Abstract
Background:
The various treatment modalities available to treat furcation involvement either maintain the existing furcation or increases access to furcation or leads to elimination of furcation (root resection, bicuspidization etc). Newer treatment modalities include regenerative procedures like placement of bone graft and organic or synthetic membranes. In this study we have evaluated the use of a new xenograft based tissue engineered bone material which provides both the inorganic and organic component; individually and in conjunction with a synthetic bioresorbable material.
Materials and Methods:
6 patients with 18 mandibular grade 2 furcations were selected after the completion of initial phase in all the patients. Selected sites were divided into control and experimental groups randomly and were treated by split mouth design. The control sites were treated with flap debridement and placement of ABM graft, whereas the experimental site received flap debridement, ABM graft and a synthetic bioresorbable membrane.
Results:
All the parameters recorded showed significant reduction from baseline to 9 months in both the experimental and control group. When compared in between the control and experimental group, all the parameters showed marginally better results in the control group, although none of them were clinically significant.
Conclusion:
The results of this study suggest that the use of ABM along with a bioresorbable membrane and without membrane is both beneficial for the treatment of grade 2 furcation. On the cost benefit basis, the bone graft alone seems to be a better choice for regenerative treatment of furcation involvement.
Keywords: Anorganic bovine mineral, bone graft, furcation stent, grade II furcation, xenograft
INTRODUCTION
An ultimate goal of periodontal therapy is predictable regeneration of a functional attachment apparatus destroyed as a result of periodontitis. Resective and regenerative are the two approaches which can be used to eliminate the anatomic defect caused as a result of periodontitis. The regenerative approach seeks to eliminate periodontal defects by creating new bone and periodontal ligament and coronally displacing gingival attachment and margin. Invasion of bifurcation and/or trifurcation of multirooted teeth are the most serious complications of periodontitis, resulting in early and frequent loss of molars than any other teeth type. Various treatment modalities involve either maintaining existing furcation (scaling and root planing) or increasing access to furcation (gingivectomy/ apically positioned flap, odontoplasty, osteoplasty/ostectomy), or it leads to the elimination of furcation (root amputation/tooth resection, bicuspidization). The newer aspect involves the utilization of regenerative procedures for the treatment of furcation defects which includes placement of bone grafts or bone substrate implants, use of certain cements in grade II and III furcation, and the use of organic or synthetic barrier membranes based on the principles of guided tissue regeneration (GTR). Xenografts have proven efficacy for bone fill, attachment gain, and pocket reduction.[1–3] PepGen P-15 (Ceramed Dental LLC) is a new tissue-engineered bone material and provides both the inorganic and organic component. Anorganic bovine material (ABM) provides the same anatomic scaffold, composition, and structure as autogenous bone necessary for cellular invasion. It is of type I collagen, a 15-amino acid sequence. It provides a tissue-engineered hospitable biomimetic habitat for cells like osteoblasts and fibroblasts and serves as a bone-like substitute for autogenous bone grafts.
PepGen P-15 demonstrates an increased expression of growth factors[4] and provides an appropriate environment for bone. It has also shown a significantly higher reduction in probing depth and gain in attachment level as compared to another xenograft, BioOss.[5] The use of GTR to treat human class II furcation defects was first reported by Gottlow et al.[6] Atrisorb barrier is a synthetic liquid polymer of Lactic acid poly (DL lactide) (PLA) dissolved N-methyl-2-pyrrolidone (NMP). It is a nontoxic, bioabsorbable, and efficacious material. A pilot study[7] and a multicenter study[8] with Atrisorb used in class II furcation in humans have indicated favorable regenerative outcomes.
Hence, the present study has been undertaken to evaluate the efficacy of peptide-enhanced bone graft (PepGen P-15) and resorbable GTR (Atrisorb) barrier in the treatment of human mandibular class II furcation both clinically and radiographically.
MATERIALS AND METHODS
The review committee of the Rajiv Gandhi University of Health Sciences approved the protocol for human subjects. The study group included patients with at least two or more grade II furcation involvement. A total of six patients (1 female and 5 male) in the age group 28-60 years were selected. A total of 18 sites were divided into control flap (debridement and placement of PepGen P-15 bone graft material) and experimental site (flap debridement, placement of PepGen P-15 bone graft material along with bioresorbable GTR barrier Atrisorb) based on split mouth design.
Inclusion criteria considered were:
Patients with no medical problems that would contraindicate routine periodontal surgery
Patients with evidence of advanced periodontitis having clinically at least two or more mandibular grade II furcation involvement present bilaterally
Nonsmokers
Patients who had not taken any type of periodontal therapy six months prior to initial examination.
The following exclusion criteria were considered:
Patients who had taken antibiotics one month prior to the study
Patients showing unacceptable oral hygiene during presurgical therapy
Patients allergic to tetracycline and/or chlorhexidine
Pregnant or lactating patients
Patients showing insufficient motivation
After informed consent was taken, the patients were included in the study and the following clinical parameters were recorded at baseline, three months, six months, and nine months postoperatively:
Vertical measurement for determination of probing depth and gingival margin (GM) position[1,5,9]
Fixed reference point (FRP) to the base of the pocket (BOP) [Figures 1 and 2][1,5,9]
FRP-GM
Direct probing into the furcation defect without stent[1,5,9]
Reference point (RP) to depth of furcation (DOF) with stent [Figures 5 and 6][1,5,9]
Figure 1.
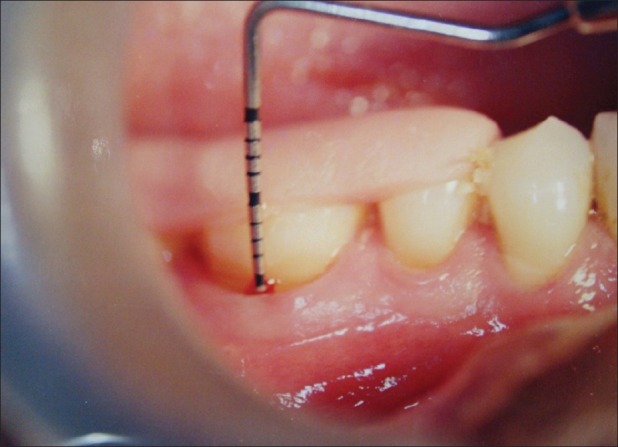
Fixed reference point to base of the pocket (baseline)
Figure 2.
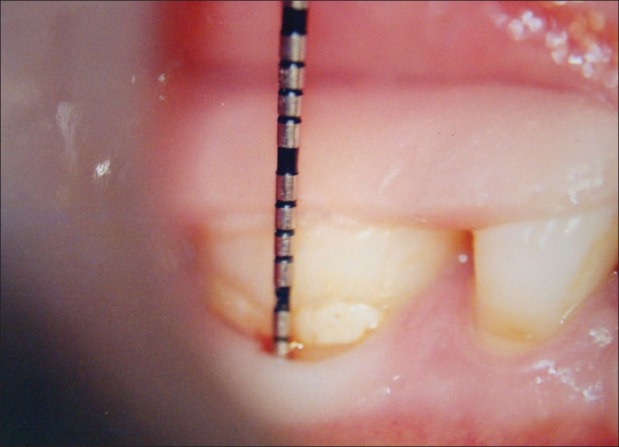
Fixed reference point to base of the pocket (9 months)
Figure 3.
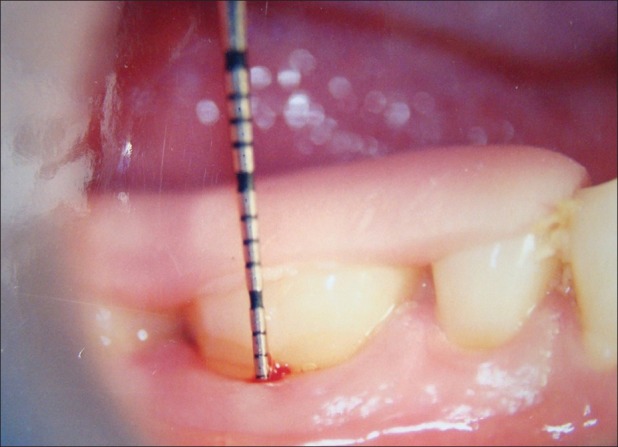
Fixed reference point to cemento-enamel junction (baseline)
Figure 4.
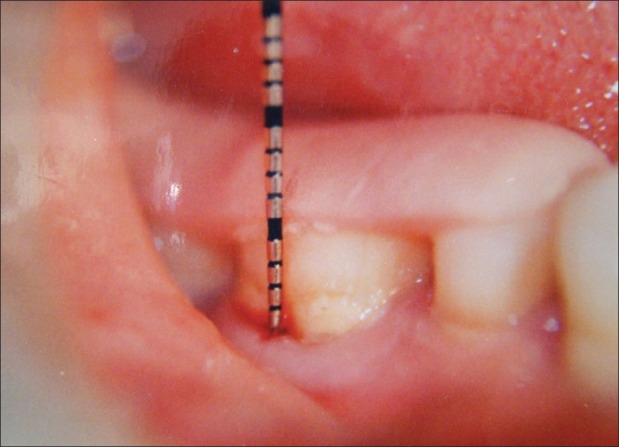
Fixed reference point to cemento-enamel junction (9 months)
Figure 5.
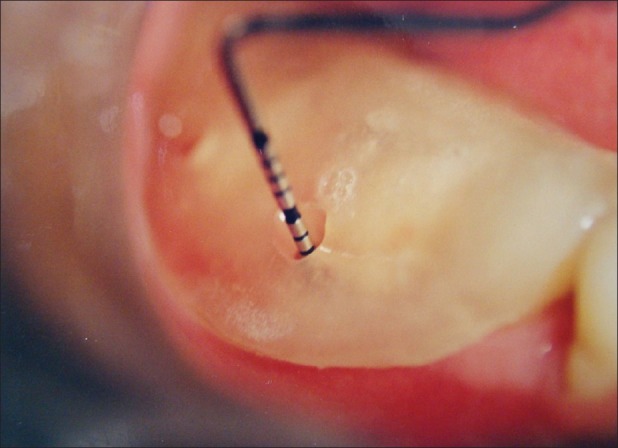
Reference point to depth of furcation (baseline)
Figure 6.
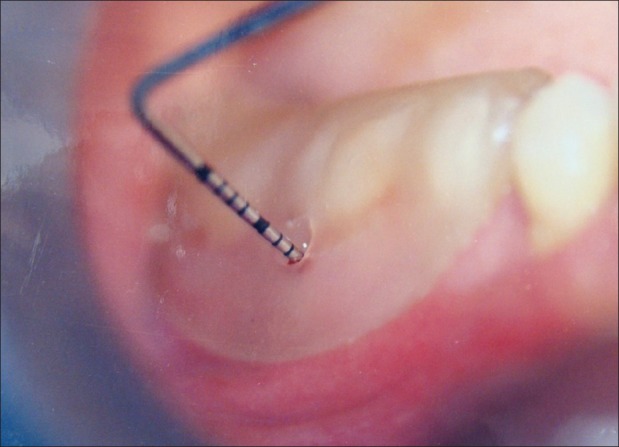
Fixed reference point to depth of furcation (9 months)
All measurements were standardized using customized acrylic stents with grooves, which were prepared on study models of the patients. Two different types of stents were made, one for vertical and the other for horizontal. Recording were made using Hu-Friedy PCP-UNC 15 probe [Figures 7 and 8].
Figure 7.
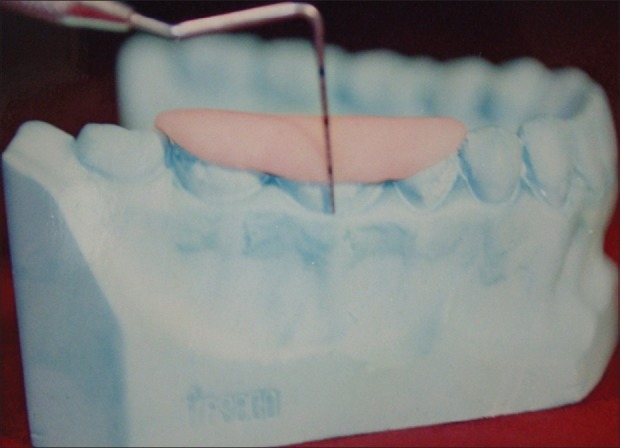
Custom-made stent for vertical probing
Figure 8.
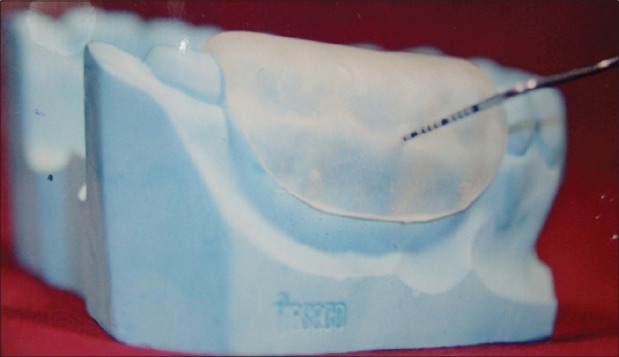
Custom-made stent for horizontal probing
The following calculation was made from clinical measurements recorded:
Radiographs were taken of each control and experimental site at baseline, and nine months postoperatively using long cone/paralleling technique. A single examiner recorded all the measurements.
Bone graft and GTR membrane used in the study
Atrisorb (a synthetic liquid polymer) was used as a resorbable barrier membrane, manufactured by Atrix Laboratories, Fort Collins, Colorado, United States of America.
PepGen P-15 [an anorganic bovine mineral (ABM) with an enhanced peptide P-15] was used as a bone graft. It is manufactured by CeraMed Dental, LLC, Lakewood, Colorado, United States of America.
Atrisorb
It is a synthetic, bioabsorbable liquid polymer formulation developed for GTR procedures. It consists of a polymer of lactic acid, poly (DL lactide) PLA dissolved in N-methyl-2-pyrrolidone. Atrisorb is prepared as a solution that coagulates or sets to a firm consistency on contact with water or other aqueous solutions. A series of research reports suggest that Atrisorb is an acceptable barrier material. Bogle et al.[10] used Atrisorb barrier to repair class II furcation defects in beagle dogs and reported regeneration of new bone, cementum, and periodontal ligament in 71% defects. It was also reported that sites treated with Atrisorb had a reduced incidence of postoperative suppuration and abscess formation. This product or polymer is supplied as a flowable formulation. It (the polymer) accounts for 37% of the formulation and the solvent for 63% by weight.
It is available in two forms:
A barrier membrane system: This is designed to partially set a barrier at the chairside that would be trimmed to the dimensions of the periodontal defect
A direct flow: In the second direct flow system, Atrisorb, which is presented in a syringe form of viscous gel, is directly applied over the treated lesion without preforming the barrier at the chairside before placement.
One additional benefit of this material is that it demonstrates an intrinsic antimicrobial property, which may help in diminishing the risk of site infection.
PepGen P-15
PepGen is an anorganic bovine-derived HA bone matrix (ABM) used for this study, and is a natural, microporous, xenogeny hydroxyapatite. The ABM/P-15 combination bone replacement graft material (using only 200 ng of P-15 with 1 g of ABM) has been shown in vitro to enhance the attachment of cells, and to promote attachment of periodontal ligament fibroblasts. The peptide component of the test material, P-15, is a synthetic clone of the 15-amino acid sequences of type I collagen. It is a highly conserved linear polypeptide with a 15-amino acid sequence identical to the sequence contained in residues 766-780 of the αl chain of type I collagen; therefore, P15 is essentially a very small synthetic fragment of the αl chain of type I collagen. The type 1 molecule is made up of 3α chains (2αl, l α2 chains). Each of the α chains consists of approximately 1000 amino acid residues; P-15 represents approximately 1.5% (15 / 1000 amino acid residues) of the size of the αl chain of 0.5% (15 / 3000 amino acid residues) of a type I collagen molecule.[11]
Presurgical procedure
All the patients were given detailed instructions in self-monitored plaque control measures and were subjected to phase I therapy. Selective grinding in cases with traumatic occlusion was considered. Two weeks after phase I, the patients were subjected to a surgical procedure.
Surgical procedure
Upon completion of initial therapy, the furcation defects were randomly assigned as either control or experimental sites. The operating site was anesthetized with 2% xylocaine with 1:80,000 adrenaline using inferior alveolar nerve block and lingual nerve block. A full-thickness mucoperiosteal flap was raised, taking care to preserve interdental papilla. After thorough surgical debridement, the surgical site was irrigated copiously.
In the control site, PepGen P-15 was loosely packed taking care to avoid overfilling [Figure 9]. On the experimental site, PepGen P-15 was placed followed by the barrier membrane in such a way so as to overlap the walls of defect by at least 2-3 mm on all sides to allow complete bone contact and to prevent connective tissue surface invasion below the material [Figure 10]. The canula tip was held 1-2 mm away from the graft and the applicator was squeezed. The expressed polymer was uniformly spread over the graft. The polymer was then mixed with sterile water or saliva for about 10-20 seconds to set the barrier. After the barrier was set, it was not touched and the flaps were gently approximated and sutured. The surgical site was protected with a non-eugenol dressing (Voco pac). The patients were prescribed systemic doxycycline Hcl 200 mg for the first day followed by 100 mg/day for another four days along with nimesulide 100 mg tablets twice daily for three days. Postoperative instructions were given to all. After 10 days, dressings and sutures were removed and the surgical sites were irrigated with normal saline. Inquiry regarding postsurgical problems was made and the area was checked for any membrane exposure. Recall appointments were made at one month, three months, six months, and nine months. At each visit, clinical parameters were recorded and radiographs were taken. Oral hygiene instructions were reinforced. The recorded data of the study were subjected to statistical analysis.
Figure 9.
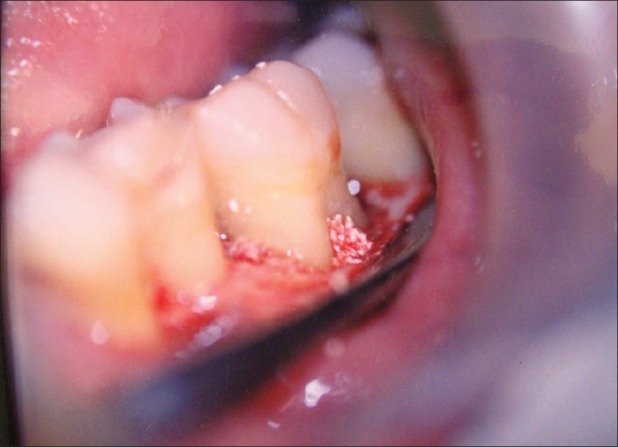
Filling the defect with bone graft
Figure 10.
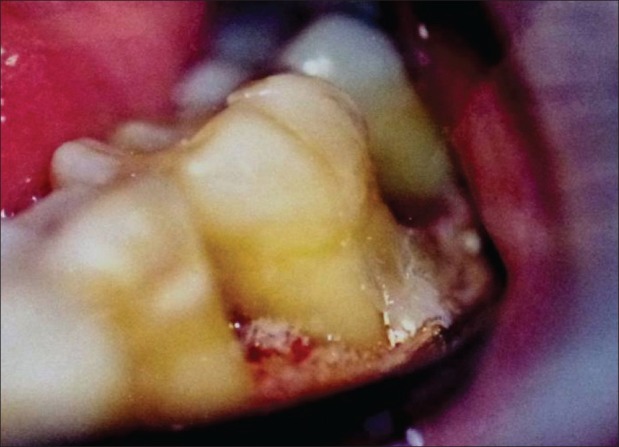
GTR application
Statistical analysis
Data are expressed as mean+SD (SD: standard deviation) and percentage of post-treatment changes. Paired t-test was used for each group to assess the significance of treatment changes. Intergroup comparisons of treatment changes were analyzed by Mann-Whitney test, where P<0.05 was considered as a statistically significant difference.
RESULTS
The study results are expressed in Tables 1–4.
Table 1.
Comparison of mean values of horizontal furcation depth of experimental and control groups at baseline and nine months post surgery measured without stent
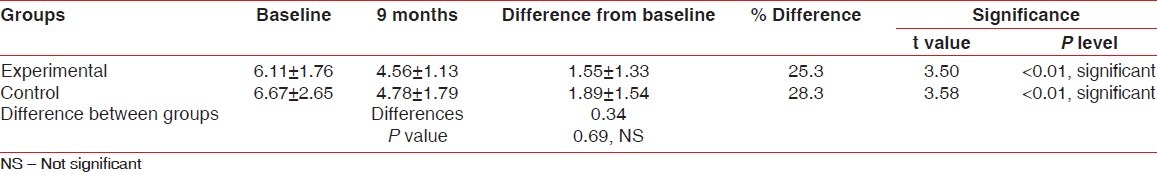
Table 4.
Comparison of mean values±SD for soft tissue measurements between experimental group & control group at baseline & 9 months post surgery

Horizontal probing depth of the furcation defects without the use of a stent
In the experimental group, mean reduction in horizontal probing depth of furcation defects from baseline to nine months was 1.55±1.33 which was statistically significant [Tables 1 and 3].
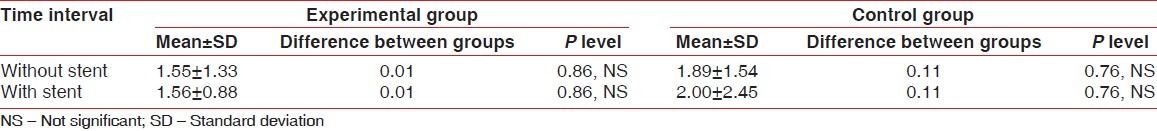
Table 3.
Comparison of furcation depth changes (baseline to 9 months) of experimental and control groups measured with and without stent
In the control group, mean reduction in horizontal probing depth of furcation defects from baseline to nine months was 1.89±1.54 which was statistically significant.
On comparison of the two groups at nine months post surgery, the mean reduction in horizontal probing depth of the furcation defects without the use of a stent was statistically not significant.
Horizontal probing depth of the furcation defects using a newly designed stent
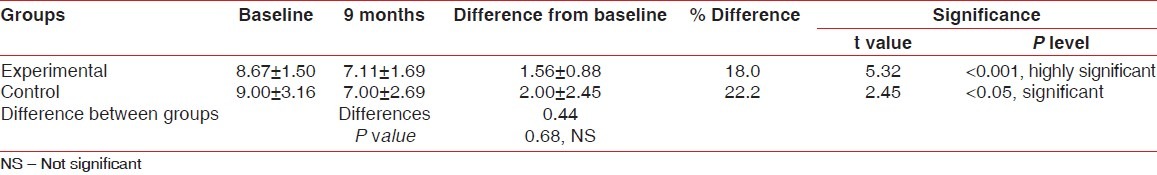
In the experimental group, mean reduction in horizontal probing depth of furcation defects using stent from baseline to nine months was 1.56±0.88 which was statistically highly significant (P<0.001). In the control group, mean reduction in horizontal probing depth of furcation defects using stent from baseline to nine months was 2.00±2.45 which was statistically significant (P<0.05) [Tables 2 and 3].
Table 2.
Comparison of mean values of horizontal furcation depth of experimental and control groups at baseline and nine months post surgery measured with stent
Changes in the pocket depth
In the experimental group, mean pocket depth reduction from baseline to nine months was 1.33±1.00 which was statistically significant (P<0.01) In the control group, mean pocket depth reduction from baseline to nine months was 2.00±1.32 which was statistically significant (P<0.01) On comparison of the two groups at nine months post surgery, the mean reduction in pocket depth was statistically not significant [Table 4].
Changes in the clinical attachment level
In the experimental group, mean clinical attachment gain from baseline to nine months was 1.45±1.13 which was statistically significant (P<0.01) [Table 4].
In the control group, mean clinical attachment gain from baseline to nine months was 2.00±1.22 which was statistically highly significant (P<0.001).
Mean gain in clinical attachment level was statistically highly significant (P<0.001) for control sites nine months post surgery and also statistically significant (P<0.01) for the experimental sites nine months post surgery.
Changes in the GM position
In the experimental group, mean change in the GM position from baseline to nine months was -0.22±0.67 which was statistically not significant.
In the control group, mean change in the GM position from baseline to nine months was 0.0±0.71 which was statistically not significant.
On comparison of the groups at nine months post surgery, mean change in the GM position and recession were both statistically not significant [Table 4].
Radiographically, in this study, a trial was made to compare Intraoral periapical (IOPA) radiographs for furcation assessment from baseline to nine months postoperatively. A total of 30 radiographs (15 baseline and 15 nine months postoperatively) were obtained which were categorized under two groups.
Group 1: Radiographs where furcation involvement was evident and changes in the furcation area were detected post surgery [Figures 11 and 12].
Figure 11.
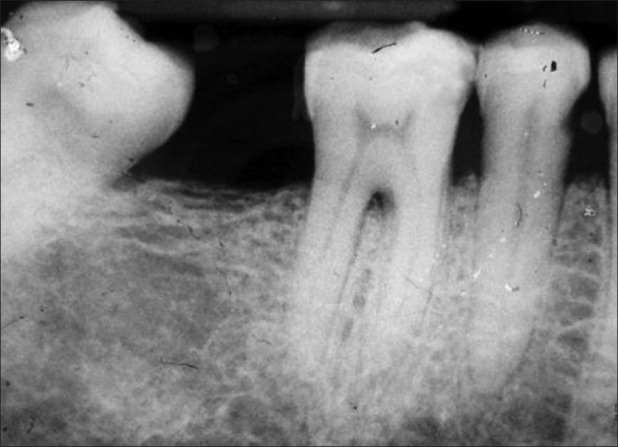
Group 1 – Baseline
Figure 12.
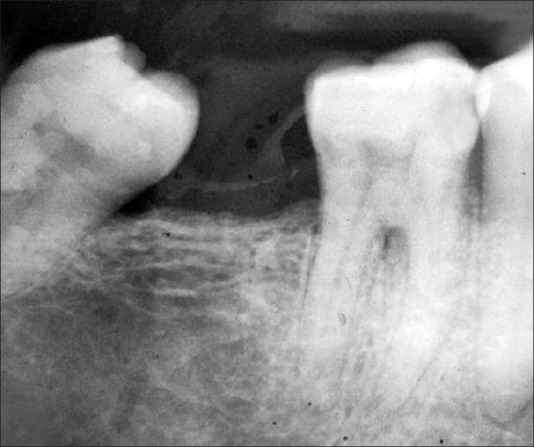
Group 1 – Nine months post surgery
Group 2: Radiographs where furcation was evident but changes in the furcation area were not detected post surgery [Figures 13 and 14].
Figure 13.
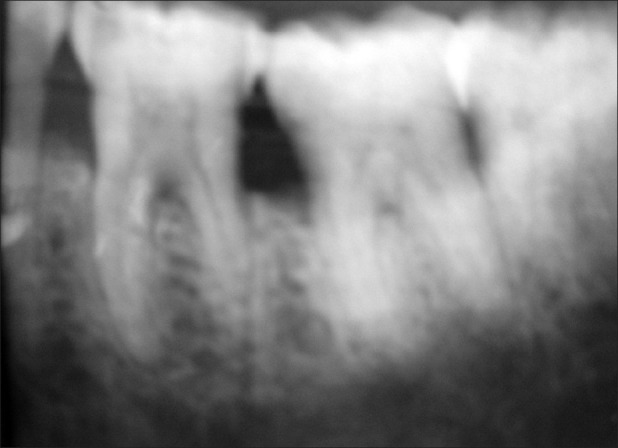
Group 2 – Baseline
Figure 14.
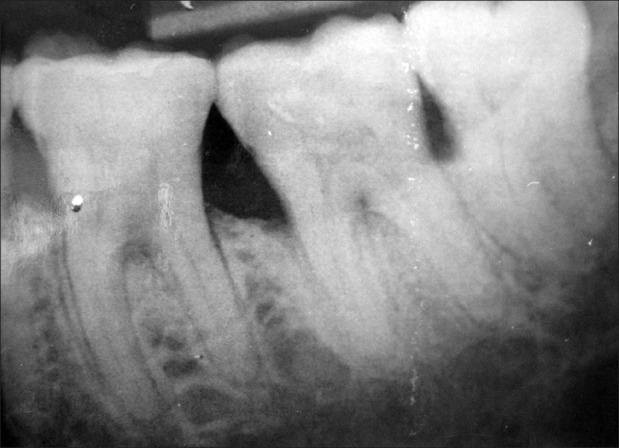
Group 2 – Nine months post surgery
Out of the total 30 radiographs taken, 75% fell into group 2 and as the majority of the radiographs fell into group 2, it was not possible to interpret the bony changes which had occurred after nine months of treatment.
DISCUSSION
In this study, positive control (control site) was grafted with PepGen P-15 alone and the experimental site was grafted with PepGen P-15 over which the Atrisorb barrier was applied. A nongraft control (negative control) was excluded as numerous studies have demonstrated a statistically significant result favoring bone grafts when compared to a nongrafted site as suggested by Bower,[12] Mellonig,[13] Richardson et al.,[14] and Camargo et al.[15] All the patients showed good compliance and the healing period was uneventful for both the experimental and control groups. Signs of inflammation or any other complication were not evident in any of the patients. During all the recall visits of the patients, membrane exposure was not evident at any interval.
In this study, only clinical parameters were compared and an attempt was made to compare the results radiographically too. The results of the study are discussed as follows:
In this study, mean reduction in horizontal probing depth of the furcation defect without a stent was statistically significant (P<0.01) for the control site nine months postoperatively similar to the findings of Yukna et al.,[16] and the mean reduction in the experimental site nine months postoperatively was also statistically significant (P<0.01) similar to the findings of Rosen and Reynolds.[17] On comparison of the control and experimental groups at nine months post surgery, the mean reduction in horizontal probing depth of the furcation defects without the use of a stent was statistically not significant.
Stent used for measuring the horizontal depth of furcation
In this study, a new stent was designed to measure the horizontal furcation depth clinically without re-entry in a reliable manner at a given interval of time. A variety of stents have been used for vertical measurement including pocket depth, clinical attachment level, and GM position.[18,19] Moreover, furcation probing with the help of stents using different methods has been done either probing during re-entry or at the time of surgical exposure.[20,21] Dr. Devraj and Dr. Vandana (2003)[5] did a comparative evaluation of BioOss and PepGen P-15 using a similar customized stent for horizontal furcation probing and found out a highly significant reduction in probing depth in the PepGen P-15 group as compared to BioOss.
Horizontal probing depth of the furcation defects using a newly designed stent
In the experimental group, mean reduction in horizontal probing depth of furcation defects using a stent from baseline to 9 months was 1.56±0.88 which was statistically highly significant (P<0.001). In the control group, mean reduction in horizontal probing depth of furcation defects using stents from baseline to nine months was 2.00±2.45 which was statistically significant (P<0.05) [Tables 2 and 3].
On comparison of the two groups at nine months post surgery, mean reduction in horizontal probing depths of the furcation defects using a stent was statistically not significant. The results suggest that the newly designed stent for horizontal measurements of the furcation depth would be a useful device clinically for standardization and reproducibility of probing measurements of the furcation defect.
On comparison of the two groups at nine months post surgery, the mean reduction in pocket depth was statistically not significant. Mean gain in clinical attachment level was statistically highly significant (P<0.001) for control sites nine months post surgery and also statistically significant (P<0.01) for the experimental sites nine months post surgery. On comparison of the groups at nine months post surgery, mean change in GM position and recession were both statistically not significant. The changes in the vertical pocket depth, clinical attachment level, and GM position that occurred in both the experimental and control sites are due to the open debridement performed at the site of the surgery.
Radiographically, in this study, a trial was made to compare IOPA radiographs for the furcation assessment from baseline to nine months postoperatively. A total of 30 radiographs (15 baseline and 15 nine months postoperatively) were obtained which were categorized under two groups.
Group 1: Radiographs where furcation involvement was evident and the changes in the furcation area were detected post surgery [Figures 11 and 12].
Group 2: Radiographs where furcation was evident but changes in the furcation area were not detected post surgery [Figures 13 and 14].
Of the total 30 radiographs taken, 75% fell into group 2 and as majority of the radiographs fell into group 2, it was not possible to interpret the bony changes which had occurred after nine months of treatment.
In this study, we decided not to use a Naber's probe because the calibrations on a UNC-15 probe are in millimeters, whereas the Naber's probe has four calibrations of 3 mm each. For our study, the changes had to be recorded in millimeters so that even a change of a single millimeter could be recorded either as it is or by rounding off to the nearest millimeter, which would not have been feasible in a Naber's probe. Moreover, it was realized that the readings of the horizontal measurement from the edge of the stent may not represent accurate millimeters due to the geometry involved when using a double-plane curved probe such as the Naber's probe. Therefore a curved Naber's probe is more useful as a diagnostic tool rather than a prognostic one, which is why it was not considered for this study.[9]
Pubmed search did not reveal any studies to compare results obtained from furcation defect treatment.
CONCLUSION
It can be concluded from this study that the reduction in furcation defect using PepGen P-15 alone and a combination of PepGen P-15 and Atrisorb were equivocal. It can be suggested that the combined use of GTR barrier and bone graft did not prove beneficial for the clinical outcome of the mandibular grade II furcation defect treatment. Hence, the cost effective and economical treatment of choice for grade II furcation defects may be bone graft alone.
Further long-term studies using larger sample sizes should be directed toward comparison of bone grafts and the combination of GTR and bone graft, and histologic evaluation would provide better insight into periodontal healing.
ACKNOWLEDGMENT
The authors wish to acknowledge and thank Dr. K.T Chandrashekhar, MDS, Professor, Department of Periodontics, College of Dental Sciences, Davangere and Dr. Rajat Mangi, Lecturer, Department of Periodontics, Sudha Rustagi College of Dental Sciences and Research, Faridabad, for their help in editing of the manuscript and valuable suggestions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Vandana KL, Khatri M. Clinical and radiographic evaluation of human mandibular grade II furcation defects treated with bovine derived xenograft(Bio-Oss) and a bioresorbable membrane. J Indian Soc Periodontal. 2003;6:125–33. doi: 10.4103/0972-124X.106917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson CR, Mellonig JT, Brunsvold MA, McDonell HT, Cochran DL. Clinical evaluation of Bio-Oss: A bovine derived xenograft for the treatment of Periodontal osseous defect in Humans. J Clin Periodontol. 1999;26:421–8. doi: 10.1034/j.1600-051x.1999.260702.x. [DOI] [PubMed] [Google Scholar]
- 3.Yukna RA, Callan DP, Krauser JT, Evans GH, Aichelmann-Reidy ME, Moore K, et al. Multicentre clinical evaluation of combination anorganic bovine derived Hydroxyapatite matrix(ABM)/Cell binding peptide(P-15) as a bone replacement graft material in human periodontal osseous defects – 6 month results. J Periodontol. 1998;69:655–63. doi: 10.1902/jop.1998.69.6.655. [DOI] [PubMed] [Google Scholar]
- 4.Bhatnagar RS, Qian JJ, Gough CA. The role in cell binding of a bend within the triple helical region in collagen 1 (1) chain; structural and Biological evidence for conformational tautomerism on fibre surface. J Biomol Struct Dyn. 1997;14:547–59. doi: 10.1080/07391102.1997.10508155. [DOI] [PubMed] [Google Scholar]
- 5.Devraj CG, Vandana KL. Comparative Evaluation of PepGen P-15 and Bio-Oss in the Treatment of Human Periodontal osseous Defects - Clinically and Radiologically. J Indian Dent Assoc. 2003;74:527–30. [Google Scholar]
- 6.Gottlow J, Nyman S, Lindhe J, Karring T, Wennström J. New attachment formation in human periodontium by GTR. Case Reports. J Clin Periodontol. 1986;13:604–16. doi: 10.1111/j.1600-051x.1986.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 7.Polson Am, Southard GL, Dunn RL, Polson AP, Billen JR, Laster LL. Initial study of GTR in class II furcation defects after use of biodegradable barriers. Int J Periodontol Rest Dent. 1994;15:43–55. [PubMed] [Google Scholar]
- 8.Polson AM, Southard GL, Dunn RL, Polson AP, Yewey GL, Swanbom DD. Periodontal Healing after GTR with Atrisorb barrier in beagle dogs. Int J Periodontol Rest Dent. 1995;15:575–89. [PubMed] [Google Scholar]
- 9.Laxman VK, Khatri M, Devaraj CG, Reddy K, Reddy R. Evaluation of a new furcation stent as a fixed reference point for class II furcation measurements. J Contemp Dent Pract. 2009;10:18–25. [PubMed] [Google Scholar]
- 10.Bogle G, Garrett S, Stoller NH, Swanbom DD, Fulfs JC, Rodgers PW, et al. Periodontal regeneration in naturally occurring class II furcation defects in beagle dogs after GTR with bioabsorbable barrier. J Periodontol. 1997;68:536–44. doi: 10.1902/jop.1997.68.6.536. [DOI] [PubMed] [Google Scholar]
- 11.Qian JJ, Bhatnagar RS, Wedrychowska A. Induction of collagenase by a synthetic Analogue of Collagen. J Dent Res. 1994;73(256):1318. [Google Scholar]
- 12.Bower RC. Furcation morphology relative to periodontal treatment furcation entrance architecture. J. Periodontol. 1989;50:23–7. doi: 10.1902/jop.1979.50.1.23. [DOI] [PubMed] [Google Scholar]
- 13.Mellonig JT. Autogenous and Allogenic grafts in periodontal therapy. Crit Rev Oral Biol. 1992;3:333–52. doi: 10.1177/10454411920030040201. [DOI] [PubMed] [Google Scholar]
- 14.Richardson CR, Mellonig JT, Brunsvold MA, McDonnell HT, Cochran DL. Clinical evaluation of Bio-Oss a bovine derived xenograft for the treatment of periodontal osseous defects in humans. J Clin Periodontol. 1999;26:421–8. doi: 10.1034/j.1600-051x.1999.260702.x. [DOI] [PubMed] [Google Scholar]
- 15.Camargo, Wolinsky LE, Wagner WR, Burgess AV, Paluk SF. A feasibility and dose –response study of the use of bovine derived bone morphogenic proteins in the treatment of mandibular class II furcation defects in humans. J Periodontol. 2000;71:1804–5. [Google Scholar]
- 16.Yukna RA. Histological regeneration in humans with pepGen P-15. J Clin Periodontol. 2000;27:195. [Google Scholar]
- 17.Rosen PS, Reynolds MA. Polymer assisted regenerative therapy; Case report of 22 consecutively treated periodontal defects with a novel combined surgical approach. J Periodontol. 1999;70:554–61. doi: 10.1902/jop.1999.70.5.554. [DOI] [PubMed] [Google Scholar]
- 18.Chung KM, Salkin LM, Stein MD, Freedman AL. Clinical evaluation of a biodegradable collagen membrane in GTR. J Periodontol. 1990;61:732–6. doi: 10.1902/jop.1990.61.12.732. [DOI] [PubMed] [Google Scholar]
- 19.Moriarty JD, Scheitler LE, Hutchens LH, Jr, Delong ER. Inter examiner reproducibility of probing pocket depth in molar furcation sites. J Clin Periodontol. 1988;15:68–72. doi: 10.1111/j.1600-051x.1988.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 20.Zappa U, Grosso L, Simona C, Graf H, Case D. Clinical furcation diagnosis and Interradicular bone defects. J Periodontol. 1993;64:219–27. doi: 10.1902/jop.1993.64.3.219. [DOI] [PubMed] [Google Scholar]
- 21.Pontoriero R, Lindhe J, Nyman S, Karring T, Rosenberg E, Sanavi F. GTR in degree II furcation – involved mandibular molars. A clinical study. Clin. Periodontol. 1988;15:247–54. doi: 10.1111/j.1600-051x.1988.tb01578.x. [DOI] [PubMed] [Google Scholar]


