Abstract
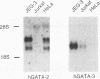
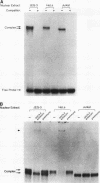
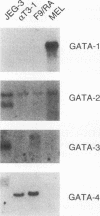
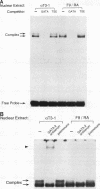
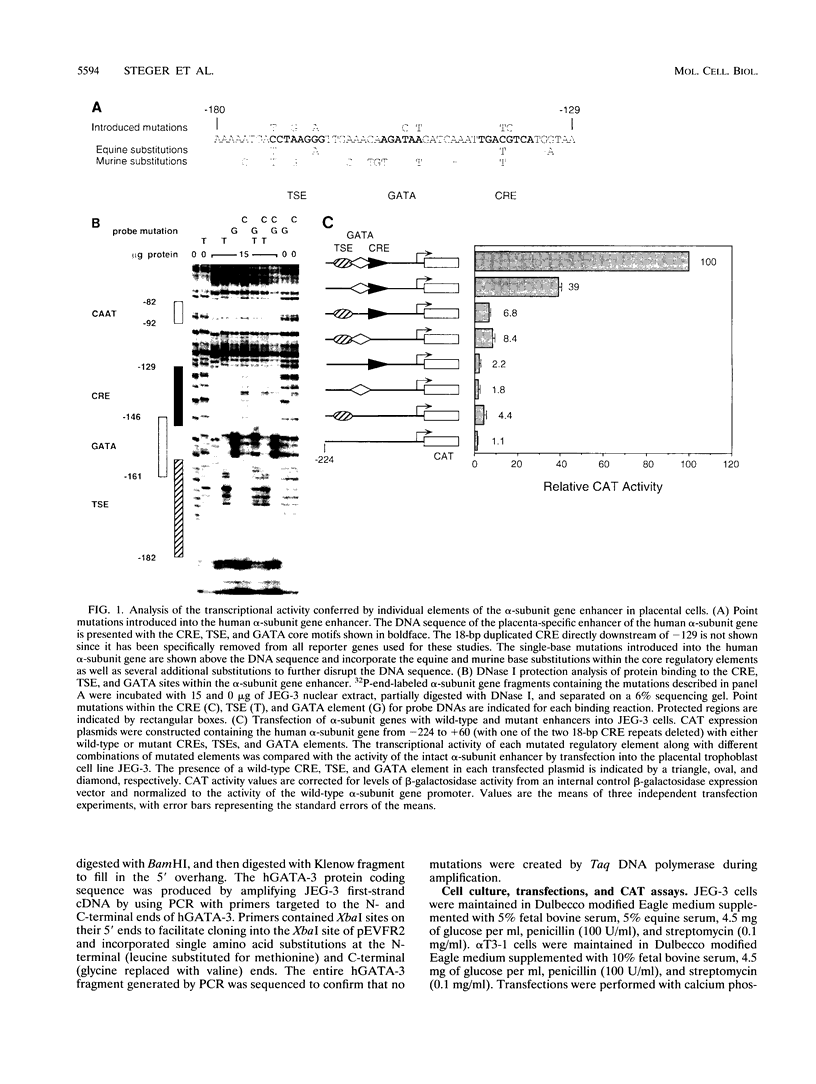
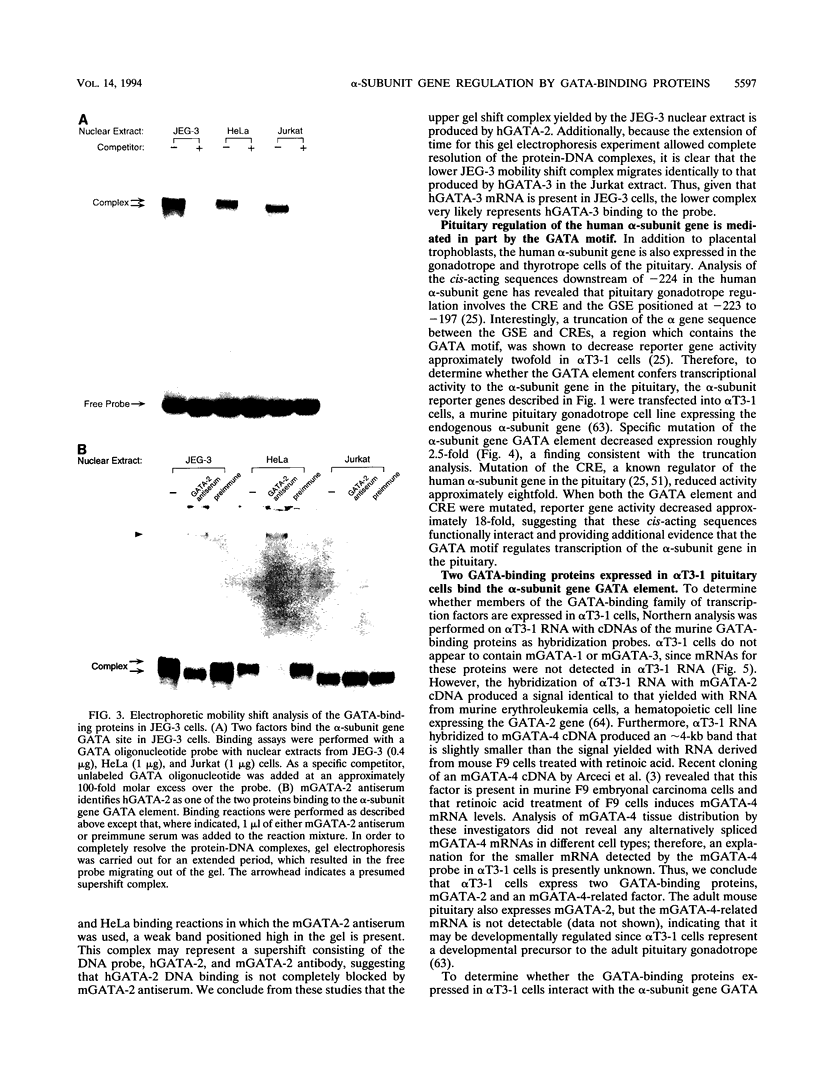
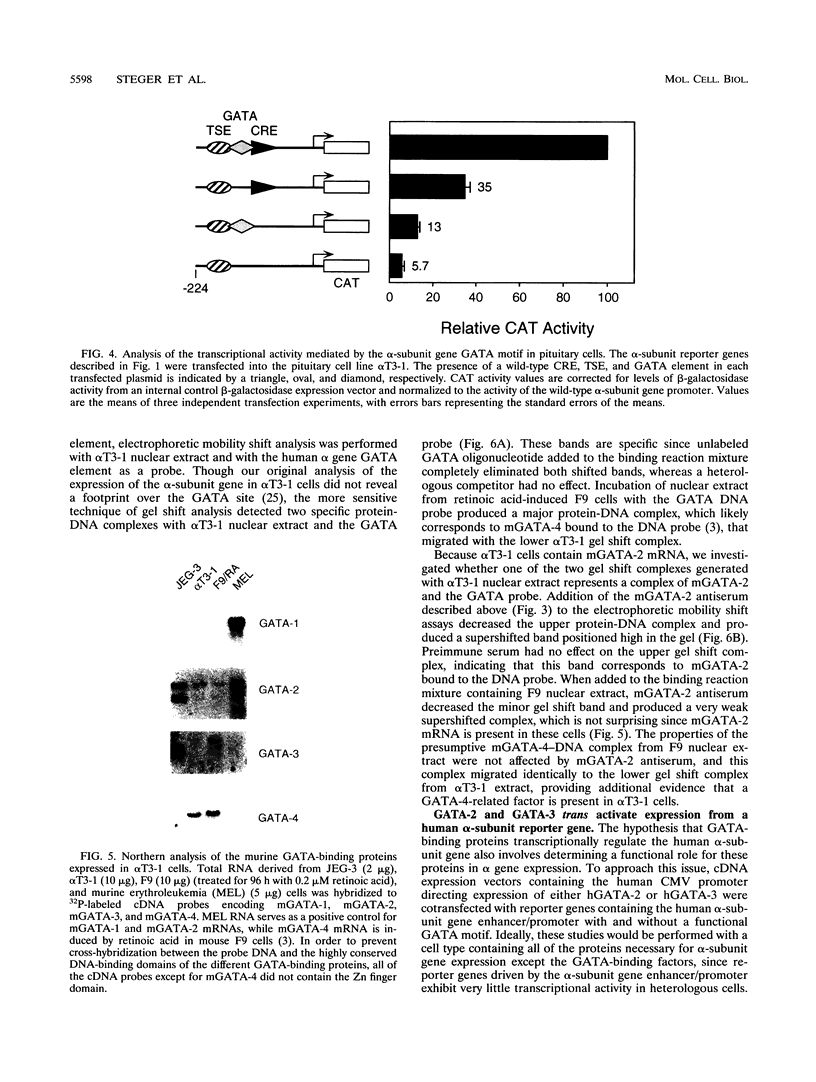
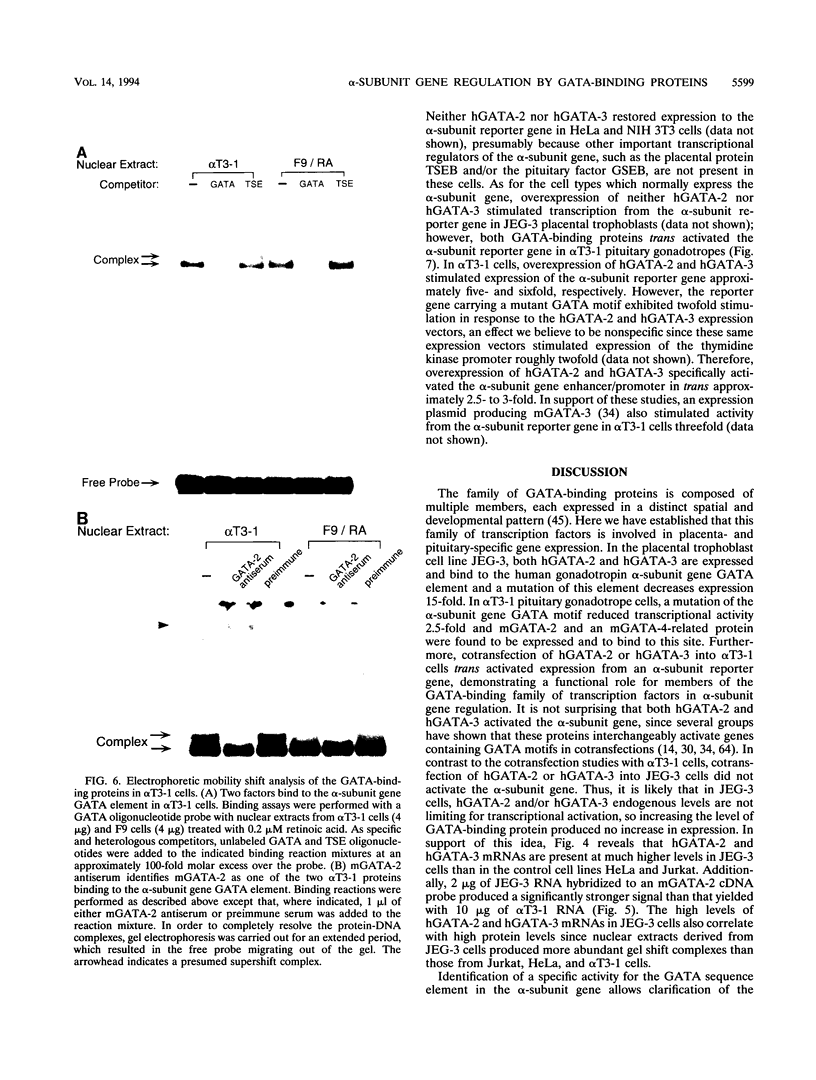
The human glycoprotein hormone alpha-subunit gene is expressed in two quite dissimilar tissues, the placenta and anterior pituitary. Tissue-specific expression is determined by combinations of elements, some of which are common and others of which are specific to each tissue. In the placenta, a composite enhancer confers specific expression. It contains four protein-binding sites: two cyclic AMP (cAMP) response elements that bind CREB, a trophoblast-specific element that binds TSEB, and a sequence motif, AGATAA, that matches the consensus binding site for a family of transcription factors termed the GATA-binding proteins. In pituitary gonadotropes, the cAMP response elements remain important for expression, TSEB is absent, and elements further upstream participate in tissue-specific expression. Here we establish a regulatory role for the GATA element in both the placenta and pituitary by demonstrating that a mutation of this element decreases alpha-subunit gene expression 15-fold in JEG-3 human placental cells and 2.5-fold in alpha T3-1 mouse pituitary gonadotropes. In JEG-3 cells, human GATA-2 (hGATA-2) and hGATA-3 are highly expressed and both proteins bind to the alpha-subunit gene GATA element. In alpha T3-1 cells, the GATA motif is bound by mouse GATA-2 (mGATA-2) and an mGATA-4-related protein. Cotransfection of hGATA-2 or hGATA-3 into alpha T3-1 cells activates the alpha-subunit gene threefold. These studies establish a role for the GATA-binding proteins in placental and pituitary alpha-subunit gene expression, significantly expanding the known target genes of GATA-2, GATA-3, and perhaps GATA-4.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerblom I. E., Ridgway E. C., Mellon P. L. An alpha-subunit-secreting cell line derived from a mouse thyrotrope tumor. Mol Endocrinol. 1990 Apr;4(4):589–596. doi: 10.1210/mend-4-4-589. [DOI] [PubMed] [Google Scholar]
- Andersen B., Kennedy G. C., Hamernik D. L., Bokar J. A., Bohinski R., Nilson J. H. Amplification of the transcriptional signal mediated by the tandem cAMP response elements of the glycoprotein hormone alpha-subunit gene occurs through several distinct mechanisms. Mol Endocrinol. 1990 Apr;4(4):573–582. doi: 10.1210/mend-4-4-573. [DOI] [PubMed] [Google Scholar]
- Arceci R. J., King A. A., Simon M. C., Orkin S. H., Wilson D. B. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol Cell Biol. 1993 Apr;13(4):2235–2246. doi: 10.1128/mcb.13.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokar J. A., Keri R. A., Farmerie T. A., Fenstermaker R. A., Andersen B., Hamernik D. L., Yun J., Wagner T., Nilson J. H. Expression of the glycoprotein hormone alpha-subunit gene in the placenta requires a functional cyclic AMP response element, whereas a different cis-acting element mediates pituitary-specific expression. Mol Cell Biol. 1989 Nov;9(11):5113–5122. doi: 10.1128/mcb.9.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briegel K., Lim K. C., Plank C., Beug H., Engel J. D., Zenke M. Ectopic expression of a conditional GATA-2/estrogen receptor chimera arrests erythroid differentiation in a hormone-dependent manner. Genes Dev. 1993 Jun;7(6):1097–1109. doi: 10.1101/gad.7.6.1097. [DOI] [PubMed] [Google Scholar]
- Chin W. W., Gharib S. D. Organization and expression of gonadotropin genes. Adv Exp Med Biol. 1986;205:245–265. doi: 10.1007/978-1-4684-5209-9_11. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins S., Altschmied J., Herbsman O., Caron M. G., Mellon P. L., Lefkowitz R. J. A cAMP response element in the beta 2-adrenergic receptor gene confers transcriptional autoregulation by cAMP. J Biol Chem. 1990 Nov 5;265(31):19330–19335. [PubMed] [Google Scholar]
- Darnell R. B., Boime I. Differential expression of the human gonadotropin alpha gene in ectopic and eutopic cells. Mol Cell Biol. 1985 Nov;5(11):3157–3167. doi: 10.1128/mcb.5.11.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delegeane A. M., Ferland L. H., Mellon P. L. Tissue-specific enhancer of the human glycoprotein hormone alpha-subunit gene: dependence on cyclic AMP-inducible elements. Mol Cell Biol. 1987 Nov;7(11):3994–4002. doi: 10.1128/mcb.7.11.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch P. J., Jameson J. L., Habener J. F. Cyclic AMP responsiveness of human gonadotropin-alpha gene transcription is directed by a repeated 18-base pair enhancer. Alpha-promoter receptivity to the enhancer confers cell-preferential expression. J Biol Chem. 1987 Sep 5;262(25):12169–12174. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman D. M., Wilson D. B., Bruns G. A., Orkin S. H. Human transcription factor GATA-2. Evidence for regulation of preproendothelin-1 gene expression in endothelial cells. J Biol Chem. 1992 Jan 15;267(2):1279–1285. [PubMed] [Google Scholar]
- Dugaiczyk A., Haron J. A., Stone E. M., Dennison O. E., Rothblum K. N., Schwartz R. J. Cloning and sequencing of a deoxyribonucleic acid copy of glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid isolated from chicken muscle. Biochemistry. 1983 Mar 29;22(7):1605–1613. doi: 10.1021/bi00276a013. [DOI] [PubMed] [Google Scholar]
- Evans T., Felsenfeld G. The erythroid-specific transcription factor Eryf1: a new finger protein. Cell. 1989 Sep 8;58(5):877–885. doi: 10.1016/0092-8674(89)90940-9. [DOI] [PubMed] [Google Scholar]
- Evans T., Reitman M., Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5976–5980. doi: 10.1073/pnas.85.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenstermaker R. A., Farmerie T. A., Clay C. M., Hamernik D. L., Nilson J. H. Different combinations of regulatory elements may account for expression of the glycoprotein hormone alpha-subunit gene in primate and horse placenta. Mol Endocrinol. 1990 Oct;4(10):1480–1487. doi: 10.1210/mend-4-10-1480. [DOI] [PubMed] [Google Scholar]
- Fiddes J. C., Goodman H. M. The gene encoding the common alpha subunit of the four human glycoprotein hormones. J Mol Appl Genet. 1981;1(1):3–18. [PubMed] [Google Scholar]
- Fiddes J. C., Talmadge K. Structure, expression, and evolution of the genes for the human glycoprotein hormones. Recent Prog Horm Res. 1984;40:43–78. doi: 10.1016/b978-0-12-571140-1.50006-2. [DOI] [PubMed] [Google Scholar]
- Gharib S. D., Wierman M. E., Shupnik M. A., Chin W. W. Molecular biology of the pituitary gonadotropins. Endocr Rev. 1990 Feb;11(1):177–199. doi: 10.1210/edrv-11-1-177. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Yamamoto K. K., Fischer W. H., Karr D., Menzel P., Biggs W., 3rd, Vale W. W., Montminy M. R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989 Feb 23;337(6209):749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Ho I. C., Vorhees P., Marin N., Oakley B. K., Tsai S. F., Orkin S. H., Leiden J. M. Human GATA-3: a lineage-restricted transcription factor that regulates the expression of the T cell receptor alpha gene. EMBO J. 1991 May;10(5):1187–1192. doi: 10.1002/j.1460-2075.1991.tb08059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffler J. P., Meyer T. E., Yun Y., Jameson J. L., Habener J. F. Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental cDNA. Science. 1988 Dec 9;242(4884):1430–1433. doi: 10.1126/science.2974179. [DOI] [PubMed] [Google Scholar]
- Horn F., Windle J. J., Barnhart K. M., Mellon P. L. Tissue-specific gene expression in the pituitary: the glycoprotein hormone alpha-subunit gene is regulated by a gonadotrope-specific protein. Mol Cell Biol. 1992 May;12(5):2143–2153. doi: 10.1128/mcb.12.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Lala D. S., Luo X., Kim E., Moisan M. P., Parker K. L. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol. 1993 Jul;7(7):852–860. doi: 10.1210/mend.7.7.8413309. [DOI] [PubMed] [Google Scholar]
- Jameson J. L., Albanese C., Habener J. F. Distinct adjacent protein-binding domains in the glycoprotein hormone alpha gene interact independently with a cAMP-responsive enhancer. J Biol Chem. 1989 Sep 25;264(27):16190–16196. [PubMed] [Google Scholar]
- Jameson J. L., Jaffe R. C., Deutsch P. J., Albanese C., Habener J. F. The gonadotropin alpha-gene contains multiple protein binding domains that interact to modulate basal and cAMP-responsive transcription. J Biol Chem. 1988 Jul 15;263(20):9879–9886. [PubMed] [Google Scholar]
- Jameson J. L., Powers A. C., Gallagher G. D., Habener J. F. Enhancer and promoter element interactions dictate cyclic adenosine monophosphate mediated and cell-specific expression of the glycoprotein hormone alpha-gene. Mol Endocrinol. 1989 May;3(5):763–772. doi: 10.1210/mend-3-5-763. [DOI] [PubMed] [Google Scholar]
- Joulin V., Bories D., Eléouet J. F., Labastie M. C., Chrétien S., Mattéi M. G., Roméo P. H. A T-cell specific TCR delta DNA binding protein is a member of the human GATA family. EMBO J. 1991 Jul;10(7):1809–1816. doi: 10.1002/j.1460-2075.1991.tb07706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley C., Blumberg H., Zon L. I., Evans T. GATA-4 is a novel transcription factor expressed in endocardium of the developing heart. Development. 1993 Jul;118(3):817–827. doi: 10.1242/dev.118.3.817. [DOI] [PubMed] [Google Scholar]
- Kemler I., Bucher E., Seipel K., Müller-Immerglück M. M., Schaffner W. Promoters with the octamer DNA motif (ATGCAAAT) can be ubiquitous or cell type-specific depending on binding affinity of the octamer site and Oct-factor concentration. Nucleic Acids Res. 1991 Jan 25;19(2):237–242. doi: 10.1093/nar/19.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy G. C., Andersen B., Nilson J. H. The human alpha subunit glycoprotein hormone gene utilizes a unique CCAAT binding factor. J Biol Chem. 1990 Apr 15;265(11):6279–6285. [PubMed] [Google Scholar]
- Ko L. J., Yamamoto M., Leonard M. W., George K. M., Ting P., Engel J. D. Murine and human T-lymphocyte GATA-3 factors mediate transcription through a cis-regulatory element within the human T-cell receptor delta gene enhancer. Mol Cell Biol. 1991 May;11(5):2778–2784. doi: 10.1128/mcb.11.5.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler P. O., Bridson W. E. Isolation of hormone-producing clonal lines of human choriocarcinoma. J Clin Endocrinol Metab. 1971 May;32(5):683–687. doi: 10.1210/jcem-32-5-683. [DOI] [PubMed] [Google Scholar]
- Lee M. E., Temizer D. H., Clifford J. A., Quertermous T. Cloning of the GATA-binding protein that regulates endothelin-1 gene expression in endothelial cells. J Biol Chem. 1991 Aug 25;266(24):16188–16192. [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Martin D. I., Orkin S. H. Transcriptional activation and DNA binding by the erythroid factor GF-1/NF-E1/Eryf 1. Genes Dev. 1990 Nov;4(11):1886–1898. doi: 10.1101/gad.4.11.1886. [DOI] [PubMed] [Google Scholar]
- Martin D. I., Tsai S. F., Orkin S. H. Increased gamma-globin expression in a nondeletion HPFH mediated by an erythroid-specific DNA-binding factor. Nature. 1989 Mar 30;338(6214):435–438. doi: 10.1038/338435a0. [DOI] [PubMed] [Google Scholar]
- Matthias P., Müller M. M., Schreiber E., Rusconi S., Schaffner W. Eukaryotic expression vectors for the analysis of mutant proteins. Nucleic Acids Res. 1989 Aug 11;17(15):6418–6418. doi: 10.1093/nar/17.15.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P., Parker V., Gluzman Y., Maniatis T. Identification of DNA sequences required for transcription of the human alpha 1-globin gene in a new SV40 host-vector system. Cell. 1981 Dec;27(2 Pt 1):279–288. doi: 10.1016/0092-8674(81)90411-6. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Ocran K. W., Sarapura V. D., Wood W. M., Gordon D. F., Gutierrez-Hartmann A., Ridgway E. C. Identification of cis-acting promoter elements important for expression of the mouse glycoprotein hormone alpha-subunit gene in thyrotropes. Mol Endocrinol. 1990 May;4(5):766–772. doi: 10.1210/mend-4-5-766. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. GATA-binding transcription factors in hematopoietic cells. Blood. 1992 Aug 1;80(3):575–581. [PubMed] [Google Scholar]
- Pevny L., Simon M. C., Robertson E., Klein W. H., Tsai S. F., D'Agati V., Orkin S. H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991 Jan 17;349(6306):257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarapura V. D., Wood W. M., Gordon D. F., Ocran K. W., Kao M. Y., Ridgway E. C. Thyrotrope expression and thyroid hormone inhibition map to different regions of the mouse glycoprotein hormone alpha-subunit gene promoter. Endocrinology. 1990 Sep;127(3):1352–1361. doi: 10.1210/endo-127-3-1352. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Van Dyke M. W., Gregor P. D., Roeder R. G. Multiple forms of the human gene-specific transcription factor USF. I. Complete purification and identification of USF from HeLa cell nuclei. J Biol Chem. 1988 Aug 25;263(24):11985–11993. [PubMed] [Google Scholar]
- Schoderbek W. E., Kim K. E., Ridgway E. C., Mellon P. L., Maurer R. A. Analysis of DNA sequences required for pituitary-specific expression of the glycoprotein hormone alpha-subunit gene. Mol Endocrinol. 1992 Jun;6(6):893–903. doi: 10.1210/mend.6.6.1379672. [DOI] [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Silver B. J., Bokar J. A., Virgin J. B., Vallen E. A., Milsted A., Nilson J. H. Cyclic AMP regulation of the human glycoprotein hormone alpha-subunit gene is mediated by an 18-base-pair element. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2198–2202. doi: 10.1073/pnas.84.8.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger D. J., Altschmied J., Büscher M., Mellon P. L. Evolution of placenta-specific gene expression: comparison of the equine and human gonadotropin alpha-subunit genes. Mol Endocrinol. 1991 Feb;5(2):243–255. doi: 10.1210/mend-5-2-243. [DOI] [PubMed] [Google Scholar]
- Steger D. J., Büscher M., Hecht J. H., Mellon P. L. Coordinate control of the alpha- and beta-subunit genes of human chorionic gonadotropin by trophoblast-specific element-binding protein. Mol Endocrinol. 1993 Dec;7(12):1579–1588. doi: 10.1210/mend.7.12.7511787. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990 Feb 9;60(3):375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Lai J. S., Herr W. Promoter-selective activation domains in Oct-1 and Oct-2 direct differential activation of an snRNA and mRNA promoter. Cell. 1992 Feb 21;68(4):755–767. doi: 10.1016/0092-8674(92)90150-b. [DOI] [PubMed] [Google Scholar]
- Taylor I. C., Workman J. L., Schuetz T. J., Kingston R. E. Facilitated binding of GAL4 and heat shock factor to nucleosomal templates: differential function of DNA-binding domains. Genes Dev. 1991 Jul;5(7):1285–1298. doi: 10.1101/gad.5.7.1285. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Martin D. I., Zon L. I., D'Andrea A. D., Wong G. G., Orkin S. H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989 Jun 8;339(6224):446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- Wall L., deBoer E., Grosveld F. The human beta-globin gene 3' enhancer contains multiple binding sites for an erythroid-specific protein. Genes Dev. 1988 Sep;2(9):1089–1100. doi: 10.1101/gad.2.9.1089. [DOI] [PubMed] [Google Scholar]
- Weintraub H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 1993 Dec 31;75(7):1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Dorfman D. M., Orkin S. H. A nonerythroid GATA-binding protein is required for function of the human preproendothelin-1 promoter in endothelial cells. Mol Cell Biol. 1990 Sep;10(9):4854–4862. doi: 10.1128/mcb.10.9.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle J. J., Weiner R. I., Mellon P. L. Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol. 1990 Apr;4(4):597–603. doi: 10.1210/mend-4-4-597. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Ko L. J., Leonard M. W., Beug H., Orkin S. H., Engel J. D. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev. 1990 Oct;4(10):1650–1662. doi: 10.1101/gad.4.10.1650. [DOI] [PubMed] [Google Scholar]