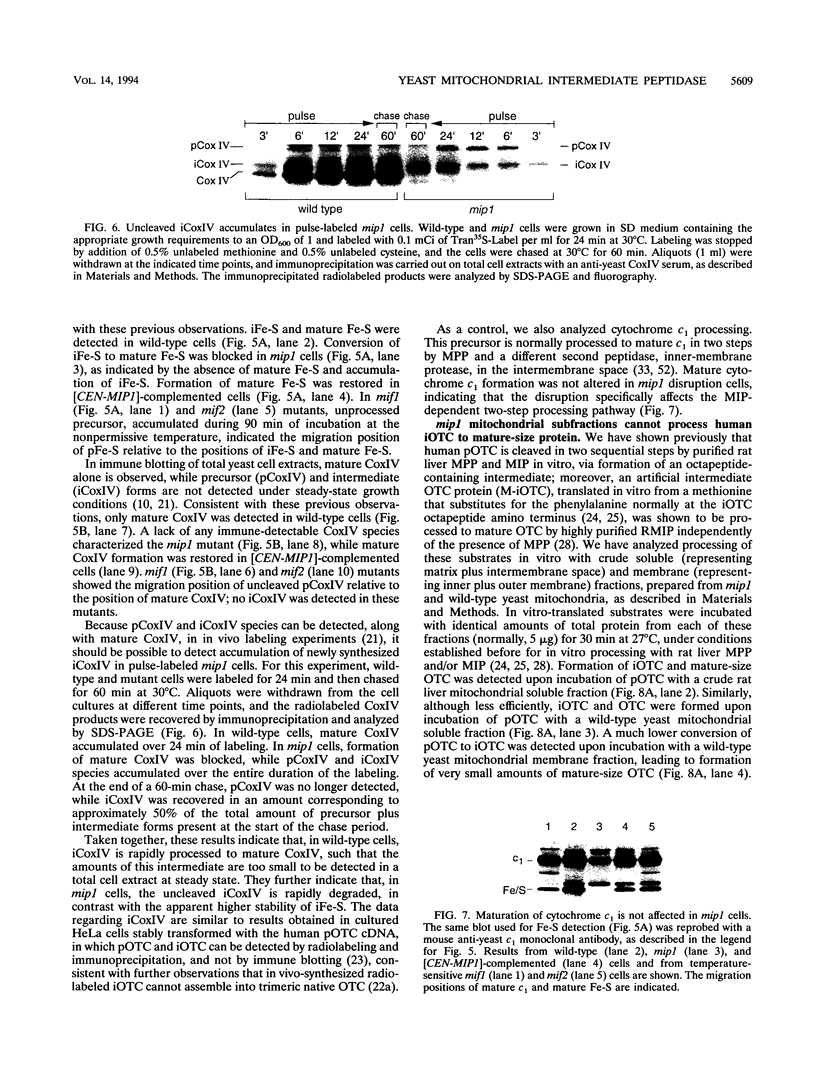
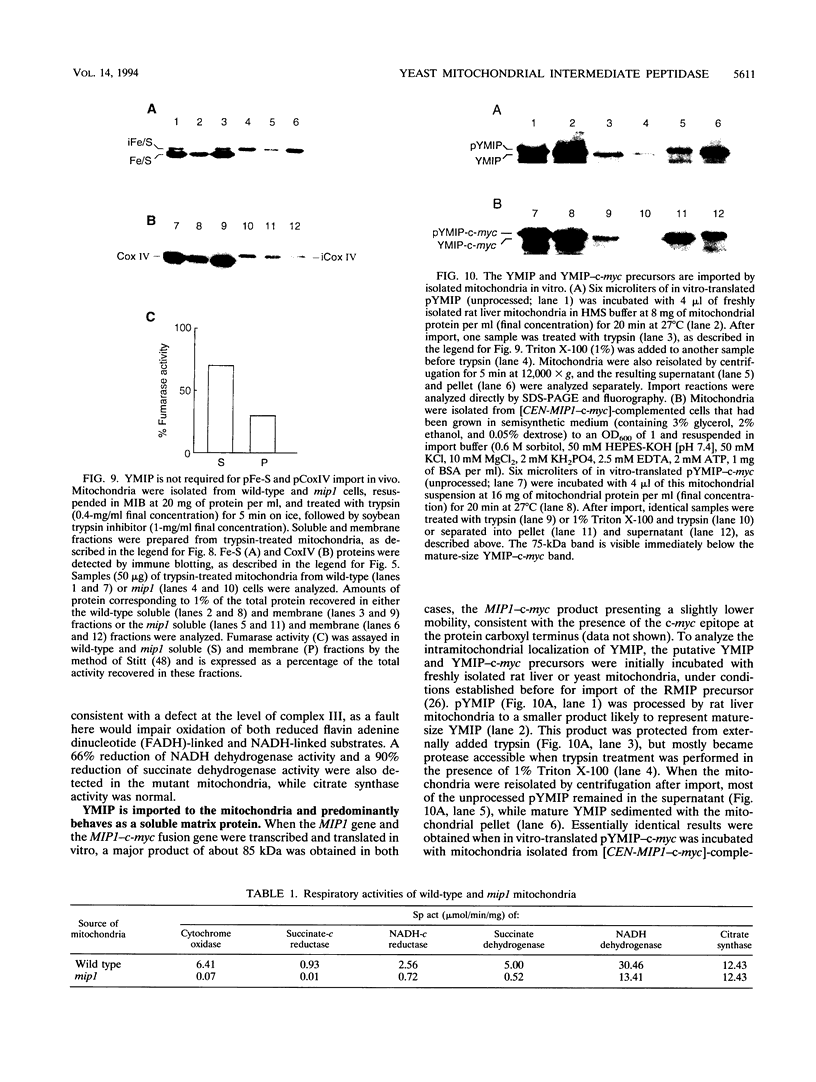
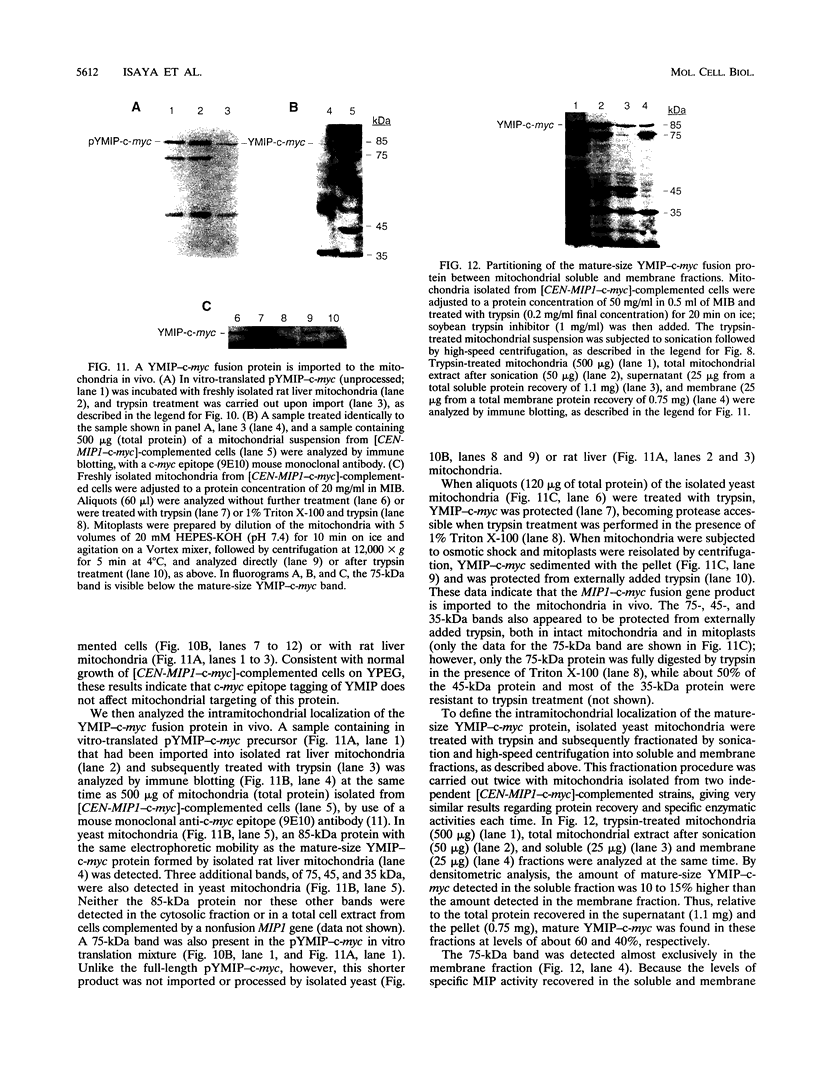
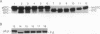Abstract
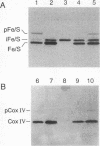
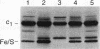
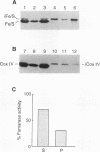
Cleavage of amino-terminal octapeptides, F/L/IXXS/T/GXXXX, by mitochondrial intermediate peptidase (MIP) is typical of many mitochondrial precursor proteins imported to the matrix and the inner membrane. We previously described the molecular characterization of rat liver MIP (RMIP) and indicated a putative homolog in the sequence predicted from gene YCL57w of yeast chromosome III. A new yeast gene, MIP1, has now been isolated by screening a Saccharomyces cerevisiae genomic library with an RMIP cDNA probe. MIP1 predicts a protein of 772 amino acids (YMIP), which is 54% similar and 31% identical to RMIP and includes a putative 37-residue mitochondrial leader peptide. RMIP and YMIP contain the sequence LFHEMGHAM HSMLGRT, which includes a zinc-binding motif, HEXXH, while the predicted YCL57w protein contains a comparable sequence with a lower degree of homology. No obvious biochemical phenotype was observed in a chromosomally disrupted ycl57w mutant. In contrast, a mip1 mutant was unable to grow on nonfermentable substrates, while a mip1 ycl57w double disruption did not result in a more severe phenotype. The mip1 mutant exhibited defects of complexes III and IV of the respiratory chain, caused by failure to carry out the second MIP-catalyzed cleavage of the nuclear-encoded precursors for cytochrome oxidase subunit IV (CoxIV) and the iron-sulfur protein (Fe-S) of the bc1 complex to mature proteins. In vivo, intermediate-size CoxIV was accumulated in the mitochondrial matrix, while intermediate-size Fe-S was targeted to the inner membrane. Moreover, mip1 mitochondrial fractions failed to carry out maturation of the human ornithine transcarbamylase intermediate (iOTC), specifically cleaved by RMIP. A CEN plasmid-encoded YMIP protein restored normal MIP activity along with respiratory competence. Thus, YMIP is a functional homolog of RMIP and represents a new component of the yeast mitochondrial import machinery.
Full text
PDF




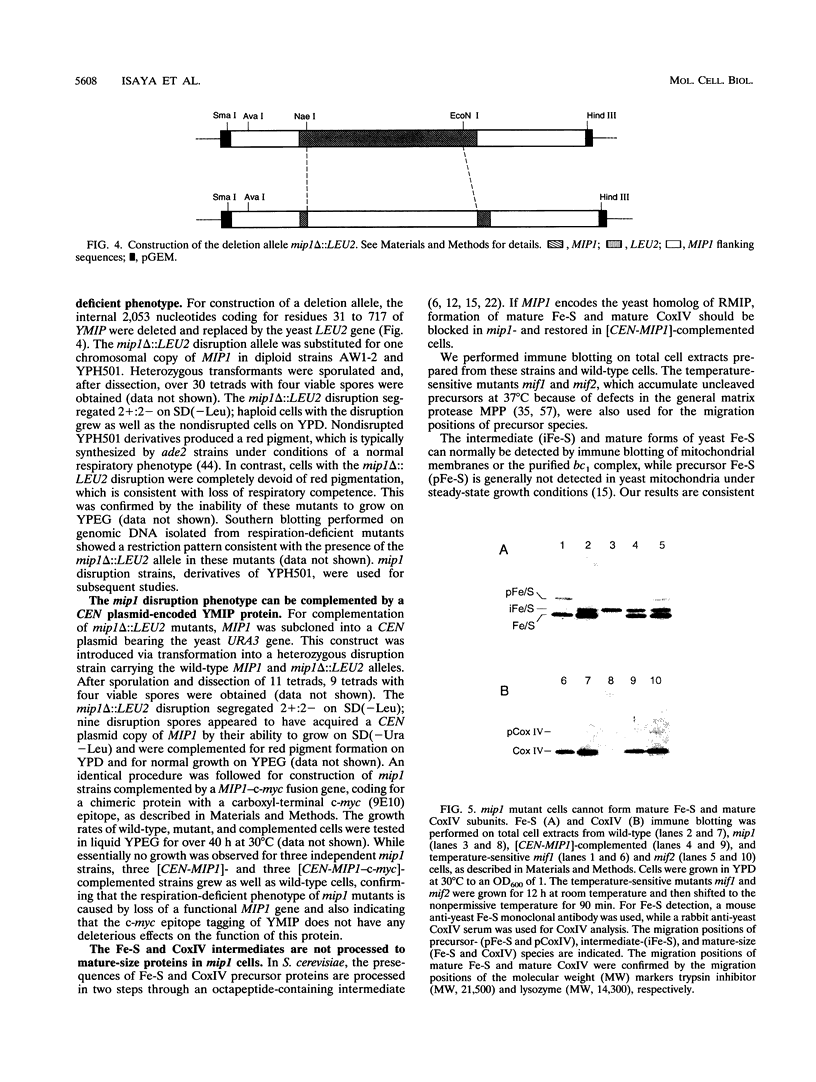





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker K. P., Schatz G. Mitochondrial proteins essential for viability mediate protein import into yeast mitochondria. Nature. 1991 Jan 17;349(6306):205–208. doi: 10.1038/349205a0. [DOI] [PubMed] [Google Scholar]
- Beckmann J. D., Ljungdahl P. O., Trumpower B. L. Mutational analysis of the mitochondrial Rieske iron-sulfur protein of Saccharomyces cerevisiae. I. Construction of a RIP1 deletion strain and isolation of temperature-sensitive mutants. J Biol Chem. 1989 Mar 5;264(7):3713–3722. [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Martin J., Pollock R. A., Kalousek F., Neupert W., Hallberg E. M., Hallberg R. L., Horwich A. L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989 Feb 16;337(6208):620–625. doi: 10.1038/337620a0. [DOI] [PubMed] [Google Scholar]
- Cheng M. Y., Pollock R. A., Hendrick J. P., Horwich A. L. Import and processing of human ornithine transcarbamoylase precursor by mitochondria from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4063–4067. doi: 10.1073/pnas.84.12.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- DiMauro S., Bonilla E., Zeviani M., Nakagawa M., DeVivo D. C. Mitochondrial myopathies. Ann Neurol. 1985 Jun;17(6):521–538. doi: 10.1002/ana.410170602. [DOI] [PubMed] [Google Scholar]
- Dowhan W., Bibus C. R., Schatz G. The cytoplasmically-made subunit IV is necessary for assembly of cytochrome c oxidase in yeast. EMBO J. 1985 Jan;4(1):179–184. doi: 10.1002/j.1460-2075.1985.tb02334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W., Japa S., Beattie D. S. Import of the iron-sulfur protein of the cytochrome b.c1 complex into yeast mitochondria. J Biol Chem. 1990 Sep 25;265(27):16541–16547. [PubMed] [Google Scholar]
- García-Alvarez N., Teichert U., Wolf D. H. Proteinase yscD mutants of yeast. Isolation and characterization. Eur J Biochem. 1987 Mar 2;163(2):339–346. doi: 10.1111/j.1432-1033.1987.tb10805.x. [DOI] [PubMed] [Google Scholar]
- Gavel Y., von Heijne G. Cleavage-site motifs in mitochondrial targeting peptides. Protein Eng. 1990 Oct;4(1):33–37. doi: 10.1093/protein/4.1.33. [DOI] [PubMed] [Google Scholar]
- Graham L. A., Brandt U., Sargent J. S., Trumpower B. L. Mutational analysis of assembly and function of the iron-sulfur protein of the cytochrome bc1 complex in Saccharomyces cerevisiae. J Bioenerg Biomembr. 1993 Jun;25(3):245–257. doi: 10.1007/BF00762586. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Pfanner N., Nicholson D. W., Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989 Jan 18;988(1):1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Schmidt B., Wachter E., Weiss H., Neupert W. Transport into mitochondria and intramitochondrial sorting of the Fe/S protein of ubiquinol-cytochrome c reductase. Cell. 1986 Dec 26;47(6):939–951. doi: 10.1016/0092-8674(86)90809-3. [DOI] [PubMed] [Google Scholar]
- Hawlitschek G., Schneider H., Schmidt B., Tropschug M., Hartl F. U., Neupert W. Mitochondrial protein import: identification of processing peptidase and of PEP, a processing enhancing protein. Cell. 1988 Jun 3;53(5):795–806. doi: 10.1016/0092-8674(88)90096-7. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Hodges P. E., Rosenberg L. E. Survey of amino-terminal proteolytic cleavage sites in mitochondrial precursor proteins: leader peptides cleaved by two matrix proteases share a three-amino acid motif. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4056–4060. doi: 10.1073/pnas.86.11.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Müller U., Schatz G. The first twelve amino acids of a yeast mitochondrial outer membrane protein can direct a nuclear-coded cytochrome oxidase subunit to the mitochondrial inner membrane. EMBO J. 1985 Dec 16;4(13A):3509–3518. doi: 10.1002/j.1460-2075.1985.tb04110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Suda K., Oppliger W., Schatz G. The first twelve amino acids (less than half of the pre-sequence) of an imported mitochondrial protein can direct mouse cytosolic dihydrofolate reductase into the yeast mitochondrial matrix. EMBO J. 1985 Aug;4(8):2061–2068. doi: 10.1002/j.1460-2075.1985.tb03892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaya G., Fenton W. A., Hendrick J. P., Furtak K., Kalousek F., Rosenberg L. E. Mitochondrial import and processing of mutant human ornithine transcarbamylase precursors in cultured cells. Mol Cell Biol. 1988 Dec;8(12):5150–5158. doi: 10.1128/mcb.8.12.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaya G., Kalousek F., Fenton W. A., Rosenberg L. E. Cleavage of precursors by the mitochondrial processing peptidase requires a compatible mature protein or an intermediate octapeptide. J Cell Biol. 1991 Apr;113(1):65–76. doi: 10.1083/jcb.113.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaya G., Kalousek F., Rosenberg L. E. Amino-terminal octapeptides function as recognition signals for the mitochondrial intermediate peptidase. J Biol Chem. 1992 Apr 15;267(11):7904–7910. [PubMed] [Google Scholar]
- Isaya G., Kalousek F., Rosenberg L. E. Sequence analysis of rat mitochondrial intermediate peptidase: similarity to zinc metallopeptidases and to a putative yeast homologue. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8317–8321. doi: 10.1073/pnas.89.17.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalousek F., Hendrick J. P., Rosenberg L. E. Two mitochondrial matrix proteases act sequentially in the processing of mammalian matrix enzymes. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7536–7540. doi: 10.1073/pnas.85.20.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalousek F., Isaya G., Rosenberg L. E. Rat liver mitochondrial intermediate peptidase (MIP): purification and initial characterization. EMBO J. 1992 Aug;11(8):2803–2809. doi: 10.1002/j.1460-2075.1992.tb05347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata S., Nakagawa K., Muta T., Iwanaga S., Davie E. W. Rabbit liver microsomal endopeptidase with substrate specificity for processing proproteins is structurally related to rat testes metalloendopeptidase 24.15. J Biol Chem. 1993 Jun 15;268(17):12498–12503. [PubMed] [Google Scholar]
- Kleiber J., Kalousek F., Swaroop M., Rosenberg L. E. The general mitochondrial matrix processing protease from rat liver: structural characterization of the catalytic subunit. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7978–7982. doi: 10.1073/pnas.87.20.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein J., Scholte H. R., Wit-Peeters E. M. A rapid and simple procedure to deplete rat-liver mitochondria of lysosomal activity. Biochim Biophys Acta. 1970 Dec 8;223(2):432–436. doi: 10.1016/0005-2728(70)90201-x. [DOI] [PubMed] [Google Scholar]
- Nunnari J., Fox T. D., Walter P. A mitochondrial protease with two catalytic subunits of nonoverlapping specificities. Science. 1993 Dec 24;262(5142):1997–2004. doi: 10.1126/science.8266095. [DOI] [PubMed] [Google Scholar]
- Oliver S. G., van der Aart Q. J., Agostoni-Carbone M. L., Aigle M., Alberghina L., Alexandraki D., Antoine G., Anwar R., Ballesta J. P., Benit P. The complete DNA sequence of yeast chromosome III. Nature. 1992 May 7;357(6373):38–46. doi: 10.1038/357038a0. [DOI] [PubMed] [Google Scholar]
- Pollock R. A., Hartl F. U., Cheng M. Y., Ostermann J., Horwich A., Neupert W. The processing peptidase of yeast mitochondria: the two co-operating components MPP and PEP are structurally related. EMBO J. 1988 Nov;7(11):3493–3500. doi: 10.1002/j.1460-2075.1988.tb03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K. M., Lemire B. D. Isolation and nucleotide sequence of the Saccharomyces cerevisiae gene for the succinate dehydrogenase flavoprotein subunit. J Biol Chem. 1992 May 15;267(14):10101–10107. [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A., Behrens M., Scherer P., Pratje E., Michaelis G., Schatz G. Inner membrane protease I, an enzyme mediating intramitochondrial protein sorting in yeast. EMBO J. 1991 Feb;10(2):247–254. doi: 10.1002/j.1460-2075.1991.tb07944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisljar U. Thimet oligopeptidase--a review of a thiol dependent metallo-endopeptidase also known as Pz-peptidase endopeptidase 24.15 and endo-oligopeptidase. Biol Chem Hoppe Seyler. 1993 Feb;374(2):91–100. [PubMed] [Google Scholar]
- Tropschug M., Nicholson D. W., Hartl F. U., Köhler H., Pfanner N., Wachter E., Neupert W. Cyclosporin A-binding protein (cyclophilin) of Neurospora crassa. One gene codes for both the cytosolic and mitochondrial forms. J Biol Chem. 1988 Oct 5;263(28):14433–14440. [PubMed] [Google Scholar]
- Vassarotti A., Chen W. J., Smagula C., Douglas M. G. Sequences distal to the mitochondrial targeting sequences are necessary for the maturation of the F1-ATPase beta-subunit precursor in mitochondria. J Biol Chem. 1987 Jan 5;262(1):411–418. [PubMed] [Google Scholar]
- West A. H., Clark D. J., Martin J., Neupert W., Hartl F. U., Horwich A. L. Two related genes encoding extremely hydrophobic proteins suppress a lethal mutation in the yeast mitochondrial processing enhancing protein. J Biol Chem. 1992 Dec 5;267(34):24625–24633. [PubMed] [Google Scholar]
- Yaffe M. P. Analysis of mitochondrial function and assembly. Methods Enzymol. 1991;194:627–643. doi: 10.1016/0076-6879(91)94046-f. [DOI] [PubMed] [Google Scholar]
- Yang M., Jensen R. E., Yaffe M. P., Oppliger W., Schatz G. Import of proteins into yeast mitochondria: the purified matrix processing protease contains two subunits which are encoded by the nuclear MAS1 and MAS2 genes. EMBO J. 1988 Dec 1;7(12):3857–3862. doi: 10.1002/j.1460-2075.1988.tb03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon A. P., Brändli A. W., Schatz G. The presequences of two imported mitochondrial proteins contain information for intracellular and intramitochondrial sorting. Cell. 1986 Mar 14;44(5):801–812. doi: 10.1016/0092-8674(86)90846-9. [DOI] [PubMed] [Google Scholar]