release chlorhexidine chip (PerioCol™ -CG) as an adjunct to scaling and root planing when compared with scaling and root planing alone in the treatment of chronic periodontitis.
PerioCol™-CG is manufactured by Eucare pharmaceuticals (P) Ltd. PerioCol™-CG is a small, orange- brown rectangular chip. It is rounded at one end for easy insertion into periodontal pockets. Each PerioCol™-CG contains approximately 2.5 mg of chlorhexidine gluconate in a biodegradeable matrix of Type 1 collagen derived from fish sources. PerioCol™-CG releases chlorhexidine in vitro with a release profile of approximately 40-45% within 24h and afterward in linear fashion for 7-8 days. The release profile may be explained by initial burst effect due to diffusion of the drug from the chip followed by release of the drug due to enzymatic degradation.
MATERIALS AND METHODS
Twenty patients, aged 35-55 years, diagnosed with generalized chronic periodontitis, having probing pocket depth ≥5mm were selected from the Department of Periodontology and Implantology, Meenakshi Ammal Dental College, Chennai, for this study which was approved by Ethical Committee.
A total number of 40 posterior sites were selected. These sites were divided into two groups in a split mouth design.
Group-A (control site) had 20 sites treated with scaling and root planing alone.
Group-B (test site) had 20 sites treated with scaling and root planing and PerioCol™-CG.
Exclusion criteria included allergy to chlorhexidine, presence of overhanging restoration, smoking, pregnant women, those who have received antibiotics or any form of periodontal treatment in the previous 6 months.
Study design
After selection of patients, a full mouth supragingival scaling was done with ultrasonic scalers and oral hygiene instructions were given. Impressions were taken and an acrylic stent was made for standardized measurements of probing pocket depth and clinical attachment level [Figure 1a, c–e].
Figure 1.
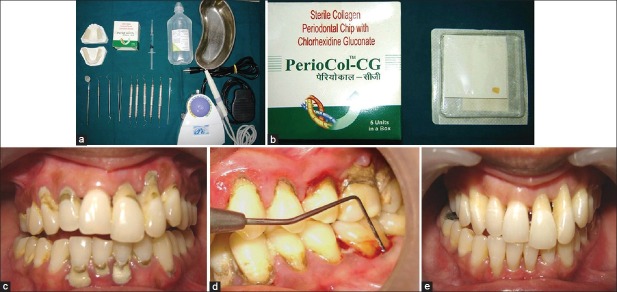
(a) Armamentarium; (b) PerioCol™-CG; (c) Chronic periodontitis; (d) Assessment of bleeding on probing; (e) After scaling and root planing
The clinical parameters (Plaque index, bleeding on probing, probing pocket depth, clinical attachment level) were recorded at baseline, 90th , and 180th day.
Baseline (0 day): After recording the clinical parameters, scaling and root planing was performed using ultrasonic scalers and Gracey curettes in control sites [Figure 2] and test sites [Figure 3a]. It was followed by the placement of PerioCol™-CG at the (test sites) Group-B [Figure 3b].
Figure 2.
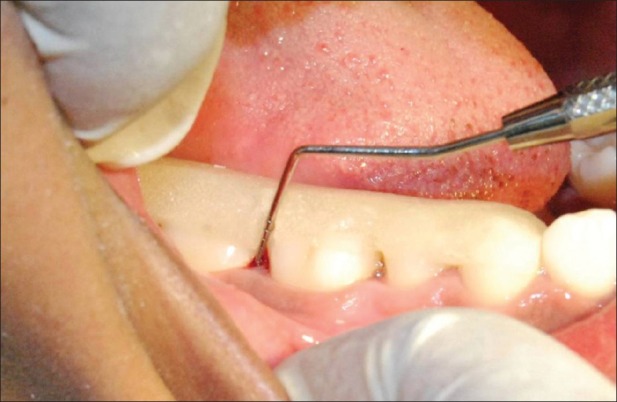
Group A - Measurement of probing pocket depth and clinical attachment level using acrylic stent
Figure 3.
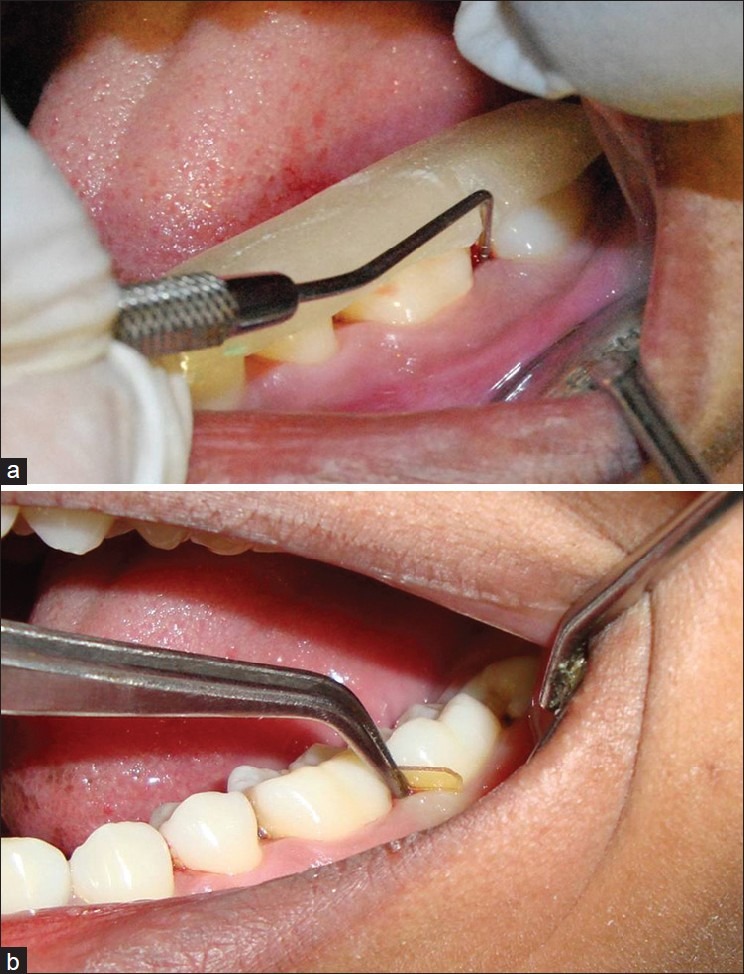
(a) Group B-Measurement of probing pocket depth and clinical attachment level using acrylic stent; (b) Placing of PerioCol™-CG
Placement of Periocol-CG: After scaling and root planing, the test sites were dried with cotton pellets. The PerioCol™-CG was taken with a sharp tweezer tip from an individual foil packet. PerioCol™-CG was moistened with normal saline. It was grasped at the flat end, and the curved end was first inserted into the periodontal pocket. It was gently pressed apically to the base of the pocket without any folds [Figure 3b]. After placement into the test sites, patients were instructed not to use interdental cleansers such as dental floss or tooth pick for the next 10 days to avoid displacement, or any kind of chemotherapeutic mouth rinses or oral irrigation device. The patients were recalled on the third and seventh day to assess the position of PerioCol™-CG.
On 90th day: The clinical parameters were recorded in both Groups A and B [Figures 2 and 3a]. Supragingival scaling was performed in both Groups A and B. PerioCol-CG™ was again placed into Group B.
On 180th day: The clinical parameters were recorded in both Groups A and B.
Statistical analysis
The mean and standard deviation were compared by using Student's paired t-test, Wilcoxon's signed rank test, and McNemar's test.
RESULTS
Overall, 40 sites were treated, 20 with SRP and 20 with SRP plus CHX chip.
Table 1 shows the comparison of mean, standard deviation, and test of significance for the Plaque index score between Groups A and B at different time points.
Table 1.
Comparison of mean, standard deviation, and test of significance for the Plaque index score between Groups A and B at different time points

At baseline, there was no statistically significant difference in the Plaque index score between the SRP alone and the CHX plus SRP group. Both groups had a significant change in the Plaque index score at 90 and 180 days. At the 90th day, the plaque index score was reduced to 1.1 in the SRP alone group and 0.9 in the SRP plus CHX chip group. Further improvements occurred in the Plaque index score at 180th day, 0.9 in SRP alone, and 0.7 in the SRP plus CHX chip group, respectively.
Table 2 shows the percentage of gingival bleeding sites in Group A and Group B at different time points.
Table 2.
Percentage of gingival bleeding sites in Group A and B at different time points
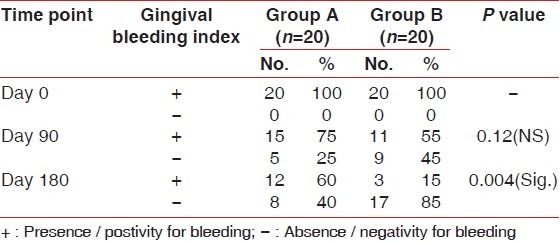
The positivity of gingival bleeding sites was 100% at baseline in both Group A and Group B. On the 90th day, there was 25% reduction in gingival bleeding sites in Group A and 45% reduction in Group B. On 180th day, there was 40% reduction in gingival bleeding sites in Group A and 85% reduction in Group B.
Table 3 shows the comparison of mean, standard deviation,and test of significance for probing pocket depth between Group A and B at different time points. At baseline, there was no statistically significant difference in probing depth between the SRP alone and CHX plus SRP group. At 90th day, PD was reduced to 6.2 mm in the SRP alone group and 5.8 mm in the SRP plus CHX chip group compared to baseline. At 180th day PD was further reduced to 4.8mm in SRP alone group and 4.2 mm in the SRP plus CHX chip group, respectively.
Table 3.
Comparison of mean, standard deviation, and test of significance for probing pocket depth between Group A and B at different time points
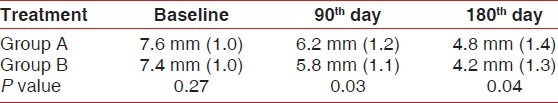
Table 4 shows the comparison of mean change in probing pocket depth between Group A and B from baseline. Mean reduction in probing pocket depth between 0 and 90th day in Group A was 1.4±0.5 mm and in Group B it was found to be 1.6±0.5 mm. Mean reduction in probing pocket depth between 180th day in Group A was 2.8±1.0 mm and in Group B it was found to be 3.2±0.6 mm.
Table 4.
Comparison of mean change in probing pocket depth between Group A and B from baseline

Table 5 shows the comparison of mean change in clinical attachment level between Group A and B from baseline. The mean gain in clinical attachment level from Day 0 to 90 in Group A was 1.3±0.5 mm and in Group B it was 1.8±0.6 mm. The mean gain in clinical attachement level from Day 0 to 180 in Group A was 2.7±1.0 mm and in Group B it was 3.2±0.9 mm. Group B showed a significant gain in the clinical attachment level as compared to Group A from baseline to 90th day and 180th day, respectively.
Table 5.
Comparison of mean change in clinical attachment level between Group A and B from baseline

DISCUSSION
In clinical trials by Soskolne[5] and Jeffcoat,[6] Periochip was used as an adjunctive to scaling and root planing in the treatment of chronic periodontitis. Both studies showed that the adjunctive use of Periochip was effective in reducing Plaque index scores, bleeding on probing, probing pocket depth, and gain in clinical attachment level when compared to scaling and root planing alone.
In the present study, supragingival plaque decreased significantly from baseline in both the groups as a result of full mouth supragingival and subgingival scaling. In Group A, the mean reduction in the Plaque index score was in accordance to studies done by Mc Nabb et al.,[7] Dahlen et al.,[8] and Ximmenez-Fyvie et al.[9] who demonstrated that repeated professional supragingival plaque control reduced both supra and sub-gingival plaque. The reduction was higher in Group B because of application of PerioCol™-CG This result was similar to the study done by Mizrak et al.[10] The plaque scores were maintained at a low level throughout the study, indicating good oral hygiene performance of all the patients and successful re-motivation and instruction in supportive periodontal care.
Both the treatment groups presented marked reduction in the percentage of bleeding sites. On comparing both these parameters between the groups, the reduction was more significant in Group B compared to Group A. The results in Group A were similar with the results of Mc Nabb et al.,[7] Dahlen et al.,[8] and Ximmenez-Fyvie et al.[9] who demonstrated that repeated professional supragingival plaque control reduced counts of both supra- and sub-gingival plaque and may have altered the inflammatory state of the periodontal tissues. In Group B, apart from supragingival scaling, the adjunctive placement of PerioCol-CG would have released chlorhexidine with drug concentration greater than 125 μg/ml into the periodontal pocket for 7-10 days. This concentration has been reported to be above the minimum inhibitory concentration for more than 99% of subgingival microorganisms. This change in microflora would have further reduced the inflammatory state of periodontal tissues, thus bringing about reduction in the bleeding on probing. The results in Group B were in accordance with the results of the study done by Killoy et al.[4]
In the present study, the mean reduction in probing pocket depth in Group A was similar to the studies done by Hill,[11] Pihlstorm,[12] and Cugini.[13] The mean reduction in probing pocket depth in Group B was similar to studies done by Soskolne,[14] Jeffcoat,[15] Azmak,[16] and Paolantonio.[17]
On comparison of the mean probing pocket depth between Group A and B, there was no statistically significant difference observed from the baseline to 90th day and baseline to 180th day. On clinical examination, Group B demonstrated a higher reduction in probing pocket depth when compared to Group A. In Group A, the probing pocket depth would have reduced because of the beneficial effects of scaling and root planing which occurred within the first 3 months followed by a period of stability aided by maintenance scaling. In Group B, the probing pocket depths reduced because of the placement of PerioCol™-CG. This indicates that there was an additional beneficial effect of chlorhexidine apart from scaling and root planing alone. The continuing efficacy of chlorhexidine was due to the fact that the periodontal pockets received a second application of chlorhexidine at 3 months with supragingival scaling while the control group received supragingival scaling only.
The mean gain in the clinical attachment level from baseline to 90th day, and baseline to 180th day was statistically significant in Group A and Group B. On comparing the mean gain in clinical attachment level between Group A and B, the gain was higher in Group B. In Group A, the improvement in clinical attachment level was due to deeper baseline probing depth in the present study. According to Kaldahl,[18] there was greater gain in clinical attachment level after scaling and root planing with PPD of >4 mm. In Group B, the sites were treated by SRP followed by treatment of periodontal pockets with PerioCol™-CG. With the additional placement of chlorhexidine chip, there was sustained exposure of chlorhexidine in pocket environment for 6-9 days which gave long-lasting effects on microbiota. This would have brought about additional gain in the clinical attachment level in Group B. These findings were similar to studies by Soskolne[14] and Jeffcoat[15] who also demonstrated gain in the clinical attachment level in test sites which received the chlorhexidine chip.
Studies done by Mizrak[10] and Azmak[16] demonstrated that after placement of Periochip there was reduction in GCF PGE2 and GCF MMP-8 levels. In this present study, the adjunctive use of chlorhexidine along with SRP resulted in a beneficial effect in both clinical and microbiological parameters which could have been due to reduction in GCF PGE2 levels and reduction in GCF MMP-8 levels. The microbiological analysis and GCF analysis after placement of the chlorhexidine chip was not performed as it was not within the scope of this study.
However, long-term studies are required with larger sample size and longer time period with microbiological analysis to evaluate the effectiveness of PerioCol™-CG.
CONCLUSION
From the results observed in this study, it can be concluded that the adjunctive use of PerioCol™-CG was safe and provided significant improvement in the Plaque index and gingival bleeding index. It was more favorable than scaling and root planing alone in the reduction of probing pocket depth and gain in the clinical attachment level.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cetin EO, Buduneli N. In vitro studies on controlled- release cellulose acetate films for local drug delivery of chlorhexidine, indomethacin and meloxicam. J Clin Periodontol. 2004;31:1117–21. doi: 10.1111/j.1600-051X.2004.00620.x. [DOI] [PubMed] [Google Scholar]
- 2.Langer R, Peppas N. Present and future applications of biomaterials in controlled drug delivery systems. Biomaterials. 1981;2:201–4. doi: 10.1016/0142-9612(81)90059-4. [DOI] [PubMed] [Google Scholar]
- 3.Langer R. New methods of drug delivery. Science. 1990;249:1527–33. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 4.Killoy WJ. The use of locally delivered chlorhexidine in the treatment of periodontitis. Clinical results. J Clin Periodontol. 1998;25:953–8. doi: 10.1111/j.1600-051x.1998.tb02397.x. [DOI] [PubMed] [Google Scholar]
- 5.Soskolne WA, Heasman PA, Stabholz A. Sustained local delivery of chlorhexidine in the treatment of periodontitis: A multi-center study. J Periodontol. 1997;68:32–8. doi: 10.1902/jop.1997.68.1.32. [DOI] [PubMed] [Google Scholar]
- 6.Jeffcoat MK, Bray Kimberly S, Ciancio Sebastian G. Adjunctive Use of a Subgingival Controlled-Release Chlorhexidine Chip Reduces Probing Depth and Improves Attachment Level Compared With Scaling and Root Planing Alone. J Periodontol. 1998;69:989–97. doi: 10.1902/jop.1998.69.9.989. [DOI] [PubMed] [Google Scholar]
- 7.McNaabb H, Monbelli A, Lang NP. Supragingival cleaning 3 times a week. The microbial effects in moderately deep pockets. J Clin Periodontol. 1992;19:348–56. doi: 10.1111/j.1600-051x.1992.tb00658.x. [DOI] [PubMed] [Google Scholar]
- 8.Dahlen G, Lindhe J, Sato K, Hanamura Y. The effects of supragingival plaque control on the subgingival microbiota in subjects with periodontal disease. J Clin Periodontol. 1992;19:802–9. doi: 10.1111/j.1600-051x.1992.tb02174.x. [DOI] [PubMed] [Google Scholar]
- 9.Ximmenez- Fyvie LA, Haffajee A, Thompson M. The effect of repeated professional supragingival plaque removal on the composition of supra and subgingival microbiota. J Clin Periodontol. 2000;27:637–47. doi: 10.1034/j.1600-051x.2000.027009637.x. [DOI] [PubMed] [Google Scholar]
- 10.Mizrak T, Güncü GN, Caglayan F, Balci TA, Aktar GS. Effect of a controlled-release chlorhexidine chip on clinical and microbiological parameters and prostaglandin E2 levels in gingival crevicular fluid. J Periodontol. 2006;77:437–43. doi: 10.1902/jop.2006.050105. [DOI] [PubMed] [Google Scholar]
- 11.Hill RW, Ramfjord SP, Morrinson EC. Four types of periodontal treatment compared over two years. J Periodontol. 1981;52:655–62. doi: 10.1902/jop.1981.52.11.655. [DOI] [PubMed] [Google Scholar]
- 12.Philstorm BL, Mc Hugh RB, Oliphant TH. Comparision of surgical and non surgical treatment of periodontal disease. A review of current studies and additional results after six and one half year. J Clin Periodontol. 1983;10:524–41. doi: 10.1111/j.1600-051x.1983.tb02182.x. [DOI] [PubMed] [Google Scholar]
- 13.Cugini MA, Haffajee A D, Smith C, Kent RL, Jr, Socransky S. The effects of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12 month results. J Clin Periodontol. 2000;27:30–6. doi: 10.1034/j.1600-051x.2000.027001030.x. [DOI] [PubMed] [Google Scholar]
- 14.Soskolne WA, Stabholz A. An in vivo study of the periochip in the gingival crevicular fluid, plasma and urine. J Clin Periodontol. 1998;25:1017–21. doi: 10.1111/j.1600-051x.1998.tb02407.x. [DOI] [PubMed] [Google Scholar]
- 15.Jeffcoat MK, Palcanis KG, Weatherford TW, Reese M, Geurs NC, Flashner M. Use of a biodegradable chlorhexidine chip in the treatment of adult periodontitis: clinical and radiographic findings. J Periodontol. 2000;71:256–62. doi: 10.1902/jop.2000.71.2.256. [DOI] [PubMed] [Google Scholar]
- 16.Azmak N, Atilla G, Luoto H, Sorsa T. The effect of subgingival controlled-release delivery of chlorhexidine chip on clinical parameters and matrix metalloproteinase-8 levels in gingival crevicular fluid. J Periodontol. 2002;73:608–15. doi: 10.1902/jop.2002.73.6.608. [DOI] [PubMed] [Google Scholar]
- 17.Paolantonio M, D’Angelo M, Grassi RF. Clinical and microbiologic effects of subgingival controlled-release delivery of chlorhexidine chip in the treatment of periodontitis: A multicenter study. J Periodontol. 2008;79:271–82. doi: 10.1902/jop.2008.070308. [DOI] [PubMed] [Google Scholar]
- 18.Kaldahl WB, Kenneth LK, Patil KD. Evaluation of four modalities of Periodontal therapy. J Periodontol. 1988;59:783–93. doi: 10.1902/jop.1988.59.12.783. [DOI] [PubMed] [Google Scholar]


