Abstract
Replication comes with a price. The molecular gymnastics that occur on DNA during its duplication frequently derive to a wide spectrum of abnormalities which are still far from understood. These are brought together under the unifying term “replicative stress” (RS) which likely stands for large and unprotected regions of single-stranded DNA (ssDNA). In addition to RS, recombinogenic stretches of ssDNA are also formed at resected DNA double strand breaks (DSB). Both situations converge on a ssDNA intermediate, which is the triggering signal for a damage situation. The cellular response in both cases is coordinated by a phosphorylation-based signaling cascade that starts with the activation of the ATR (ATM and Rad3-related) kinase. Given that ATR is essential for replicating cells, understanding the consequences of a defective ATR response for a mammalian organism has been limited until recent years. We here discuss on the topic and review the findings that connect ATR to ageing and cancer.
Keywords: ATR, Chk1, cancer, DNA damage response, ageing
1. Introduction
Exogenous and endogenous sources are constantly challenging the integrity of DNA. To protect their genomes, cells have evolved a large network of caretakers which detect, signal and repair the lesions. Among the different types of damage, and due to its potential to promote chromosome rearrangements, DSB are particularly toxic for the cell [1]. Work done particularly in the last 15 years, and which arguably started with the identification of ATM (Ataxia Telangiectasia Mutated) as the protein kinase that was mutated in patients of Ataxia Telangiectasia [2], has provided a fairly comprehensive view of how cells respond to DSB. Their presence triggers a coordinated signaling response which stimulates DNA repair and limits the expansion of the damaged cell by apoptotic or cytostatic mechanisms. This network is what is generally known as the “DNA damage response” (DDR) [3].
Most of the early works on the DDR focused on the understanding of how ATM responded to DSB. However, we now know that the DDR is not only stimulated by DSB, but also by regions of ssDNA that can be generated both at stalled replication forks (RF) and at resected DSB. This second response is the one coordinated by the ATM-related kinase ATR, and is critical to protect the genome from RS [4]. In the next pages, we provide an overview of the ATR-dependent DDR, and discuss the recent data on the impact that a deficient ATR response has on a mammalian organism.
2. The DDR brotherhood: ATM, ATR and DNA-PK
ATR and ATM belong to the PIKK (phosphatidylinositol-3-kinase related kinases) family [5]. Whereas related to PI3K, these enzymes are protein kinases and do not phosphorylate lipids. A third member of the family, the catalityc subunit of the DNA-dependent protein kinase holoenzyme (DNA-PKcs), is also activated by DSB. However, and in contrast to ATM and ATR, its role seems to be restricted to the stimulation of DNA Repair by Non-Homologous End-Joining (NHEJ) rather than in coordinating a global signaling response [6]. Noteworthy, recent evidences suggest that DNA-PK might also have some role in checkpoint signaling, which is only evident in the absence of ATM [7, 8]. In fact, the simultaneous elimination of ATM and DNA-PK is essential for mouse development [9]. Other members of the family include kinases involved in nutrient signaling such as mTOR (mammalian Target Of Rapamycin) or mRNA metabolism as SMG1 (Suppressor with Morphological effect on Genitalia).
Members of the family are large proteins (more than 300 kDa), with a related domain structure. All of them contain a large region of repeated domains in the N-terminus (HEAT domains); followed by a FAT domain, a catalytic domain homologue to that in PI-3-Kinase (PI3K), a PIKK regulatory domain (PRD) and a FATC domain at the C-terminus [10]. The HEAT, FAT and FATC domains are thought to be involved in favoring the interactions of these proteins with their substrates and modulators [11]. In contrast, the recently described PRD (at least in the case of ATR) seems to be directly involved in stimulating the kinase activity [12]. Whereas the basal activity does not require an intact PRD, this is required for ATR stimulation by its alosteric activator TopBP1 [13]. There are hints suggesting that the PRD might also operate in other members of the family. However, it remains to be determined to what extent the PRD works similarly on the other PIKKs.
Upon activation, ATM and ATR coordinate a cellular response which affects a wide variety of cellular processes including transcription, replication, differentiation, apoptosis or cell division. With this broad scope, it is not surprising that the number of ATM/ATR targets is very big, with more than 700 hundred of substrates having been identified in one single proteomic search [14]. The majority of the targets can be phosphorylated by both kinases, so that the cellular outcomes to ATM or ATR activation are shared to a large extent. However, the two key checkpoint-related substrates, Chk1 and Chk2, are specific of ATR or ATM, respectively [15]. Accordingly, Chk1 phosphorylation is performed by ATR even in response to ionizing radiation (IR) [16, 17]. In contrast, ATR fails to phosphorylate Chk2 even when its activity is unleashed by artificial means [18]. The reason for this strict specificity for Chk1/2 seems to be given by mediator proteins that restrict the interaction of ATM and ATR with checkpoint kinases. For example, Chk1 phosphorylation by ATR is mediated by the interacting protein Claspin [19]. Whether a “Claspin-like” protein mediates the ATM/Chk2 relationship is still not known, but we favor this idea given that ATR is unable to phosphorylate Chk2 in vivo, even when its activity is stimulated by TopBP1 [18].
In addition to their specificity for checkpoint kinases, the key difference between ATM and ATR is the signal that activates them. ATM is activated exclusively by DSBs, which can arise from exogenous (IR, genotoxic drugs) or endogenous (reactive oxygen species, eroded telomeres, intermediates of immune and meiotic recombination, etc.) sources. Accordingly, ATM deficiency in both mouse and humans concurs with sterility, immune deficiency and a high sensitivity to radiation [1]. In contrast, ATR is activated by ssDNA, which can occur at persistent DSB, but more extensively on stalled RFs [4]. In addition, whereas DSB activate ATM at any stage of the cell cycle, the response of ATR to DSB is restricted to S and G2 phases [16, 17]. The reason for this specificity is that the resection of DSB ends is restricted to S/G2, likely influenced by CDK-mediated stimulation of resection-regulators, such as Mre11, Exo1 and CtIP [20-22]. Interestingly, activation of ATR in G1 is sufficient to activate the G1/S checkpoint in ATM deficient cells [18], once again reinforcing that both ATM and ATR trigger a similar response, the main difference being when or where these are activated. We now center our work in describing the molecular mechanism of ATR activation in more detail.
3. ATR activation step by step
As mentioned, RPA-coated ssDNA is the actual triggering signal for ATR activation [23]. Previous to this work in mammalian cells, work done in yeast had already identified RPA and ssDNA as important mediators of the checkpoint response [24, 25]. The actual role of ssDNA is to recruit ATR to the sites of the lesion by direct binding of RPA-ssDNA to the ATR partner ATRIP (ATR interacting protein) [26]. Of note, ATR and ATRIP seem to be constitutively bound, so that free ATR might not exist within cells. In fact, as is the case with many constitutive complexes, depletion of either ATR or ATRIP also depletes the other member. In this scenario, situations that lead to the generation of ssDNA are triggers for ATR activation. These include RS, resected DSB, telomeres and gaps generated during Nucleotide Excision Repair (NER) [4]. Among these situations, is fair to say that RS is the less understood. This term unifies all “problems” that arise at a RF and lead to recombinogenic stretches of ssDNA. One nice mechanistic proposal was the one made by Karlene Cimprich which suggested that RS could due to uncoupling helicase and polymerase activities [27]. It would be interesting to determine whether this is a mechanism by which oncogenes promote RS and DDR activation [28]. However, this is not the only way to accumulate ssDNA at the RF and work done in yeast is paving the way for the discovery of such structures [29].
ssDNA accumulation is only the first step towards activating ATR. In Figure 1 we provide a model that summarizes the stepwise activation of ATR. First, RPA-coated ssDNA recruits ATR-ATRIP complex [26]. This brings ATR to the lesion but in order to be activated it still needs to interact with its activator TopBP1. This occurs by an independent cascade or recruitments that are also initiated by ssDNA-RPA. The first step is to recruit Rad17, which is conserved with members of the Replication Factor C (RFC) that loads PCNA onto primed DNA during replication. Then, the clamp loader Rad17 loads the PCNA-like heterotrimeric ring 9-1-1 (Rad9-Rad1-Hus1) to the damage sites [30]. Finally, 9-1-1 brings TopBP1 in close proximity to ATR-ATRIP, unleashing its activity [31]. In addition to bringing TopBP1, Rad17 also directs the loading of Claspin to ssDNA [32]. Thus, the Rad17/9-1-1 complex not only coordinates the loading of ATR but is also is key to translate ATR activation into a checkpoint signal.
Figure 1. Activating ATR.
The figure illustrates the steps that lead to the activation of ATR. The common signal for initiating an ATR dependent response is the presence of ssDNA. Due to the work of replicative helicases, ssDNA is most abundant during S phase, but there are other situations that can lead to ssDNA such as exposing the telomeric overhang, double-nicks generated during NER and resected DSB. ssDNA is rapidly coated by stabilizing proteins. RPA-coated ssDNA independently brings together the two components of the pathway. One one hand, RPA recruits ATRIP, which is in a complex with ATR and thus brings the kinase to the lesion. On the other hand, RPA recruits Rad17, which loads the 9-1-1. This complex is then essential to recruit and position the alosteric activator TopBP1. On close proximity, TopBP1 activates ATR.
4. Consequences of ATR signaling
4.1 Checkpoints: All about Chk1
The first and mostly studied role of ATR is on regulating checkpoints induced by DNA damage. In fact, the cloning of ATR identified this protein as the ortologue of Sacharomyces cerevisiae Mec1p, which is the main checkpoint regulator in yeast [33]. In spite of the numerous substrates of ATR, the key event that translates ATR activity into a checkpoint signal may mostly depend on one single target, which is the phosphorylation and activation of Chk1 [34]. Like ATR, Chk1 activation is restricted to S and G2, so that it only regulates S and G2 checkpoints [35]. Complementarily, whereas Chk2 activation by ATM has a reported role in the G1/S checkpoint [36, 37], no clear defect in the S and G2/M checkpoints were detected in Chk2 deficient mouse cells [38]. Notably, whereas most ATR targets are phosphorylated at the lesion site the phosphorylation of Chk1 drives activated Chk1 away from chromatin, consistent with the notion of being the transmitter of the alarm signal [39]. Once activated and free from chromatin, Chk1 activity translates into growth arrest by modulating the function of CDK regulators [39, 40].
Ultimately, the way by which Chk1 activation leads to growth arrest is through the control of inhibitory phosphorylations on CDKs. On one hand, Chk1 phosphorylates and activates Wee1; which is the kinase that delivers inhibitory phosphorylations on CDKs [41]. On the other hand, Chk1 also targets Cdc25A, which is the phosphatase that counteracts Wee1-mediated phosphorylations on CDKs. This phosphorylation marks Cdc25A for its degradation by the proteasome, so that depletion of Cdc25A is an early event after the exposure to DNA damage [42, 43]. Significantly, recent data suggest that the Cdc25A regulation is related to the essential role of Chk1. Chk1 depletion leads to massive amounts of DNA damage and cellular lethality [44]. However, simultaneous depletion of Cdc25A significantly alleviates the deleterious effects of Chk1 knockdown in human cells [45]. These observations might be very relevant, since they offer a cue to explore how ATR/Chk1 losses could be tolerated in vivo at the cellular or even organism level.
Whereas of pivotal importance, the ATR/Chk1/Cdc25A pathway is not the only way by which ATR activation leads to growth arrest. For instance, recent work identified NEK11 as a Chk1-regulated kinase which also influences Cdc25A degradation [46]. In addition, all of the above are related to the early activation of cell-cycle checkpoints. Upon more persistent damage, ATR and Chk1 also phosphorylate p53, which contributes to the upregulation of p53 levels and becomes important for the maintenance of the cell-cycle arrest and/or the activation of apoptosis [47]. This pathway might be critical for the induction of senescence under conditions of persistent DNA damage, which could be the case for the damage induced by oncogenes [48-50]. In agreement with this idea, prolonged ATR activation, even in the absence of DNA damage, is enough to promote senescence in a p53-dependent fashion [18]. To what extent the ATR-p53 axis is of relevance for the protection against human cancer is still a matter of debate (discussed below).
4.2 RF dynamics: Collapse and re-start
In addition to the control of cell-cycle transitions, ATR has an essential and local function regulating the stability of the RF. Most of the knowledge came originally from yeast studies and, to some extent, have also been observed in mammals. The original observation started with the analysis of how RF progression in yeast cells was affected by alkylating agents [51]. In wild type yeast, RFs stalled when cells were grown in the presence of methyl methanesulphonate (MMS). In contrast, the presence of MMS led to a collapse of RF on checkpoint mutants (i.e. defective in the ATR ortologue Mec1), which greatly compromised cell viability. To this date, it is still a mystery what “collapse” actually means or how it arises; but its outcome is that DSB are generated at the RF. We now know that RF can also collapse in wild type cells upon prolonged stalling. This has been nicely shown in mammalian cells where the nuclease Mus81 seems to be responsible for breaking the stalled forks [52]. An attractive hypothesis is that Mus81-generated DSB are created in order to restart the fork by promoting homologous recombination (HR), which would be the actual meaning of collapse. Given that RF collapse is more abundant on ATR/Chk1 mutants, one possibility is that the ATR pathway restricts the activity of Mus81 at RF and therefore limits promiscuous HR. Significantly, despite to multiple studies on the role of ATR regulating fork stability the targets responsible for this remain largely elusive [53].
Once again, Chk1 seems to be a key factor in regulating the stability of the RF. Accordingly, Chk1 depletion leads to massive DNA damage in replicating cells [44] and, like ATR [54, 55], Chk1 deletion is early embryonic lethal in the mouse [34, 56]. Noteworthy, severe ATR hypomorphism is tolerated in humans but leads to a disease known as the Seckel Syndrome which is associated with dwarfism and craniofacial abnormalities [57]. We recently modeled this syndrome in the mouse and showed that low levels of ATR lead a dramatic increase in DNA damage during replication, most prominently during embryogenesis [58]. To what extent Chk1 is responsible for the phenotypes associated to the dampening of ATR function in mammals remains to be determined.
There are evidences suggesting that replisome regulation by ATR might also operate through Chk1-independent signaling [59]. For instance, recent data showed that the yeast Claspin ortologue Mrc1 regulates RF dynamics in a Mec1-dependent but Chk1-independent fashion [60]. In addition, helicases of the RecQ family such as Sgs1 in yeast, and BLM and WRN helicases in human, have been shown to be critical targets of ATR in safeguarding the integrity of RF [61-63]. However, it should also be noted that Chk1 is non essential in yeast, so that it is hard to translate the role of Chk1 to mammalian cells. Moreover, it remains possible that helicases are also influenced by Chk1 in mammalian cells so that the intimate details of how the ATR-helicase connection operates at the RF are far from being solved.
In addition to maintaining the stability of the fork, ATR is also involved in the regulation of origins of replication. Whereas the activation of ATR promotes a dampening of replication rates and a global inhibition of origin firing, it can also promote new origin firing near stalled RF [4, 64]. These apparent contradictory functions may ensure complete genome replication once DNA damage is repaired or cells are adapted to it [64]. Importantly, ATR limits origin activity even in the absence of exogenous DNA damage [65]. Their observations led the authors to propose that an “ATR signaling domain” might always be generated at each origin, which would be responsible for preventing the firing of more origins in the vicinity. A very attractive proposal that deserves to be further explored. As in the case of RF stability, and even though many replication-related targets have been discovered, the means by which ATR regulates origin dynamics are far from understood.
4.3 A role for ATR in DNA repair
Studies of the role of ATR in DNA repair have been limited by the essential nature of the kinase. In what regards to DSB, existing data suggest that ATR is more involved in the repair of DNA breaks by HR rather than NHEJ [66]. In this regard, ATR regulates BRCA1 in somatic and meiotic cells [67-69]. In addition, Chk1 has been shown to stimulate HR by phosphorylation of Rad51 [70]. However, it is possible that the role of ATR in stimulating HR is restricted to DSB arising at RF. In fact, ATR only confers a mild sensitivity to IR, so that the relevance of the ATR-response to DSB is not clear.
Besides HR, ATR might play an important role on Fanconi Anemia (FA) pathway which is involved in the repair of DNA crosslinks. Exposure of ATR deficient cells to mytomicin-C leads to the accumulation of radial structures on chromosomes, a hallmark of FA. This is thought to be due to a failure to monoubiquitinate FANCD2, a key event for crosslink repair [71]. Moreover, a recent work also showed a reverse connection between ATR and FA proteins; namely that FancM is necessary for the activation of ATR [72]. The links between ATR and FA are still being discovered, and it is likely that they might not be completely separated from the roles of ATR in HR.
5. The role of ATR beyond the single cell
Organisms can avoid exposure to external agents that damage DNA such as sunlight, but one thing they cannot avoid is to replicate the DNA of their cells. As explained, there is no such a thing as “damage-less” replication and ATR/Chk1 signaling is necessary to prevent the accumulation of RS even in the absence of exogenous DNA damage. This makes the ATR response essential for embryonic development and limited the use of knockout mouse strains [54, 55]. Recent developments in modeling ATR function in the mouse have allowed evaluating the consequences of a defective ATR response. We are now beginning to understand how the accumulation of RS impacts mammalian physiology. Previous work has already reviewed the phenotypes associated to ATR hypomorphism such as craniofacial abnormalities [73]. We here focus specifically on how the RS-response provided by ATR impacts on ageing and cancer development.
5.1. ATR and cancer: a dose effect
The DDR has an unquestionable role in cancer. Accordingly, mutations in DDR members such as ATM or Chk2 have been found in human tumors and mouse models of the DDR are frequently associated with an increased tumor incidence. The question is HOW the DDR protects us from tumors. As mentioned, the DDR potentiates DNA repair and activates checkpoints. Which of the two activities is essential for cancer protection? The original view was simple. Cancer arises from mutations and DNA repair activities obviously limit their accumulation. In this view, DDR members would be caretakers. However, a new view has emerged [28].
Extensive analyses of human biopsies found evidences of an activated DDR in early stages of malignant transformation [74, 75]. The authors proposed that this could reflect an activation of the DDR by some sort of DNA damage generated by oncogenes, which would trigger the checkpoints and limit the expansion of these premalignant cells. In this context, the DDR would play the role as a gatekeeper. Later studies reported that, in fact, oncogene activation can lead to DNA damage which will promote a DDR-dependent senescent response [48-50]. The key issue is that the damage generated by oncogenes is linked to replication. Therefore, although much emphasis is put on the ATM response, it is reasonable to believe that ATR should be the main responder to the DNA damage induced by oncogenes. Nevertheless, since as explained above persistent RS can also derive into fork collapse and DSB formation, ATM would also be finally activated by this secondary damage. In agreement with this, ATM deletion accelerates lymphomas initiated by a c-myc transgene [76, 77]. However, we would want to note that ATM has a very limited role in oncogene-induced senescence in a mouse model of Ras-induced lung tumors [78]. It is likely that not all oncogenes, and not all tumors, are associated to the same degree of RS, so that the oncogene-induced DNA damage model might only apply for rapidly proliferating tumors (as is the case for myc-induced lymphomas).
In what regards specifically to the RS-response, both ATR and Chk1 are halpoinsufficient tumor suppressors in the mouse. The absence of one allele leads to a modest increase in tumor incidence [34, 54], which is greatly enhanced in combination with other cancer-prone mutations [79, 80]. In addition, ATR activation leads to senescence [18], so that potentiating the ATR-response could limit the expansion of premalignant cells. Still, direct proof of the role of ATR/Chk1 in the oncogene-induced DDR model is still missing. Paradoxically, even though half dosage of Chk1 promotes tumors, Chk1 inhibitors are currently being tested for cancer treatment [81]. The reason for this is that Chk1 inhibition is particularly toxic for cells lacking p53, providing one example of synthetic lethality [82]. But why is that and what about potential ATR inhibitors?
The reason for these contradictory observations lies on the levels (Figure 2). While lowering the amounts of ATR/Chk1 by half might lead to more mutations and cancer, a severe inhibition of this response might be particularly deleterious for cells that replicate very fast, such as is the case for many cancer cells. In this regard, and after analyzing more than 200 mutant mice, we have never observed a tumor on ATR hypomorphic mice [58]. Moreover, and as it has also been observed with a conditional knockout allele, deletion of p53 not only does not promote tumors but is actually very toxic in conditions of low ATR levels [58, 83]. In other words, and as shown for Chk1 inhibitors, ATR and p53 loss display a synthetic lethal interaction. Despite numerous efforts, there are no ATR inhibitors good enough for clinic use. It is possible, however, that the cancer protective effects exerted by caffeine (a non-specific PI3-kinase inhibitor) could be related to the inhibition of ATR [84, 85]. To what extent ATR or Chk1 show also synthetic lethal interactions with other cancer related mutations remains to be probed. Our prediction is that such inhibitors would be of particular use for any tumor that has high replication rates and, as a consequence, high constitutive amounts of RS.
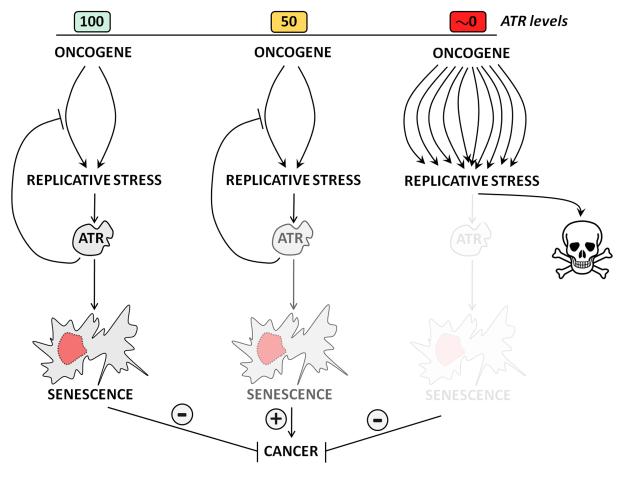
Figure 2. ATR and cancer: Friend or Foe?
ATR can protect from cancer but, at the same time, its inhibition might be used for the killing of cells with high levels of RS. On one hand, the checkpoint response of ATR might limit the expansion of precancerous cells in which oncogenes generate RS (green). In this context, a partial reduction of ATR might allow the expansion of pretumoral cells and thus lead to cancer development (red). However, if ATR levels were very limiting (for instance, with the use of an inhibitor) this would generate lethal amounts of RS and thus lead to cell lethality (red). Importantly, such an approach would be particularly toxic for cells with high levels of RS, such as those expressing oncogenes or lacking tumor suppressors.
5.2. Organism senescence: Replication-driven ageing
Ageing research has revealed that the accumulation of DNA damage during lifetime is probably the major driving force of the ageing process [86, 87]. In agreement with this, several studies have shown that aged tissues or stem cells (SC) present an activated DDR [88, 89], and DDR-related diseases frequently suffer from premature ageing. However, which types of DNA damage (RS, eroded telomeres, ROS…) are most relevant for normal ageing remains to be understood.
In regard to ATR, constitutive ATR hypomorphism or conditional deletion of ATR in adult mice leads to accelerated ageing [58, 90]. Likewise, ageing-associated phenotypes have also been noted in human patients of the Seckel Syndrome, some of whom present ATR hypomorphism [57]. It is possible that both situations lead to ageing due to different mechanisms. Conditional ATR elimination in adult mice is lethal for proliferating cells, so that it promotes the exhaustion of SC in tissues with high turnover rates [90]. In contrast, ATR-Seckel mice display very high levels of RS during embryogenesis, which might affect SC niches and compromise ageing for the later life of the animals [58]. This led us to propose the concept of “intrauterine programming of ageing” (discussed in [91]), which states that stresses that affect embryonic development (i.e. RS) could accelerate ageing rates. Of course, mammals are not going to replicate faster or more frequently than during embryogenesis, which explains why a proficient ATR response is particularly critical at this stage. The concept of intrauterine programming has important implications since it suggests that strategies directed exclusively to the intrauterine period could be worth to be explored as life-extending strategies. Notoriously, p53 deletion accelerated ageing phenotypes in both ATR mouse models, once again as evidence of the synthetic lethal interaction described above [58, 83]. Of course, all of these are extreme models of ATR hypomorphism and RS. To what extent RS contributes to natural ageing is also not known. Perhaps a way to answer this question is to determine whether a better RS-response is able to extend lifespan in a mammalian model.
6. Concluding remarks
We now have a rudimentary knowledge of what RS is, and of the consequences for an organism of an improper ATR-response. The dual role of ATR in cancer suggests that inhibitors of ATR and Chk1 could be used for the killing of tumors with high levels of RS, but having in mind that an insufficient inhibition might be tumor prone. This is of course no different to any other genotoxic chemotherapy, and it offers the advantage of synthetic lethality with p53 deficient cells. Still, these facts should always be considered when thinking about such an approach. In what regards to ageing, the question is whether we could ever use the information we’ve learned about ATR and RS to live longer, but of course, still being able to replicate…
ACKNOWLEDGEMENTS
Work in O. F.’s laboratory is supported by grants from the Spanish Ministry of Science (CSD2007-00017 and SAF2008-01596), Miguel Catalan Award from the Community of Madrid, EMBO Young Investigator Programme and the European Research Council (ERC-210520).
REFERENCES
- [1].Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle DA, Smith S, Uziel T, Sfez S, Ashkenazi M, Pecker I, Frydman M, Harnik R, Patanjali SR, Simmons A, Clines GA, Sartiel A, Gatti RA, Chessa L, Sanal O, Lavin MF, Jaspers NG, Taylor AM, Arlett CF, Miki T, Weissman SM, Lovett M, Collins FS, Shiloh Y. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- [3].Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- [4].Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- [6].Meek K, Dang V, Lees-Miller SP. DNA-PK: the means to justify the ends? Adv Immunol. 2008;99:33–58. doi: 10.1016/S0065-2776(08)00602-0. [DOI] [PubMed] [Google Scholar]
- [7].Callen E, Jankovic M, Wong N, Zha S, Chen HT, Difilippantonio S, Di Virgilio M, Heidkamp G, Alt FW, Nussenzweig A, Nussenzweig M. Essential role for DNA-PKcs in DNA double-strand break repair and apoptosis in ATM-deficient lymphocytes. Mol Cell. 2009;34:285–297. doi: 10.1016/j.molcel.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stiff T, O’Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- [9].Gurley KE, Kemp CJ. Synthetic lethality between mutation in Atm and DNA-PK(cs) during murine embryogenesis. Curr Biol. 2001;11:191–194. doi: 10.1016/s0960-9822(01)00048-3. [DOI] [PubMed] [Google Scholar]
- [10].Lovejoy CA, Cortez D. Common mechanisms of PIKK regulation. DNA Repair (Amst) 2009;8:1004–1008. doi: 10.1016/j.dnarep.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9:759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- [12].Mordes DA, Glick GG, Zhao R, Cortez D. TopBP1 activates ATR through ATRIP and a PIKK regulatory domain. Genes Dev. 2008;22:1478–1489. doi: 10.1101/gad.1666208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kumagai A, Lee J, Yoo HY, Dunphy WG. TopBP1 activates the ATR-ATRIP complex. Cell. 2006;124:943–955. doi: 10.1016/j.cell.2005.12.041. [DOI] [PubMed] [Google Scholar]
- [14].Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- [15].Chen Y, Poon RY. The multiple checkpoint functions of CHK1 and CHK2 in maintenance of genome stability. Front Biosci. 2008;13:5016–5029. doi: 10.2741/3060. [DOI] [PubMed] [Google Scholar]
- [16].Cuadrado M, Martinez-Pastor B, Murga M, Toledo LI, Gutierrez-Martinez P, Lopez E, Fernandez-Capetillo O. ATM regulates ATR chromatin loading in response to DNA double-strand breaks. J Exp Med. 2006;203:297–303. doi: 10.1084/jem.20051923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jackson SP. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- [18].Toledo LI, Murga M, Gutierrez-Martinez P, Soria R, Fernandez-Capetillo O. ATR signaling can drive cells into senescence in the absence of DNA breaks. Genes Dev. 2008;22:297–302. doi: 10.1101/gad.452308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kumagai A, Dunphy WG. Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol Cell. 2000;6:839–849. doi: 10.1016/s1097-2765(05)00092-4. [DOI] [PubMed] [Google Scholar]
- [20].Paull TT, Gellert M. The 3′ to 5′ exonuclease activity of Mre 11 facilitates repair of DNA double-strand breaks. Mol Cell. 1998;1:969–979. doi: 10.1016/s1097-2765(00)80097-0. [DOI] [PubMed] [Google Scholar]
- [21].Nimonkar AV, Ozsoy AZ, Genschel J, Modrich P, Kowalczykowski SC. Human exonuclease 1 and BLM helicase interact to resect DNA and initiate DNA repair. Proc Natl Acad Sci U S A. 2008;105:16906–16911. doi: 10.1073/pnas.0809380105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sartori AA, Lukas C, Coates J, Mistrik M, Fu S, Bartek J, Baer R, Lukas J, Jackson SP. Human CtIP promotes DNA end resection. Nature. 2007;450:509–514. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- [24].Garvik B, Carson M, Hartwell L. Single-stranded DNA arising at telomeres in cdc13 mutants may constitute a specific signal for the RAD9 checkpoint. Mol Cell Biol. 1995;15:6128–6138. doi: 10.1128/mcb.15.11.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lee SE, Moore JK, Holmes A, Umezu K, Kolodner RD, Haber JE. Saccharomyces Ku70, mre11/rad50 and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell. 1998;94:399–409. doi: 10.1016/s0092-8674(00)81482-8. [DOI] [PubMed] [Google Scholar]
- [26].Cortez D, Guntuku S, Qin J, Elledge SJ. ATR and ATRIP: partners in checkpoint signaling. Science. 2001;294:1713–1716. doi: 10.1126/science.1065521. [DOI] [PubMed] [Google Scholar]
- [27].Byun TS, Pacek M, Yee MC, Walter JC, Cimprich KA. Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 2005;19:1040–1052. doi: 10.1101/gad.1301205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319:1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- [29].Branzei D, Foiani M. Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol. 11:208–219. doi: 10.1038/nrm2852. [DOI] [PubMed] [Google Scholar]
- [30].Yang XH, Zou L. Recruitment of ATR-ATRIP, Rad17, and 9-1-1 complexes to DNA damage. Methods Enzymol. 2006;409:118–131. doi: 10.1016/S0076-6879(05)09007-5. [DOI] [PubMed] [Google Scholar]
- [31].Lee J, Kumagai A, Dunphy WG. The Rad9-Hus1-Rad1 checkpoint clamp regulates interaction of TopBP1 with ATR. J Biol Chem. 2007;282:28036–28044. doi: 10.1074/jbc.M704635200. [DOI] [PubMed] [Google Scholar]
- [32].Wang X, Zou L, Lu T, Bao S, Hurov KE, Hittelman WN, Elledge SJ, Li L. Rad17 phosphorylation is required for claspin recruitment and Chk1 activation in response to replication stress. Mol Cell. 2006;23:331–341. doi: 10.1016/j.molcel.2006.06.022. [DOI] [PubMed] [Google Scholar]
- [33].Cimprich KA, Shin TB, Keith CT, Schreiber SL. cDNA cloning and gene mapping of a candidate human cell cycle checkpoint protein. Proc Natl Acad Sci U S A. 1996;93:2850–2855. doi: 10.1073/pnas.93.7.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, Donehower LA, Elledge SJ. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- [35].Huertas P, Jackson SP. Human CtIP mediates cell cycle control of DNA end resection and double strand break repair. J Biol Chem. 2009;284:9558–9565. doi: 10.1074/jbc.M808906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hirao A, Cheung A, Duncan G, Girard PM, Elia AJ, Wakeham A, Okada H, Sarkissian T, Wong JA, Sakai T, De Stanchina E, Bristow RG, Suda T, Lowe SW, Jeggo PA, Elledge SJ, Mak TW. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol. 2002;22:6521–6532. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Takai H, Naka K, Okada Y, Watanabe M, Harada N, Saito S, Anderson CW, Appella E, Nakanishi M, Suzuki H, Nagashima K, Sawa H, Ikeda K, Motoyama N. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 2002;21:5195–5205. doi: 10.1093/emboj/cdf506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fernandez-Capetillo O, Chen HT, Celeste A, Ward I, Romanienko PJ, Morales JC, Naka K, Xia Z, Camerini-Otero RD, Motoyama N, Carpenter PB, Bonner WM, Chen J, Nussenzweig A. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat Cell Biol. 2002;4:993–997. doi: 10.1038/ncb884. [DOI] [PubMed] [Google Scholar]
- [39].Smits VA, Reaper PM, Jackson SP. Rapid PIKK-dependent release of Chk1 from chromatin promotes the DNA-damage checkpoint response. Curr Biol. 2006;16:150–159. doi: 10.1016/j.cub.2005.11.066. [DOI] [PubMed] [Google Scholar]
- [40].Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–429. doi: 10.1016/s1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- [41].Guardavaccaro D, Pagano M. Stabilizers and destabilizers controlling cell cycle oscillators. Mol Cell. 2006;22:1–4. doi: 10.1016/j.molcel.2006.03.017. [DOI] [PubMed] [Google Scholar]
- [42].Jin J, Shirogane T, Xu L, Nalepa G, Qin J, Elledge SJ, Harper JW. SCFbeta-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev. 2003;17:3062–3074. doi: 10.1101/gad.1157503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Xiao Z, Chen Z, Gunasekera AH, Sowin TJ, Rosenberg SH, Fesik S, Zhang H. Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents. J Biol Chem. 2003;278:21767–21773. doi: 10.1074/jbc.M300229200. [DOI] [PubMed] [Google Scholar]
- [44].Syljuasen RG, Sorensen CS, Hansen LT, Fugger K, Lundin C, Johansson F, Helleday T, Sehested M, Lukas J, Bartek J. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol Cell Biol. 2005;25:3553–3562. doi: 10.1128/MCB.25.9.3553-3562.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Beck H, Nahse V, Larsen MS, Groth P, Clancy T, Lees M, Jorgensen M, Helleday T, Syljuasen RG, Sorensen CS. Regulators of cyclin-dependent kinases are crucial for maintaining genome integrity in S phase. J Cell Biol. 188:629–638. doi: 10.1083/jcb.200905059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Melixetian M, Klein DK, Sorensen CS, Helin K. NEK11 regulates CDC25A degradation and the IR-induced G2/M checkpoint. Nat Cell Biol. 2009;11:1247–1253. doi: 10.1038/ncb1969. [DOI] [PubMed] [Google Scholar]
- [47].Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- [48].Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Orntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VG. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- [49].Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre M, Nuciforo PG, Bensimon A, Maestro R, Pelicci PG, d’Adda di Fagagna F. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- [50].Mallette FA, Gaumont-Leclerc MF, Ferbeyre G. The DNA damage signaling pathway is a critical mediator of oncogene-induced senescence. Genes Dev. 2007;21:43–48. doi: 10.1101/gad.1487307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tercero JA, Diffley JF. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature. 2001;412:553–557. doi: 10.1038/35087607. [DOI] [PubMed] [Google Scholar]
- [52].Hanada K, Budzowska M, Davies SL, Drunen E. van, Onizawa H, Beverloo HB, Maas A, Essers J, Hickson ID, Kanaar R. The structure-specific endonuclease Mus81 contributes to replication restart by generating double-strand DNA breaks. Nat Struct Mol Biol. 2007;14:1096–1104. doi: 10.1038/nsmb1313. [DOI] [PubMed] [Google Scholar]
- [53].Friedel AM, Pike BL, Gasser SM. ATR/Mec1: coordinating fork stability and repair. Curr Opin Cell Biol. 2009;21:237–244. doi: 10.1016/j.ceb.2009.01.017. [DOI] [PubMed] [Google Scholar]
- [54].Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- [55].de Klein A, Muijtjens M, van Os R, Verhoeven Y, Smit B, Carr AM, Lehmann AR, Hoeijmakers JH. Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr Biol. 2000;10:479–482. doi: 10.1016/s0960-9822(00)00447-4. [DOI] [PubMed] [Google Scholar]
- [56].Takai H, Tominaga K, Motoyama N, Minamishima YA, Nagahama H, Tsukiyama T, Ikeda K, Nakayama K, Nakanishi M. Aberrant cell cycle checkpoint function and early embryonic death in Chk1(−/−) mice. Genes Dev. 2000;14:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- [57].O’Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet. 2003;33:497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- [58].Murga M, Bunting S, Montana MF, Soria R, Mulero F, Canamero M, Lee Y, McKinnon PJ, Nussenzweig A, Fernandez-Capetillo O. A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nat Genet. 2009;41:891–898. doi: 10.1038/ng.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Cobb JA, Bjergbaek L, Shimada K, Frei C, Gasser SM. DNA polymerase stabilization at stalled replication forks requires Mec1 and the RecQ helicase Sgs1. EMBO J. 2003;22:4325–4336. doi: 10.1093/emboj/cdg391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lou H, Komata M, Katou Y, Guan Z, Reis CC, Budd M, Shirahige K, Campbell JL. Mrc1 and DNA polymerase epsilon function together in linking DNA replication and the S phase checkpoint. Mol Cell. 2008;32:106–117. doi: 10.1016/j.molcel.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134:981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Davies SL, North PS, Hickson ID. Role for BLM in replication-fork restart and suppression of origin firing after replicative stress. Nat Struct Mol Biol. 2007;14:677–679. doi: 10.1038/nsmb1267. [DOI] [PubMed] [Google Scholar]
- [63].Pirzio LM, Pichierri P, Bignami M, Franchitto A. Werner syndrome helicase activity is essential in maintaining fragile site stability. J Cell Biol. 2008;180:305–314. doi: 10.1083/jcb.200705126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ge XQ, Jackson DA, Blow JJ. Dormant origins licensed by excess Mcm2-7 are required for human cells to survive replicative stress. Genes Dev. 2007;21:3331–3341. doi: 10.1101/gad.457807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Shechter D, Costanzo V, Gautier J. ATR and ATM regulate the timing of DNA replication origin firing. Nat Cell Biol. 2004;6:648–655. doi: 10.1038/ncb1145. [DOI] [PubMed] [Google Scholar]
- [66].Wang H, Powell SN, Iliakis G, Wang Y. ATR affecting cell radiosensitivity is dependent on homologous recombination repair but independent of nonhomologous end joining. Cancer Res. 2004;64:7139–7143. doi: 10.1158/0008-5472.CAN-04-1289. [DOI] [PubMed] [Google Scholar]
- [67].Foray N, Marot D, Gabriel A, Randrianarison V, Carr AM, Perricaudet M, Ashworth A, Jeggo P. A subset of ATM- and ATR-dependent phosphorylation events requires the BRCA1 protein. EMBO J. 2003;22:2860–2871. doi: 10.1093/emboj/cdg274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tibbetts RS, Cortez D, Brumbaugh KM, Scully R, Livingston D, Elledge SJ, Abraham RT. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes Dev. 2000;14:2989–3002. doi: 10.1101/gad.851000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Turner JM, Aprelikova O, Xu X, Wang R, Kim S, Chandramouli GV, Barrett JC, Burgoyne PS, Deng CX. BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr Biol. 2004;14:2135–2142. doi: 10.1016/j.cub.2004.11.032. [DOI] [PubMed] [Google Scholar]
- [70].Sorensen CS, Hansen LT, Dziegielewski J, Syljuasen RG, Lundin C, Bartek J, Helleday T. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol. 2005;7:195–201. doi: 10.1038/ncb1212. [DOI] [PubMed] [Google Scholar]
- [71].Andreassen PR, D’Andrea AD, Taniguchi T. ATR couples FANCD2 monoubiquitination to the DNA-damage response. Genes Dev. 2004;18:1958–1963. doi: 10.1101/gad.1196104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Collis SJ, Ciccia A, Deans AJ, Horejsi Z, Martin JS, Maslen SL, Skehel JM, Elledge SJ, West SC, Boulton SJ. FANCM and FAAP24 function in ATR-mediated checkpoint signaling independently of the Fanconi anemia core complex. Mol Cell. 2008;32:313–324. doi: 10.1016/j.molcel.2008.10.014. [DOI] [PubMed] [Google Scholar]
- [73].O’Driscoll M. Mouse models for ATR deficiency. DNA Repair (Amst) 2009;8:1333–1337. doi: 10.1016/j.dnarep.2009.09.001. [DOI] [PubMed] [Google Scholar]
- [74].Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Orntoft T, Lukas J, Bartek J. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- [75].Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA, Jr., Kastrinakis NG, Levy B, Kletsas D, Yoneta A, Herlyn M, Kittas C, Halazonetis TD. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- [76].Pusapati RV, Rounbehler RJ, Hong S, Powers JT, Yan M, Kiguchi K, McArthur MJ, Wong PK, Johnson DG. ATM promotes apoptosis and suppresses tumorigenesis in response to Myc. Proc Natl Acad Sci U S A. 2006;103:1446–1451. doi: 10.1073/pnas.0507367103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Reimann M, Loddenkemper C, Rudolph C, Schildhauer I, Teichmann B, Stein H, Schlegelberger B, Dorken B, Schmitt CA. The Myc-evoked DNA damage response accounts for treatment resistance in primary lymphomas in vivo. Blood. 2007;110:2996–3004. doi: 10.1182/blood-2007-02-075614. [DOI] [PubMed] [Google Scholar]
- [78].Efeyan A, Murga M, Martinez-Pastor B, Ortega-Molina A, Soria R, Collado M, Fernandez-Capetillo O, Serrano M. Limited role of murine ATM in oncogene-induced senescence and p53-dependent tumor suppression. PLoS One. 2009;4:e5475. doi: 10.1371/journal.pone.0005475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Fang Y, Tsao CC, Goodman BK, Furumai R, Tirado CA, Abraham RT, Wang XF. ATR functions as a gene dosage-dependent tumor suppressor on a mismatch repair-deficient background. EMBO J. 2004;23:3164–3174. doi: 10.1038/sj.emboj.7600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lam MH, Liu Q, Elledge SJ, Rosen JM. Chk1 is haploinsufficient for multiple functions critical to tumor suppression. Cancer Cell. 2004;6:45–59. doi: 10.1016/j.ccr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- [81].Tao ZF, Lin NH. Chk1 inhibitors for novel cancer treatment. Anticancer Agents Med Chem. 2006;6:377–388. doi: 10.2174/187152006777698132. [DOI] [PubMed] [Google Scholar]
- [82].Koniaras K, Cuddihy AR, Christopoulos H, Hogg A, O’Connell MJ. Inhibition of Chk1-dependent G2 DNA damage checkpoint radiosensitizes p53 mutant human cells. Oncogene. 2001;20:7453–7463. doi: 10.1038/sj.onc.1204942. [DOI] [PubMed] [Google Scholar]
- [83].Ruzankina Y, Schoppy DW, Asare A, Clark CE, Vonderheide RH, Brown EJ. Tissue regenerative delays and synthetic lethality in adult mice after combined deletion of Atr and Trp53. Nat Genet. 2009;41:1144–1149. doi: 10.1038/ng.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Heffernan TP, Kawasumi M, Blasina A, Anderes K, Conney AH, Nghiem P. ATR-Chk1 pathway inhibition promotes apoptosis after UV treatment in primary human keratinocytes: potential basis for the UV protective effects of caffeine. J Invest Dermatol. 2009;129:1805–1815. doi: 10.1038/jid.2008.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kerzendorfer C, O’Driscoll M. UVB and caffeine: inhibiting the DNA damage response to protect against the adverse effects of UVB. J Invest Dermatol. 2009;129:1611–1613. doi: 10.1038/jid.2009.99. [DOI] [PubMed] [Google Scholar]
- [86].Garinis GA, van der Horst GT, Vijg J, Hoeijmakers JH. DNA damage and ageing: new-age ideas for an age-old problem. Nat Cell Biol. 2008;10:1241–1247. doi: 10.1038/ncb1108-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- [88].Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- [89].Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol. 2004;6:168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- [90].Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Fernandez-Capetillo O. Intrauterine programming of ageing. EMBO Rep. 11:32–36. doi: 10.1038/embor.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]