Abstract
Bullous pemphigoid (BP) is an autoimmune blistering skin disease induced by pathogenic autoantibodies against a type II transmembrane protein (BP180, collagen type XVII, or BPAG2). In animal models, BP180 autoantibody-antigen interaction appears insufficient to develop blisters, but involvement of complement and neutrophils is required. However, cultured keratinocytes treated with BP-IgG exhibit a reduction in the adhesive strength and a loss of expression of BP180, suggesting that the autoantibodies directly affect epidermal cell–extracellular matrix integrity. In this study, we explored the consequences of two distinct epithelial cells treated with BP-IgG, particularly the fate of BP180. First, we followed the distribution of green fluorescent protein–tagged BP180 in an epithelial cell line, 804G, and normal human epidermal keratinocytes after autoantibody clustering. After BP-IgG treatment, the adhesive strength of the cells to their substrate was decreased, and BP180 was internalized in both cell types, together with the early endosomal antigen-1. By using various endocytosis inhibitors and a fluid-uptake assay, we demonstrated that BP-IgG–induced BP180 internalization is mediated via a macropinocytic pathway. Moreover, a macropinocytosis inhibitor rescued a BP-IgG–induced reduction in the adhesive strength of the cells from their substrate. The results of this study suggest that BP180 internalization induced by BP-IgG plays an important role in the initiation of disease pathogenesis.
Bullous pemphigoid (BP) is one of the most common autoimmune blistering diseases and is characterized by tense inflammatory subepidermal bullae caused by anti-basement membrane zone autoantibodies.1,2 BP autoantigens are two major hemidesmosomal components, BP180/type XVII collagen/BPAG2 and BP230/BPAG1e.3–6
Whether anti-BP230 autoantibodies directly contribute to BP pathogenesis is controversial.7,8 In contrast, several studies using experimental animal models have suggested that IgG anti-BP180 antibodies contribute to BP blister formation. Specifically, passive transfer of rabbit IgG antibodies against the murine homologue of human BP180 NC16A into healthy mice induces subepidermal blisters.9 Moreover, injection of IgG anti-human BP180 antibodies induces subepidermal blisters in BP180-knockout mice engineered to express human BP180.10
Although there is overwhelming evidence that binding of anti-BP180 autoantibodies to BP180 initiates disease, such antibody-antigen interaction appears insufficient to induce blisters in various BP animal models. Indeed, there is evidence that BP pathogenesis requires activation of complement and the presence of neutrophils and mast cells, although the role of mast cells has been questioned by a recent study.11–14 BP autoantibodies binding to hemidesmosomes are assumed not to disrupt epidermal cell-connective tissue interaction. However, contrary to this assumption, recent studies indicate that cultured keratinocytes treated with IgG from patients with BP (BP-IgG) exhibit a reduction in the adhesive strength and a loss in BP180 expression.15,16 Moreover, we have demonstrated that the velocity of keratinocytes is increased by BP-IgG treatment in vitro.17 These findings raise the question as to how BP autoantibodies influence keratinocyte adhesion and migration without involvement of various inflammatory processes. Hence, in this study, we examined the functions of BP-IgG by following the fate of green fluorescent protein (GFP)–tagged full-length BP180 (GFP-BP180) after BP-IgG treatment in live 804G epithelial cells, which are known to assemble bona fide hemidesmosomes,18 and normal human epidermal keratinocytes (NHEKs).
Materials and Methods
IgGs from Patients with BP
In accordance with Osaka City University Hospital (Osaka, Japan) and Kurume University Hospital (Fukuoka, Japan) bylaws, we obtained patient consent for experimental procedures to be performed in Osaka City University Hospital and Kurume University Hospital from each participating patient on his or her first visit to the hospital. BP-IgGs were purified from the serum samples of 20 patients with BP who were diagnosed as having BP based on the presence of typical clinical, histological, and immunopathological findings. Their enzyme-linked immunosorbent assay (ELISA) index values for BP180 NC16A autoantibodies were >100, whereas their ELISA index values for BP230 autoantibodies did not exceed 6. ELISAs were performed using commercial kits (MESACUP BP180 test and BP230 ELISA kit; MBL, Nagoya, Japan). IgGs were affinity purified from the serum samples using HiTrap Protein G HP Columns (GE Healthcare, Buckinghamshire, UK). The medical ethical committee of Osaka City University approved all studies performed in this study. The study was conducted according to the principles of the Declaration of Helsinki.
Preparation of HiLyte Fluor 647–Conjugated IgG and IgG F(ab′)2 and IgG Fab Fragments
HiLyte Fluor 647–conjugated BP-IgG and nonpathological IgG were prepared using the HiLyte Fluor 647 Labeling Kit-NH2 (Dojindo, Kumamoto, Japan), according to the manufacturer’s protocol. BP-IgG F(ab′)2 and BP-IgG Fab fragments were generated by pepsin and papain, respectively, using commercial kits (Pierce, Rockford, IL), according to the manufacturer’s protocols.
Antibodies and Immunofluorescence Probes
The rabbit anti-GFP monoclonal antibody (mAb; A11122) was obtained from Invitrogen (Carlsbad, CA). The horseradish peroxidase–conjugated goat anti-rabbit IgG polyclonal antibody (pAb) was purchased from KPL (Gaithersburg, MD). The rat mAb GoH3, a blocking antibody directed against the extracellular portion of human α6 integrin, was obtained from eBioscience (San Diego, CA). The mouse mAb 3E1 (MAB1964), a blocking antibody against human β4 integrin, was purchased from Millipore (Billerica, MA). The mouse anti-human β4 integrin mAb (555722) was purchased from BD Biosciences (San Diego, CA). The mouse anti-rat β4 integrin (ab29042) was purchased from AbCam (Cambridge, UK). The methods for the production and characterization of 5E, a human mAb against BP230, mAb-233, a mouse mAb against the extracellular domain of BP180, and J17, a rabbit pAb against the N-terminal domain of BP180, were described elsewhere.19–22 The antibodies 610457 (BD Biosciences), 610500 (BD Biosciences), and 3238 (Cell Signaling Technology Inc., Beverly, MA) recognize early endosomal antigen-1 (EEA-1), clathrin, and caveolin-1, respectively. The Alexa Fluor (AF) 488– and AF 597–labeled secondary antibodies were obtained from Invitrogen. The mouse anti-EGFR pAb (ab2430) was purchased from AbCam.
Cells and Culture Conditions
The 804G cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen), supplemented with 10% fetal bovine serum (Invitrogen) and 50 U/mL penicillin and 50 μg/mL streptomycin at 37°C, in a humidified atmosphere containing 5% CO2. NHEKs from neonate (Invitrogen) were cultured first in HuMedia-KG2 (Kurabo, Osaka, Japan). When used for experiments, NHEKs were cultured in HuMedia-KG2 containing 1.8 mmol/L CaCl2 for 12 to 24 hours.
IgG Stimulation
To examine the function of BP-IgG in 804G cells and NHEKs, BP-IgG and normal IgG were added at final concentrations of 2.0 mg/mL in the culture media. Final concentrations of GoH3 and 3E1 were 30 μg/mL in the culture media. Final concentrations of 1.47 mg/mL BP-IgG F(ab′)2 and 1.33 mg/mL BP-IgG Fab fragments in the culture media were used in certain studies.
Endocytosis Inhibitors
The 1 mmol/L N-ethylmaleimide (NEM; Sigma-Aldrich, St. Louis, MO), 0.4 mol/L sucrose (Sigma-Aldrich), 50 μmol/L nystatin (Sigma-Aldrich), 100 μmol/L genistein (Wako, Osaka, Japan), 2 μmol/L cytochalasin D (Sigma-Aldrich), and 200 μmol/L 5-(N-ethyl-N-isopropyl) amiloride (EIPA; Sigma-Aldrich) were added in the culture media 30 minutes before the experiments (except for nystatin with 1-hour treatment). The effects of these inhibitors of general, clathrin-dependent, and caveolae-dependent endocytosis were confirmed by preliminary experiments using 804G cells, which were incubated with 1 μg/mL recombinant rat EGF (R&G Systems, Inc., Minneapolis, MN) for 1 hour to trigger EGFR internalization, a typical clathrin-dependent endocytic event, or with 3 μg/mL AF 594–conjugated cholera toxin subunit B (CTB; Invitrogen) for 30 minutes to trigger internalization of CTB itself, a typical caveolae-dependent endocytosis. EGFR was visualized by immunofluorescence staining with anti-EGFR pAb, and AF 594–conjugated CTB was monitored directly by a TCS SP5 confocal microscope (Leica Microsystems, Mannheim, Germany), after treatment with the indicated inhibitors. Microscope images were exported as .tif files, and figures were generated using Adobe Photoshop CS5 (Adobe Systems, San Jose, CA).
Immunofluorescence Analyses
The cells grown on glass coverslips were fixed in 3.7% formaldehyde in PBS for 8 minutes, washed thoroughly in PBS, and permeabilized in 0.1% (v/v) Triton X-100 in PBS for 10 minutes. Primary antibodies were overlaid onto the cells, and the preparations were incubated at 37°C for 1 hour. The cells on coverslips were washed with PBS, and conjugated secondary antibodies were applied for 1 hour at 37°C. After being washed with PBS, coverslips were mounted onto slides. All preparations were examined by the TCS SP5 confocal microscope.
Western Blot and SDS-PAGE Analyses
For Western blot analyses of whole-cell lysates, the cells were lysed in RIPA Lysis buffer (Upstate, Lake Placid, NY) and boiled in NuPAGE LDS sample buffer (Invitrogen) containing 2.5% 2-mercaptoethanol (Wako). Each sample was processed for SDS-PAGE in 3% to 8% NuPAGE Tris-Acetate Mini Gels (Invitrogen). The separated proteins were transferred to Hybond-P polyvinylidene difluoride membranes (GE Healthcare). These membranes were probed with anti-GFP antibody or J17, washed, and probed with horseradish peroxidase–conjugated goat anti-rabbit pAb. Signals were detected with Super Signal West Dura Substrate (Thermo Fisher Scientific, Rockford, IL).
Samples of F(ab′)2 and Fab fragments in LDS sample buffer were processed for SDS-PAGE in 4% to 12% NuPAGE Bis-Tris Mini Gels (Invitrogen) to confirm the absence of the undigested IgG in the solutions. The membrane, to which the proteins were transferred, was stained with amido-black (Sigma-Aldrich).
Plasmid Constructs
mRNAs were extracted from subconfluent cultures of NHEKs using the RNeasy Plus Mini Kit (Qiagen, Mississauga, ON, Canada), and cDNAs were generated using the High Capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). cDNAs encoding the 5′ half and 3′ half of human BP180 were generated by PCR from the cDNA library using the following primers: 5′-CGCGGATCCATGGARGRAACCAAGAAAAACAA-3′ (forward) and 5′-TTCACCTTTTATTCCTGGTCG-3′ (reverse) and 5′-CCCAAGCTTCGGCTTGACAGCAATACT-3′ (forward) and 5′-AGGAAAGCCAGGTCTCACAG-3′ (reverse), respectively. After gel purification and restriction digestion with BamHI, KpnI, and NotI, both cDNA fragments were ligated into the BamHI/NotI sites of Kikume Green-Red, mammalian expression vector (MBL). The construct was sequenced using the ABI3130xl sequencer (Applied Biosystems) to ensure that it was in frame and had no errors. After restriction digestion with NheI, NotI, and DraI, the cDNA fragments were ligated into the NheI/NotI site of pcDNA 3.1/NT-GFP-TOPO mammalian expression vector (Invitrogen). A stop codon was inserted at the end of the part of BP180 by a KOD mutagenesis kit (Toyobo, Osaka, Japan) using a forward primer, 5′-TGAAAGGGCGAATTCTGCAGATATCCATCACA-3′, and a reverse primer, 5′-CGGCTTGACAGCAATACTTCTTCTCCTTCTC-3′, and the final clone was designated GFP-BP180. GFP-BP180 was sequenced again to ensure that it was in frame and had no errors. A construct PmKate2-zyxin was purchased from Evrogen (Moscow, Russia). A construct encoding GFP-tagged β4 integrin was characterized elsewhere.23
Transfection Procedures
At 24 hours before transfection, the culture media for 804G cells and NHEKs were changed to Dulbecco’s modified Eagle’s medium and HuMedia without antibiotics, respectively. Transfections were performed using the Lipofectamine 2000 reagent (Invitrogen) or polyethyleneimine (Sigma-Aldrich) in OPTI-MEM (Gibco, Grand Island, NY) with the vectors. Six hours later, the media were replaced with normal media. At 24 hours after transfection, the cells were suspended and seeded onto triple-well glass-bottomed dishes (Asahi Techno Glass Co, Tokyo, Japan). At 24 hours after seeding, 804G cells were immediately processed for live cell imaging, whereas NHEKs were further cultured in high Ca2+ medium for 12 to 24 hours before imaging. For Western blot analyses, GFP-positive NHEKs were sorted by using an FACS Vantage SE sorter (BD Biosciences), whereas transfected 804G cells were selected in 1000 μg/mL G418 (Sigma-Aldrich).
Live Cell Imaging
Time-lapse microscopy of the cells on triple-well glass-bottomed dishes was performed with the confocal laser scanning microscope, type TCS SP5. Subconfluent cultures of the cells were scanned every 5 minutes, and confluent cultures were scanned every 15 or 30 minutes.
Fluid-Phase Uptake Assay
Subconfluent cultures of GFP-BP180–transfected 804G cells on glass-bottomed dishes were incubated with 2.0 mg/mL BP-IgG and 0.5 mg/mL 10-kDa dextran–AF 594 (Invitrogen), a fluid-phase marker, for 10 minutes. The cells were fixed in 3.7% formaldehyde in PBS for 8 minutes. After being washed with PBS, the cells were observed with the TCS SP5 confocal microscope.
Detachment of Colonies from the Bottom of the Culture Plate
The strength of adhesion was measured as previously detailed.15 Briefly, NHEKs cultured for 50 hours were treated with 2.0 mg/mL BP-IgG or normal IgG for 6 hours, followed by pre-incubation with or without EIPA for 30 minutes. After vortex mixing for 30 minutes, the numbers of attached cells were counted.
Statistical Analysis
Data are expressed as means ± SDs from three different measurements for individual samples from five different experiments. Statistical significance was determined by the Student’s t-test. P ≤ 0.01 was considered significant.
Results
GFP-BP180 Expression in 804G Cells and NHEKs without BP-IgG Treatment
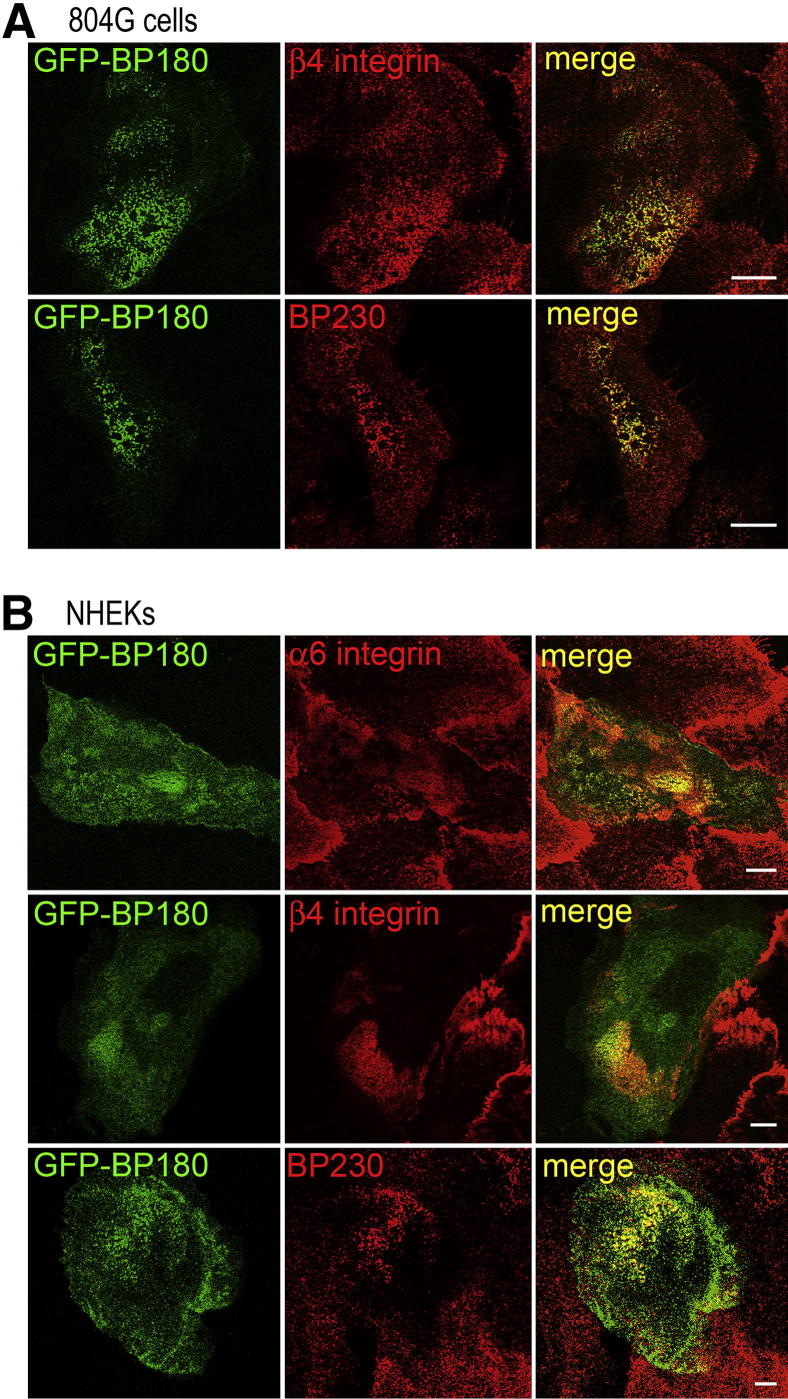
In 804G cells, transfected GFP-BP180 codistributed with other hemidesmosomal components, including β4 integrin and BP230, in a cat paw–like pattern at the substratum-attached surface of the cells (Figure 1A). In NHEKs, transfected GFP-BP180 mainly localized at the substratum-attached surface of the cells, where it colocalized with α6 integrin, β4 integrin, and BP230 (Figure 1B). Western blot analyses of extracts from the GFP-BP180–transfected 804G cells and NHEKs were performed to confirm the appropriate expression of GFP-BP180 protein. In transfected 804G cells, anti-BP180 antibody recognized strongly endogenous BP180 and weakly a 207-kDa polypeptide consistent with recombinant BP180 fused with a 27-kDa GFP tag, whereas anti-GFP mAb recognized only the 207-kDa recombinant BP180 (Supplemental Figure S1A). In transfected NHEKs, a similar expression pattern was found (Supplemental Figure S1B).
Figure 1.
GFP-BP180 incorporates into hemidesmosome-like structures in 804G cells and NHEKs. A: GFP-BP180–expressing 804G cells were stained using antibody against rat β4 integrin or 5E antibody against BP230. GFP-BP180 localized precisely with β4 integrin and BP230 in a cat paw–like pattern at the substratum-attached surface. B: Immunofluorescence analyses of GFP-BP180–expressing NHEKs using GoH3 mAb against human α6 integrin, antibody against human β4 integrin, or 5E antibody against BP230. GFP-BP180 colocalized with other hemidesmosomal components. Scale bars: 10 μm.
BP-IgG Treatment Induces an Internalization of GFP-BP180
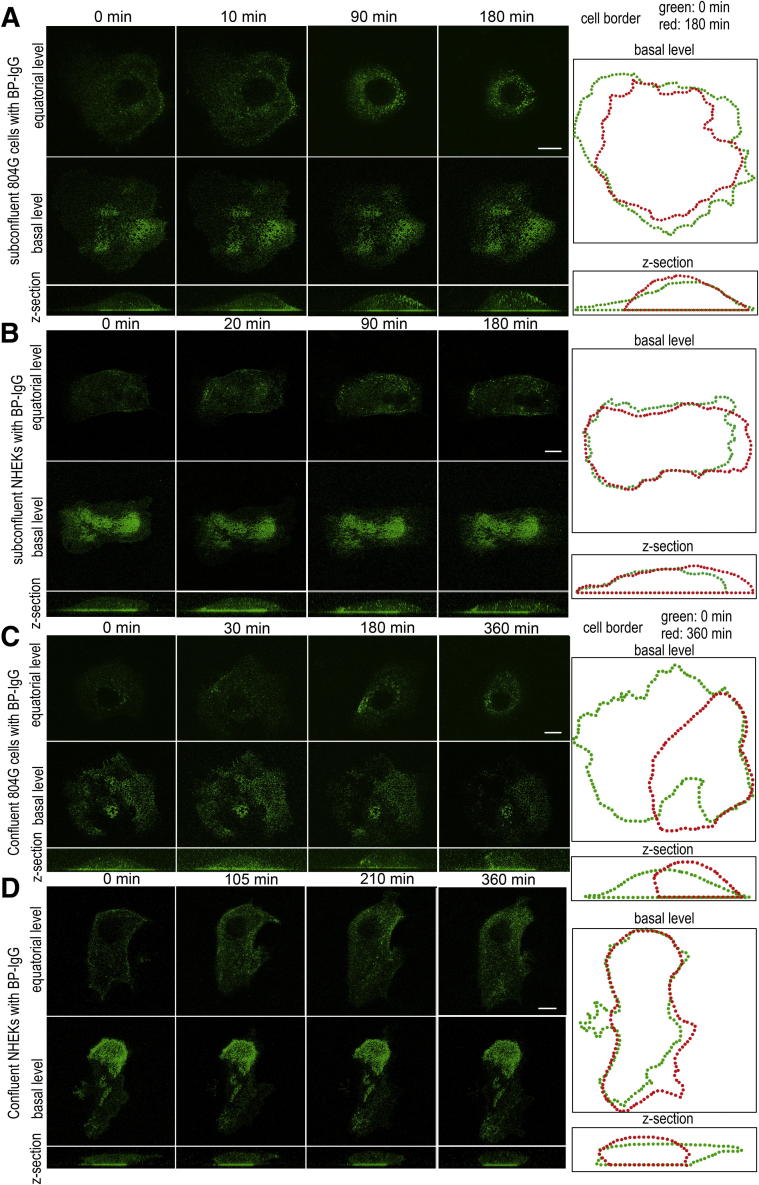
To observe the dynamics of BP-IgG–bound BP180, we followed GFP-BP180 in subconfluent cultures of 804G cells by live cell imaging, after treatment with BP-IgG at a concentration of 2.0 mg/mL (Figure 2A and Supplemental Movie S1). Before BP-IgG treatment, GFP-BP180 localized mainly at the substratum-attached surface of the cells in a distinct patch-like pattern, whereas some GFP-BP180 localized diffusely in the cytoplasm. Approximately 10 minutes after BP-IgG treatment, GFP-BP180 was detected in the cytoplasm as defined spots. By 90 minutes, GFP-BP180–positive cytoplasmic inclusions redistributed centripetally toward the perinuclear region. Interestingly, the patches of GFP-BP180 localized along the substratum-attached surface of the cell maintained their form throughout the observation period (up to 180 minutes). At 180 minutes after treatment with BP-IgG, the cells rounded up (Figure 2A). However, the treated cells did not detach from the dish during the observation period. There was neither obvious GFP-BP180 dynamic movement nor observable morphological changes in subconfluent cultures of 804G cells treated with normal IgG during the same time period (Supplemental Figure S2A).
Figure 2.
BP-IgG–induced GFP-BP180 internalization in live 804G cells and NHEKs. Live cell imaging studies of subconfluent (A) and confluent (C) cultures of 804G cells and subconfluent (B) and confluent (D) cultures of NHEKs. GFP-BP180–expressing cells were visualized by confocal microscopy, either along their substrate-attached surface (basal level) or at a higher focal plane (equatorial level), at various intervals after BP-IgG treatment. In both cell types and conditions, GFP-BP180 was internalized and redistributed centripetally toward the perinuclear region (A–D). Z-sections of the cells are shown at the bottom of each column. At the right, images of the cell border at time 0 and the end of the observation are shown in green and red, respectively. In 804G cells, rounding up of the cells was observed (A and C). Scale bars: 10 μm.
In subconfluent cultures of NHEKs treated with BP-IgG, GFP-BP180 was internalized from the substratum-attached surface into the cytoplasm as spot-like structures within approximately 20 minutes (Figure 2B and Supplemental Movie S2). Most of the internalized GFP-BP180 showed a centripetal redistribution by 90 minutes. Compared with the results in 804G cells, less GFP-BP180 localized in the cytoplasm at 180 minutes after BP-IgG treatment. The cells did not show obvious rounding up at 180 minutes after BP-IgG treatment, in contrast to what was observed under the same conditions using 804G cells (Figure 2B). Control IgG showed no particular effects on GFP-BP180 expression and the morphological characteristics in NHEKs (Supplemental Figure S2B).
GFP-BP180 expressed in NHEKs treated with inhibitory antibodies against the hemidesmosome proteins α6 integrin (GoH3) or β4 integrin (3E1) failed to become cytoplasmic, although the treated cells changed shapes and partially detached from their substrate (Supplemental Figure S2, C and D). Moreover, in NHEKs expressing either GFP-tagged β4 integrin or pmKate2-zyxin, a focal contact component, there was no internalization of either tagged protein after BP-IgG treatment (Supplemental Figure S2, E and F). However, the amount of tagged zyxin was reduced at 135 minutes after BP-IgG treatment.
Next, we followed the fate of GFP-BP180 for 6 hours in confluent cultures of 804G cells and NHEKs treated with BP-IgG (Figure 2, C and D, and Supplemental Movies S3 and S4). GFP-BP180 was internalized, as was the case in subconfluent cultures, although the time course and the numbers of internalized dot-like structures were distinct. GFP-BP180 was internalized in confluent cultures of 804G cells and NHEKs within 30 and 105 minutes, respectively, after treatment with BP-IgG. Moreover, in both 804G cells and NHEKs, a centripetal redistribution of GFP-BP180 was observed at 180 and 210 minutes, respectively. Compared with our observations on subconfluent cultures, less GFP-BP180 was internalized in confluent cultures of both cell types after BP-IgG treatment. As was the case in subconfluent cultures, BP-IgG–induced cell rounding of NHEKs in confluent cultures had a much less dramatic effect than that of 804G cells in confluent cultures (Figure 2, C and D).
Treatments with BP-IgG F(ab′)2 and BP-IgG Fab Fragments Induce an Internalization of GFP-BP180
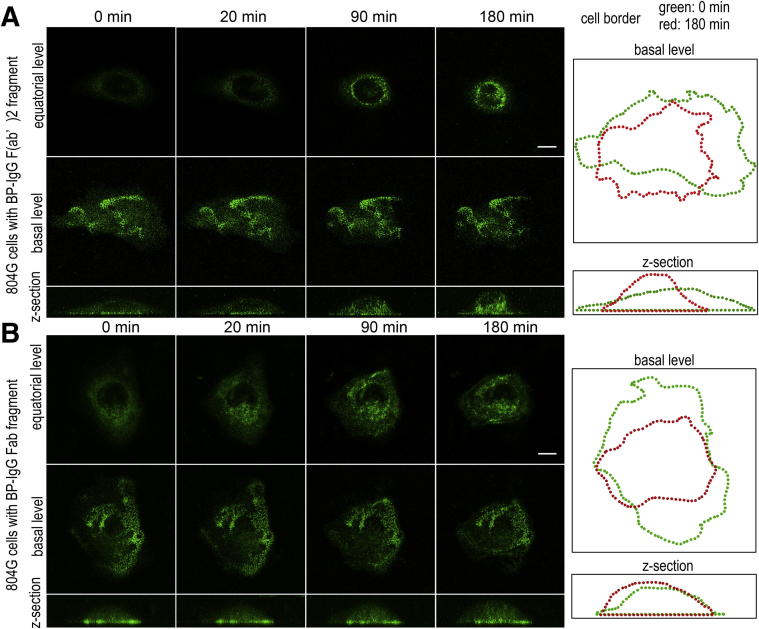
The live cell imaging observations shown in Figure 2 suggested that BP-IgG treatment induces BP180 internalization. If it was mediated through a noninflammatory pathway, the Fc portion of BP-IgG might not be required. Therefore, we next examined whether GFP-BP180 is internalized after treatments with BP-IgG F(ab′)2 and BP-IgG Fab fragments. To generate F(ab′)2 and Fab fragments, BP-IgG was digested by pepsin and papain, respectively. We confirmed the absence of undigested IgG and the high purity of F(ab′)2 and Fab fragments by SDS-PAGE (Supplemental Figure S3). Live cell imaging observations confirmed that both F(ab′)2 and Fab fragments induced GFP-BP180 internalization in subconfluent cultures of 804G cells (Figure 3, A and B). The results suggested that BP-IgG–induced BP180 internalization is regulated by an FcR-independent mechanism. For this and some following studies, we used only GFP-BP180–expressing 804G cells, because these cells showed a more prominent internalized tagged protein.
Figure 3.
BP-IgG F(ab′)2 and BP-IgG Fab fragments induce GFP-BP180 internalization in live 804G cells. Live cell imaging studies of subconfluent cultures of 804G cells treated with BP-IgG F(ab′)2 (A) and BP-IgG Fab (B) fragments. GFP-BP180 was internalized and redistributed centripetally toward the perinuclear region after treatment with both fragments. The fragments induced some cell rounding, as indicated by the outlines of the treated cells at times 0 and 180 minutes, shown to the right. Scale bars: 10 μm.
BP-IgG–Induced BP180 Internalization Is Mediated through a Clathrin-, Caveolae-, and Tyrosine Kinase–Independent Endocytic Pathway
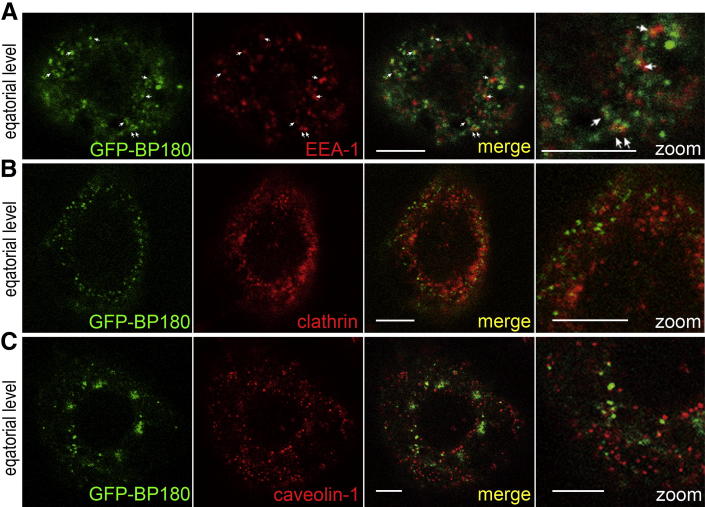
To test whether BP180 internalization is mediated through an endocytic pathway, we performed immunofluorescence analyses using representative molecules for the endocytic pathway. We examined localization of EEA-1 at 1 hour after BP-IgG treatment, and clathrin and caveolin-1 at 2 hours after BP-IgG treatment. Approximately 15% of internalized GFP-BP180 colocalized with EEA-1 (Figure 4A). In contrast, internalized GFP-BP180 did not colocalize with either clathrin or caveolin-1 (Figure 4, B and C). These results suggested that BP-IgG–induced BP180 internalization occurs via a clathrin- and caveolae-independent endocytic pathway.
Figure 4.
BP-IgG–induced GFP-BP180 internalization is mediated through endocytosis. A: After the incubation with BP-IgG for 1 hour, GFP-BP180–expressing 804G cells were stained for EEA-1, which showed some colocalization with GFP-BP180 in the cytoplasm (arrows). In contrast, after a 2-hour incubation with BP-IgG, GFP failed to colocalize with either clathrin (B) or caveolin-1 (C). Scale bars: 5 μm.
We next examined the effect of NEM, hypertonic sucrose, nystatin, and genistein. We confirmed the activity of these inhibitors of general, clathrin-dependent, and caveolae-dependent endocytosis in 804G cells by analyzing the localization of EGFR, which undergoes clathrin-dependent endocytosis, and CTB, which undergoes caveolae-dependent endocytosis (Supplemental Figure S4).24
NEM dramatically inhibited both GFP-BP180 internalization and the morphological changes in 804G cells induced by BP-IgG treatment, suggesting that BP-IgG–induced BP180 internalization is mediated through an endocytic pathway (Figure 5A). In contrast, pre-incubation with hypertonic sucrose, a clathrin-dependent endocytosis inhibitor, failed to prevent BP180 internalization (Figure 5B). Similarly, nystatin (Figure 5C) and genistein (Figure 5D) did not inhibit BP-IgG–induced BP180 internalization. Because nystatin is a caveolae-dependent endocytosis inhibitor and genistein is a tyrosine kinase inhibitor, these results confirmed that BP180 endocytosis is mediated through clathrin-, caveolae-, and tyrosine kinase–independent mechanisms.
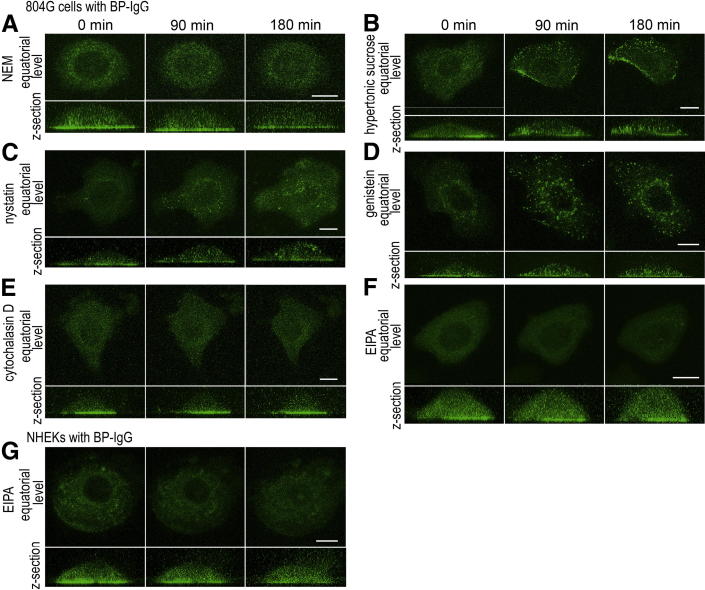
Figure 5.
Pre-incubation with the inhibitors of general endocytosis and macropinocytosis prevents BP-IgG–induced GFP-BP180 internalization. Live cell imaging studies for GFP-BP180–expressing 804G cells (A–F) and NHEKs (G) treated with BP-IgG and endocytosis inhibitors. A: Pre-incubation with NEM inhibited BP-IgG–induced GFP-BP180 internalization and cell morphological changes in 804G cells. Hypertonic sucrose (B), nystatin (C), and genistein (D) failed to prevent GFP-BP180 internalization in BP-IgG–treated 804G cells. Cytochalasin D (E) and EIPA (F) inhibited GFP-BP180 internalization in 804G cells. G: In NHEKs, EIPA prevented BP-IgG–induced GFP-BP180 internalization at 180 minutes. Scale bars:10 μm.
BP-IgG Induces BP180 Internalization via a Macropinocytic Pathway
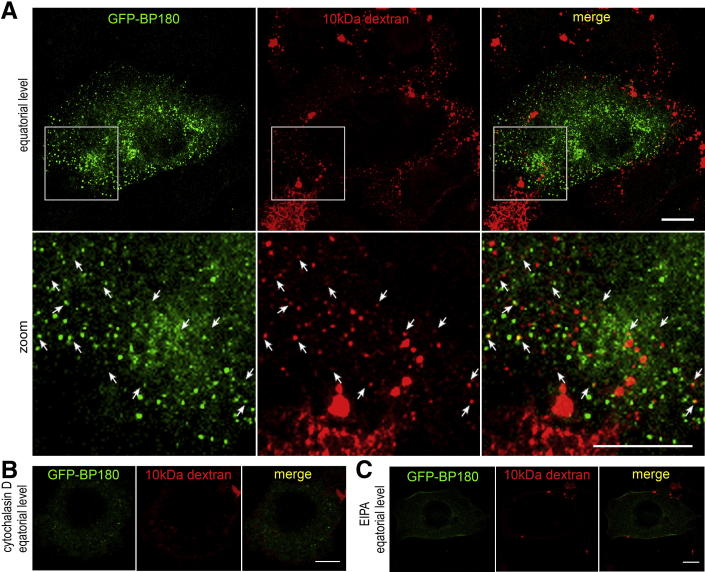
We next investigated whether BP-IgG–induced BP180 internalization involved macropinocytosis. Macropinocytosis can be distinguished by an involvement of actin dynamics and Na+/H+ exchanger and non-specific fluid uptake.25,26 In this study, GFP-BP180–expressing 804G cells were treated with BP-IgG in the presence of either cytochalasin D (Figure 5E) or EIPA (Figure 5F). These macropinocytosis inhibitors blocked GFP-BP180 internalization in 804G cells. Moreover, in NHEKs, GFP-BP180 internalization was inhibited by EIPA (Figure 5G). We further confirmed that some of the internalized BP180 colocalized with 10-kDa dextran–AF 594 in 804G cells (Figure 6A). The internalization of the fluid-phase marker was inhibited by pre-incubation with cytochalasin D and EIPA (Figure 6, B and C). These results demonstrated the participation of macropinocytosis in BP-IgG–induced BP180 internalization.
Figure 6.
Internalized BP180 colocalizes with the fluid-phase marker. After incubation with BP-IgG and 10-kDa dextran–AF 594 for 10 minutes, GFP-BP180–expressing 804G cells were fixed and viewed. A: Boxed areas are shown at a higher power; 10-kDa dextran–AF 594 was internalized and colocalized with some GFP-BP180 as spot-like structures near the cell surface (arrows). Pre-incubation with cytochalasin D (B) and EIPA (C) inhibited the internalization of GFP-BP180 and dextran. Scale bars: 10 μm.
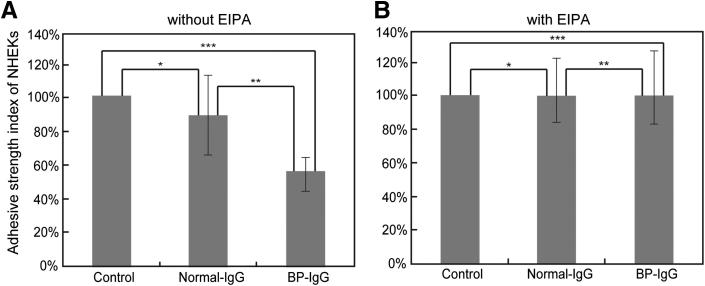
Macropinocytosis Inhibitor Rescues BP-IgG–Induced Reduction in the Adhesive Strength of the Cells
A previous study has shown that BP-IgG treatment reduces the adhesive strength of the cells to their substrate.15 Hence, we assessed whether EIPA inhibits this phenomenon. Non-treated, normal IgG–treated, and BP-IgG–treated NHEKs were vortex mixed after pre-incubation with or without EIPA, and then the numbers of adherent cells were counted. BP-IgG treatment decreased the adhesive strength of the cells, which was consistent with the result of the previous study.15 In contrast, after pre-incubation with EIPA, BP-IgG–treated NHEKs did not exhibit a reduction in the adhesive strength, compared with normal IgG-treated or non-treated cells (Figure 7).
Figure 7.
BP-IgG treatment increases the detachment of NHEKs from their substrate after vortex mixing, and the effect is inhibited by pre-incubation with EIPA. After pre-incubation with (B) or without (A) EIPA, NHEKs were treated with or without normal IgG and BP-IgG for 6 hours. The cells were vortex mixed, and the numbers of adherent cells were counted. The y axis depicts the percentage of adherent cells after agitation. The control index value without IgG addition was calculated as 100%. A: Without pre-incubation with EIPA, the adhesive strength of BP-IgG–treated cells was reduced to approximately 60% compared with normal IgG-treated cells. *P = 0.38 (not significant); **P = 0.0017; ***P = 0.0061. B: After pre-incubation with EIPA for 30 minutes, the adhesive strength of BP-IgG–treated cells was comparable to normal IgG-treated and non-treated cells. *P = 0.96; **P = 1.00; ***P = 0.96 (no significant differences).
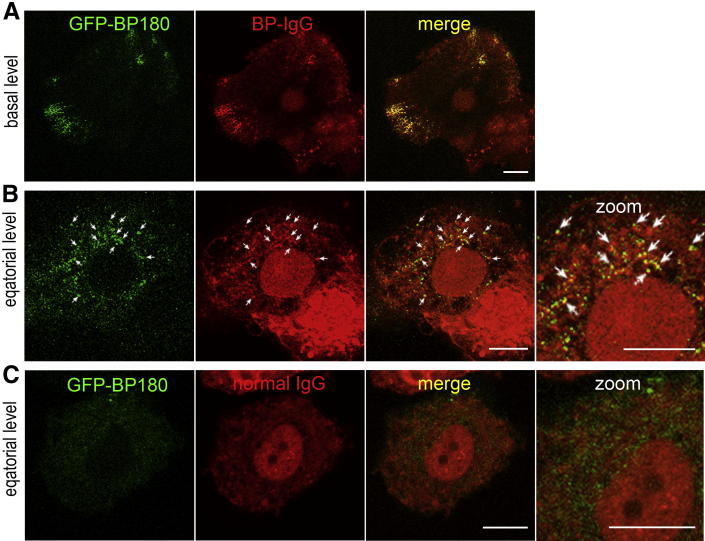
BP180 Is Internalized along with BP-IgG
We next examined whether BP-IgG is internalized along with its antigen, BP180. For this purpose, 804G cells expressing GFP-BP180 were incubated with HiLyte Fluor 647–conjugated BP-IgG for 30 minutes, washed, and then observed. HiLyte Fluor 647–conjugated BP-IgG was clustered along the cell substratum–attached surface, together with GFP-BP180 (Figure 8A), and distributed as cytoplasmic spots (Figure 8B). Some of the internalized BP-IgG spots colocalized with GFP-BP180. In contrast, after incubation with HiLyte Fluor 647–conjugated normal IgG, neither GFP-BP180 nor normal IgG was internalized (Figure 8C). These results suggested that a complex of BP-IgG bound to its BP180 antigen is internalized.
Figure 8.
HiLyte Fluor 647–conjugated BP-IgG is internalized along with BP180. HiLyte Fluor 647–conjugated BP-IgG (A and B) and HiLyte Fluor 647–conjugated normal IgG (C) were observed in GFP-BP180–expressing 804G cells after incubation for 30 minutes. A: HiLyte Fluor 647–conjugated BP-IgG colocalized with GFP-BP180 into hemidesmosome-like structures at the cell substratum–attached surface. B: At the equatorial plane, internalized BP-IgG was visible and colocalized with internalized GFP-BP180 (arrows). C: HiLyte Fluor 647–conjugated normal IgG failed to induce internalization of both IgG and GFP-BP180. Scale bars: 10 μm.
Intracellular and Extracellular Domains of BP180 Are Internalized Simultaneously
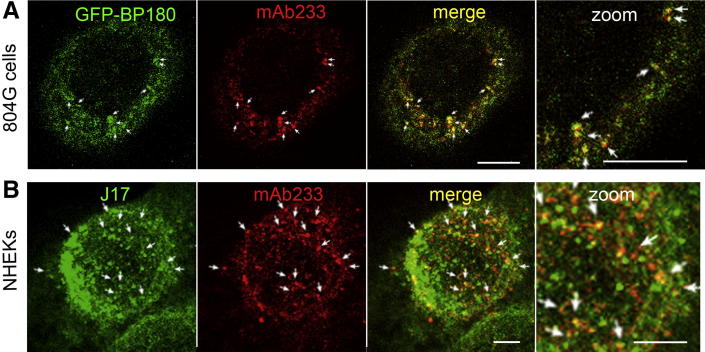
BP180 undergoes proteolytic cleavage during migration and other pathological conditions.27–30 The precise cleavage sites are unclear but may exist in the NC16A domain.27,29,30 To examine whether proteolytic cleavage of BP180 occurs during BP-IgG–induced internalization, BP-IgG–treated GFP-BP180–expressing 804G cells were stained with antibody mAb233, which recognizes the extracellular domain of BP180. BP-IgG–treated untransfected NHEKs were doubly stained with mAb233 and antibody J17, which recognizes the cytoplasmic domain of BP180. These studies showed that both intracellular and extracellular domains of internalized BP180 colocalized as cytoplasmic spots in both cell types (Figure 9, A and B).
Figure 9.
The intracellular domain of BP180 is internalized, along with the extracellular domain of BP180. A: GFP-BP180–expressing 804G cells were stained using anti-extracellular BP180 domain antibody mAb233, 2 hours after the incubation with BP-IgG. B: NHEKs were doubly stained using mAb233 and anti-cytoplasmic BP180 domain antibody J17. These studies showed that both intracellular and extracellular domains of internalized GFP-BP180 colocalized as cytoplasmic spots (arrows). Scale bars: 5 μm.
Discussion
The aim of the present study was to assess the role of BP-IgG in the initiation of noninflammatory blister formation in BP in an in vitro system. Our results indicate the following: i) BP-IgG causes BP180 internalization and morphological changes by an FcR-independent mechanism, which is consistent with the results of the previous studies,15,16,31,32 ii) BP-IgG–induced BP180 internalization and BP-IgG–induced reduction in the adhesive strength of the cells require macropinocytosis, iii) BP180 is internalized, along with BP-IgG, and iv) both intracellular and extracellular domains of BP180 are internalized after binding with BP-IgG.
In subconfluent cultures of both 804G cells and NHEKs, BP180 internalization induced by BP-IgG occurs rapidly, within <30 minutes. Interestingly, there are some differences in the fates of BP180 and the morphological characteristics between 804G cells and NHEKs. The 804G cells showed more prominent GFP-BP180 internalization and cell rounding than in NHEKs. This may reflect the different amino acid sequences between rat BP180 and human BP180, specifically in the binding site to α6 integrin.33 This raises the possibility that, in 804G cells, GFP-human BP180 may not be constrained at the plasma membrane by α6 integrin, resulting in higher internalization and less adhesion.
We confirmed that BP-IgG–induced BP180 internalization also occurs in confluent cultures, although the process appears less efficient than in subconfluent cultures. This result is important, because confluent cultures mimic more the in vivo situation. Less efficient internalization may reflect lower accessibility of BP-IgG to the substratum-attached surface of the cells in confluent cultures.
Based on the results of our fluid-uptake and live cell imaging assays using cytochalasin D and EIPA, which inhibit actin polymerization and transmembrane Na+ transport systems, respectively, we conclude that BP180 internalization occurs via macropinocytosis. Moreover, macropinocytosis of BP180 reduces cell-substrate adhesion, because EIPA rescues BP-IgG–induced detachment of the cells after vortex mixing. Interestingly, we also showed that a tyrosine kinase is unlikely to be involved in BP-IgG–induced BP180 internalization. This is not contrary to the literature because the role of tyrosine kinases in macropinocytosis is controversial.25,34–36
Our results have an interesting parallel with those involving the pathogenesis of another blistering skin disease, pemphigus vulgaris (PV). The PV antigen, desmoglein 3 (Dsg3), undergoes endocytosis after PV autoantibody binding.24,37,38 Such as the BP180 internalization shown herein, PV-IgG–induced Dsg3 internalization is mediated by a clathrin- and dynamin-independent mechanism.24 However, in contrast to BP180 internalization, both nystatin and genistein inhibit PV-IgG–induced Dsg3 internalization. Therefore, Dsg3 and BP180 internalizations are considered to be controlled by distinct endocytic pathways. The difference in cytoplasmic domains between Dsg3 and BP180 may specify the distinct endocytic pathways by which these internalizations are regulated.24
Some reports have suggested that ectodomain shedding of BP180 occurs in BP, although its pathogenic role is unclear.29,30,39–41 In this study, we detected both intracellular and extracellular domains in the internalized BP180, suggesting that ectodomain shedding of BP180 does not necessarily occur in BP180 internalization and may not be essential to BP disease onset.
Some previous studies have reported that BP-IgG F(ab′)2 and BP-IgG Fab fragments are insufficient to induce blisters in mouse models of the disease.11,42,43 In contrast, herein, we have shown that whole IgG and BP-IgG F(ab′)2 and BP-IgG Fab fragments cause BP180 internalization and cell morphological changes. One possible explanation for these contradictory results is that BP-IgG–induced BP180 internalization is insufficient to induce blister formation, although it reduces cell-extracellular matrix adhesive strength. We speculate that blistering requires various inflammatory responses at the cell-extracellular matrix zone, where it is first weakened by BP180 internalization, further leading to a BP-specific split at the lamina lucida. In other words, we suspect that blister formation in BP requires both BP-IgG–induced BP180 internalization and FcR-independent and FcR-dependent immune responses.
In conclusion, the results of the present study suggest that BP180 internalization is an early event in disease pathogenesis of BP and occurs via a macropinocytic pathway. This new understanding of disease pathogenesis should provide us with new approaches for the treatment of BP.
Footnotes
Supported by The Osaka Medical Research Foundation for Intractable Diseases grant 15-23 (S.H.), the Japanese Dermatological Association Shiseido Award23-13, the Ministry of Education, Culture, Sports, Science and Technology of Japan Grants-in-Aid for Science Research and Strategic Research Basis Formation Supporting Project J112640081 (D.T.), and NIH grant R01 AR054184 (J.C.R.J.).
Supplemental Data
GFP-BP180 expression in 804G cells and NHEKs, as determined by using Western blot analyses. Extracts of GFP-BP180 untransfected and transfected 804G cells (A) and NHEKs (B) were processed for Western blot analyses using antibodies against GFP (lanes 1 and 2) or BP180 (lanes 3 and 4). Anti-GFP antibody recognized a single 207-kDa polypeptide in lane 2. Anti-BP180 antibody recognized the 180-kDa polypeptide in lane 3 but the 180- and 207-kDa polypeptides in lane 4.
Control experiments for live cell imaging analyses of BP-IgG–induced BP180 internalization. GFP-BP180–expressing 804G cells (A) and NHEKs (B) were incubated with normal IgG for 180 minutes. There was no obvious GFP-BP180 internalization and cell morphological change. After incubation with anti-α6 integrin antibody GoH3 (C) and anti-β4 integrin antibody 3E1 (D), GFP-BP180–expressing NHEKs partially detached from the substrate, but no internalized BP180 was observed 135 minutes after antibody incubation. NHEKs expressing either GFP-tagged β4 integrin (E) or PmKate2-zyxin (F) were incubated with BP-IgG. At 135 minutes after the incubation, no obvious internalization of GFP-BP180 was observed. Scale bar = 10 μm.
The absence of undigested IgG and the high purity of F(ab′)2 and Fab fragments are confirmed by SDS-PAGE. Whole IgG and digestion products were subjected to SDS-PAGE under nonreducing conditions. Molecular weight standards were indicated at both sides. The sample of F(ab′)2 fragment showed a single band at 110 kDa, and the sample of Fab fragment had only two bands near 50 and 20 kDa. They correspond to F(ab′)2, Fab, and reduced Fab fragments and/or IgG light chain, respectively.
The internalization of EGFR and CTB in 804G cells confirms the activity of these inhibitors of general, clathrin-dependent, and caveolae-dependent endocytosis. Internalization of EGFR after incubation with EGF, a typical clathrin-dependent endocytic event, and internalization of AF 594–conjugated CTB after incubation with CTB itself, a typical caveolae-dependent endocytosis, were monitored after treatment with the indicated inhibitors. NEM (1 mmol/L), a general endocytosis inhibitor, inhibited the internalization of both EGFR and CTB. However, 0.4 mol/L hypertonic sucrose, a clathrin-dependent endocytosis inhibitor, prevented EGFR, but not CTB, internalization. On the contrary, 50 μmol/L nystatin, a caveolae-dependent endocytosis inhibitor, and 100 μmol/L genistein, a tyrosine kinase inhibitor, inhibited CTB internalization but failed to prevent EGFR internalization. Thus, these inhibitors worked as expected. Scale bar = 10 μm.
Live cell imaging of subconfluent culture of GFP-BP180–expressing 804G cells incubated with BP-IgG. Images were taken every 5 minutes for 3 hours.
Live cell imaging of subconfluent culture of GFP-BP180–expressing NHEKs incubated with BP-IgG. Images were taken every 5 minutes for 3 hours.
Live cell imaging of confluent culture of GFP-BP180–expressing 804G cells incubated with BP-IgG. Images were taken every 30 minutes for 6 hours.
Live cell imaging of confluent culture of GFP-BP180–expressing NHEKs incubated with BP-IgG. Images were taken every 15 minutes for 6 hours.
References
- 1.Lever W.F. Pemphigus. Medicine (Baltimore) 1953;32:1–123. doi: 10.1097/00005792-195302000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Jordon R.E., Beutner E.H., Witebsky E., Blumental G., Hale W.L., Lever W.F. Basement zone antibodies in bullous pemphigoid. JAMA. 1967;200:751–756. [PubMed] [Google Scholar]
- 3.Labib R.S., Anhalt G.J., Patel H.P., Mutasim D.F., Diaz L.A. Molecular heterogeneity of the bullous pemphigoid antigens as detected by immunoblotting. J Immunol. 1986;136:1231–1235. [PubMed] [Google Scholar]
- 4.Diaz L.A., Ratrie H., 3rd, Saunders W.S., Futamura S., Squiquera H.L., Anhalt G.J., Giudice G.J. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera: immunolocalization of this protein to the hemidesmosome. J Clin Invest. 1990;86:1088–1094. doi: 10.1172/JCI114812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanley J.R., Hawley-Nelson P., Yuspa S.H., Shevach E.M., Katz S.I. Characterization of bullous pemphigoid antigen: a unique basement membrane protein of stratified squamous epithelia. Cell. 1981;24:897–903. doi: 10.1016/0092-8674(81)90115-x. [DOI] [PubMed] [Google Scholar]
- 6.Stanley J.R., Tanaka T., Mueller S., Klaus-Kovtun V., Roop D. Isolation of complementary DNA for bullous pemphigoid antigen by use of patients’ autoantibodies. J Clin Invest. 1988;82:1864–1870. doi: 10.1172/JCI113803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall R.P., 3rd, Murray J.C., McCord M.M., Rico M.J., Streilein R.D. Rabbits immunized with a peptide encoded for by the 230-kD bullous pemphigoid antigen cDNA develop an enhanced inflammatory response to UVB irradiation: a potential animal model for bullous pemphigoid. J Invest Dermatol. 1993;101:9–14. doi: 10.1111/1523-1747.ep12358276. [DOI] [PubMed] [Google Scholar]
- 8.Kiss M., Husz S., Janossy T., Marczinovits I., Molnar J., Korom I., Dobozy A. Experimental bullous pemphigoid generated in mice with an antigenic epitope of the human hemidesmosomal protein BP230. J Autoimmun. 2005;24:1–10. doi: 10.1016/j.jaut.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z., Diaz L.A., Troy J.L., Taylor A.F., Emery D.J., Fairley J.A., Giudice G.J. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92:2480–2488. doi: 10.1172/JCI116856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishie W., Sawamura D., Goto M., Ito K., Shibaki A., McMillan J.R., Sakai K., Nakamura H., Olasz E., Yancey K.B., Akiyama M., Shimizu H. Humanization of autoantigen. Nat Med. 2007;13:378–383. doi: 10.1038/nm1496. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z., Giudice G.J., Swartz S.J., Fairley J.A., Till G.O., Troy J.L., Diaz L.A. The role of complement in experimental bullous pemphigoid. J Clin Invest. 1995;95:1539–1544. doi: 10.1172/JCI117826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z., Giudice G.J., Zhou X., Swartz S.J., Troy J.L., Fairley J.A., Till G.O., Diaz L.A. A major role for neutrophils in experimental bullous pemphigoid. J Clin Invest. 1997;100:1256–1263. doi: 10.1172/JCI119639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen R., Ning G., Zhao M.L., Fleming M.G., Diaz L.A., Werb Z., Liu Z. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J Clin Invest. 2001;108:1151–1158. doi: 10.1172/JCI11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feyerabend T.B., Weiser A., Tietz A., Stassen M., Harris N., Kopf M., Radermacher P., Moller P., Benoist C., Mathis D., Fehling H.J., Rodewald H.R. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity. 2011;35:832–844. doi: 10.1016/j.immuni.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Iwata H., Kamio N., Aoyama Y., Yamamoto Y., Hirako Y., Owaribe K., Kitajima Y. IgG from patients with bullous pemphigoid depletes cultured keratinocytes of the 180-kDa bullous pemphigoid antigen (type XVII collagen) and weakens cell attachment. J Invest Dermatol. 2009;129:919–926. doi: 10.1038/jid.2008.305. [DOI] [PubMed] [Google Scholar]
- 16.Messingham K.N., Srikantha R., DeGueme A.M., Fairley J.A. FcR-independent effects of IgE and IgG autoantibodies in bullous pemphigoid. J Immunol. 2011;187:553–560. doi: 10.4049/jimmunol.1001753. [DOI] [PubMed] [Google Scholar]
- 17.Ozawa T., Tsuruta D., Jones J.C., Ishii M., Ikeda K., Harada T., Aoyama Y., Kawada A., Kobayashi H. Dynamic relationship of focal contacts and hemidesmosome protein complexes in live cells. J Invest Dermatol. 2010;130:1624–1635. doi: 10.1038/jid.2009.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langhofer M., Hopkinson S.B., Jones J.C. The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro. J Cell Sci. 1993;105(Pt 3):753–764. doi: 10.1242/jcs.105.3.753. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto T., Amagai M., Ebihara T., Gamou S., Shimizu N., Tsubata T., Hasegawa A., Miki K., Nishikawa T. Further analyses of epitopes for human monoclonal anti-basement membrane zone antibodies produced by stable human hybridoma cell lines constructed with Epstein-Barr virus transformants. J Invest Dermatol. 1993;100:310–315. doi: 10.1111/1523-1747.ep12469916. [DOI] [PubMed] [Google Scholar]
- 20.Owaribe K., Nishizawa Y., Franke W.W. Isolation and characterization of hemidesmosomes from bovine corneal epithelial cells. Exp Cell Res. 1991;192:622–630. doi: 10.1016/0014-4827(91)90084-8. [DOI] [PubMed] [Google Scholar]
- 21.Nishizawa Y., Uematsu J., Owaribe K. HD4, a 180 kDa bullous pemphigoid antigen, is a major transmembrane glycoprotein of the hemidesmosome. J Biochem. 1993;113:493–501. doi: 10.1093/oxfordjournals.jbchem.a124072. [DOI] [PubMed] [Google Scholar]
- 22.Hopkinson S.B., Riddelle K.S., Jones J.C. Cytoplasmic domain of the 180-kD bullous pemphigoid antigen, a hemidesmosomal component: molecular and cell biologic characterization. J Invest Dermatol. 1992;99:264–270. doi: 10.1111/1523-1747.ep12616615. [DOI] [PubMed] [Google Scholar]
- 23.Tsuruta D., Hopkinson S.B., Lane K.D., Werner M.E., Cryns V.L., Jones J.C. Crucial role of the specificity-determining loop of the integrin beta4 subunit in the binding of cells to laminin-5 and outside-in signal transduction. J Biol Chem. 2003;278:38707–38714. doi: 10.1074/jbc.M301637200. [DOI] [PubMed] [Google Scholar]
- 24.Delva E., Jennings J.M., Calkins C.C., Kottke M.D., Faundez V., Kowalczyk A.P. Pemphigus vulgaris IgG-induced desmoglein-3 endocytosis and desmosomal disassembly are mediated by a clathrin- and dynamin-independent mechanism. J Biol Chem. 2008;283:18303–18313. doi: 10.1074/jbc.M710046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerr M.C., Teasdale R.D. Defining macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 26.Mercer J., Helenius A. Virus entry by macropinocytosis. Nat Cell Biol. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 27.Hirako Y., Nishizawa Y., Sitaru C., Opitz A., Marcus K., Meyer H.E., Butt E., Owaribe K., Zillikens D. The 97-kDa (LABD97) and 120-kDa (LAD-1) fragments of bullous pemphigoid antigen 180/type XVII collagen have different N-termini. J Invest Dermatol. 2003;121:1554–1556. doi: 10.1046/j.1523-1747.2003.12607.x. [DOI] [PubMed] [Google Scholar]
- 28.Franzke C.W., Tasanen K., Schacke H., Zhou Z., Tryggvason K., Mauch C., Zigrino P., Sunnarborg S., Lee D.C., Fahrenholz F., Bruckner-Tuderman L. Transmembrane collagen XVII, an epithelial adhesion protein, is shed from the cell surface by ADAMs. EMBO J. 2002;21:5026–5035. doi: 10.1093/emboj/cdf532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishie W., Lamer S., Schlosser A., Licarete E., Franzke C.W., Hofmann S.C., Jackow J., Sitaru C., Bruckner-Tuderman L. Ectodomain shedding generates Neoepitopes on collagen XVII, the major autoantigen for bullous pemphigoid. J Immunol. 2010;185:4938–4947. doi: 10.4049/jimmunol.1001524. [DOI] [PubMed] [Google Scholar]
- 30.Lin L., Betsuyaku T., Heimbach L., Li N., Rubenstein D., Shapiro S.D., An L., Giudice G.J., Diaz L.A., Senior R.M., Liu Z. Neutrophil elastase cleaves the murine hemidesmosomal protein BP180/type XVII collagen and generates degradation products that modulate experimental bullous pemphigoid. Matrix Biol. 2012;31:38–44. doi: 10.1016/j.matbio.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitajima Y., Hirako Y., Owaribe K., Mori S., Yaoita H. Antibody-binding to the 180-kD bullous pemphigoid antigens at the lateral cell surface causes their internalization and inhibits their assembly at the basal cell surface in cultured keratinocytes. J Dermatol. 1994;21:838–846. doi: 10.1111/j.1346-8138.1994.tb03299.x. [DOI] [PubMed] [Google Scholar]
- 32.Kitajima Y., Nojiri M., Yamada T., Hirako Y., Owaribe K. Internalization of the 180 kDa bullous pemphigoid antigen as immune complexes in basal keratinocytes: an important early event in blister formation in bullous pemphigoid. Br J Dermatol. 1998;138:71–76. doi: 10.1046/j.1365-2133.1998.02028.x. [DOI] [PubMed] [Google Scholar]
- 33.Hopkinson S.B., Findlay K., deHart G.W., Jones J.C. Interaction of BP180 (type XVII collagen) and alpha6 integrin is necessary for stabilization of hemidesmosome structure. J Invest Dermatol. 1998;111:1015–1022. doi: 10.1046/j.1523-1747.1998.00452.x. [DOI] [PubMed] [Google Scholar]
- 34.Mercer J., Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 35.Liu X., Wu J., Yammine M., Zhou J., Posocco P., Viel S., Liu C., Ziarelli F., Fermeglia M., Pricl S., Victorero G., Nguyen C., Erbacher P., Behr J.P., Peng L. Structurally flexible triethanolamine core PAMAM dendrimers are effective nanovectors for DNA transfection in vitro and in vivo to the mouse thymus. Bioconjug Chem. 2011;22:2461–2473. doi: 10.1021/bc200275g. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt F.I., Bleck C.K., Helenius A., Mercer J. Vaccinia extracellular virions enter cells by macropinocytosis and acid-activated membrane rupture. EMBO J. 2011;30:3647–3661. doi: 10.1038/emboj.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calkins C.C., Setzer S.V., Jennings J.M., Summers S., Tsunoda K., Amagai M., Kowalczyk A.P. Desmoglein endocytosis and desmosome disassembly are coordinated responses to pemphigus autoantibodies. J Biol Chem. 2006;281:7623–7634. doi: 10.1074/jbc.M512447200. [DOI] [PubMed] [Google Scholar]
- 38.Sato M., Aoyama Y., Kitajima Y. Assembly pathway of desmoglein 3 to desmosomes and its perturbation by pemphigus vulgaris-IgG in cultured keratinocytes, as revealed by time-lapsed labeling immunoelectron microscopy. Lab Invest. 2000;80:1583–1592. doi: 10.1038/labinvest.3780168. [DOI] [PubMed] [Google Scholar]
- 39.Roh J.Y., Yee C., Lazarova Z., Hall R.P., Yancey K.B. The 120-kDa soluble ectodomain of type XVII collagen is recognized by autoantibodies in patients with pemphigoid and linear IgA dermatosis. Br J Dermatol. 2000;143:104–111. doi: 10.1046/j.1365-2133.2000.03598.x. [DOI] [PubMed] [Google Scholar]
- 40.Schumann H., Baetge J., Tasanen K., Wojnarowska F., Schacke H., Zillikens D., Bruckner-Tuderman L. The shed ectodomain of collagen XVII/BP180 is targeted by autoantibodies in different blistering skin diseases. Am J Pathol. 2000;156:685–695. doi: 10.1016/S0002-9440(10)64772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egan C.A., Taylor T.B., Meyer L.J., Petersen M.J., Zone J.J. Bullous pemphigoid sera that contain antibodies to BPAg2 also contain antibodies to LABD97 that recognize epitopes distal to the NC16A domain. J Invest Dermatol. 1999;112:148–152. doi: 10.1046/j.1523-1747.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 42.Sitaru C., Schmidt E., Petermann S., Munteanu L.S., Brocker E.B., Zillikens D. Autoantibodies to bullous pemphigoid antigen 180 induce dermal-epidermal separation in cryosections of human skin. J Invest Dermatol. 2002;118:664–671. doi: 10.1046/j.1523-1747.2002.01720.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang G., Ujiie H., Shibaki A., Nishie W., Tateishi Y., Kikuchi K., Li Q., McMillan J.R., Morioka H., Sawamura D., Nakamura H., Shimizu H. Blockade of autoantibody-initiated tissue damage by using recombinant fab antibody fragments against pathogenic autoantigen. Am J Pathol. 2010;176:914–925. doi: 10.2353/ajpath.2010.090744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GFP-BP180 expression in 804G cells and NHEKs, as determined by using Western blot analyses. Extracts of GFP-BP180 untransfected and transfected 804G cells (A) and NHEKs (B) were processed for Western blot analyses using antibodies against GFP (lanes 1 and 2) or BP180 (lanes 3 and 4). Anti-GFP antibody recognized a single 207-kDa polypeptide in lane 2. Anti-BP180 antibody recognized the 180-kDa polypeptide in lane 3 but the 180- and 207-kDa polypeptides in lane 4.
Control experiments for live cell imaging analyses of BP-IgG–induced BP180 internalization. GFP-BP180–expressing 804G cells (A) and NHEKs (B) were incubated with normal IgG for 180 minutes. There was no obvious GFP-BP180 internalization and cell morphological change. After incubation with anti-α6 integrin antibody GoH3 (C) and anti-β4 integrin antibody 3E1 (D), GFP-BP180–expressing NHEKs partially detached from the substrate, but no internalized BP180 was observed 135 minutes after antibody incubation. NHEKs expressing either GFP-tagged β4 integrin (E) or PmKate2-zyxin (F) were incubated with BP-IgG. At 135 minutes after the incubation, no obvious internalization of GFP-BP180 was observed. Scale bar = 10 μm.
The absence of undigested IgG and the high purity of F(ab′)2 and Fab fragments are confirmed by SDS-PAGE. Whole IgG and digestion products were subjected to SDS-PAGE under nonreducing conditions. Molecular weight standards were indicated at both sides. The sample of F(ab′)2 fragment showed a single band at 110 kDa, and the sample of Fab fragment had only two bands near 50 and 20 kDa. They correspond to F(ab′)2, Fab, and reduced Fab fragments and/or IgG light chain, respectively.
The internalization of EGFR and CTB in 804G cells confirms the activity of these inhibitors of general, clathrin-dependent, and caveolae-dependent endocytosis. Internalization of EGFR after incubation with EGF, a typical clathrin-dependent endocytic event, and internalization of AF 594–conjugated CTB after incubation with CTB itself, a typical caveolae-dependent endocytosis, were monitored after treatment with the indicated inhibitors. NEM (1 mmol/L), a general endocytosis inhibitor, inhibited the internalization of both EGFR and CTB. However, 0.4 mol/L hypertonic sucrose, a clathrin-dependent endocytosis inhibitor, prevented EGFR, but not CTB, internalization. On the contrary, 50 μmol/L nystatin, a caveolae-dependent endocytosis inhibitor, and 100 μmol/L genistein, a tyrosine kinase inhibitor, inhibited CTB internalization but failed to prevent EGFR internalization. Thus, these inhibitors worked as expected. Scale bar = 10 μm.
Live cell imaging of subconfluent culture of GFP-BP180–expressing 804G cells incubated with BP-IgG. Images were taken every 5 minutes for 3 hours.
Live cell imaging of subconfluent culture of GFP-BP180–expressing NHEKs incubated with BP-IgG. Images were taken every 5 minutes for 3 hours.
Live cell imaging of confluent culture of GFP-BP180–expressing 804G cells incubated with BP-IgG. Images were taken every 30 minutes for 6 hours.
Live cell imaging of confluent culture of GFP-BP180–expressing NHEKs incubated with BP-IgG. Images were taken every 15 minutes for 6 hours.