Abstract
Despite innovations in HIV counseling and testing (HCT), important gaps remain in understanding linkage to care. We followed a cohort diagnosed with HIV through a community-based HCT campaign that trained persons living with HIV/AIDS (PLHA) as navigators. Individual, interpersonal, and institutional predictors of linkage were assessed using survival analysis of self-reported time to enrollment. Of 483 persons consenting to follow-up, 305 (63.2%) enrolled in HIV care within 3 months. Proportions linking to care were similar across sexes, barring a sub-sample of men aged 18–25 years who were highly unlikely to enroll. Men were more likely to enroll if they had disclosed to their spouse, and women if they had disclosed to family. Women who anticipated violence or relationship breakup were less likely to link to care. Enrollment rates were significantly higher among participants receiving a PLHA visit, suggesting that a navigator approach may improve linkage from community-based HCT campaigns.
Keywords: Linkage to care, Antiretroviral treatment, Community-based testing, HIV-1, Sub-Saharan Africa, Survival analysis, HIV counseling and testing
The advent of antiretroviral therapy (ART) has increased quality of life and life expectancy for persons living with HIV/AIDS (PLHA) [1–3]. However, those in resource-constrained settings tend to start ART treatment with more advanced disease than those in resource-rich settings [4]. Late diagnosis is associated with HIV-related morbidity and mortality in sub-Saharan Africa [5], as are late presentation into care [6] and delayed ART initiation [5, 7–16]. Delays in diagnosis, linkage to care, and subsequent ART initiation also pose a higher risk of HIV transmission to others, since treatment reduces viral load and infectivity [17–21].
Understanding the dynamics of linkage to care in sub-Saharan Africa is particularly salient amidst recent calls for universal HIV testing and immediate treatment [22]. In alignment with a ‘test and treat’ approach, new strategies for HIV counseling and testing (HCT), such as community-based testing via mobile clinics, have proven to be cost-effective and successful at reaching previously untested individuals [23–28]. At the same time, these innovative testing strategies may increase the challenges of linking individuals to the healthcare facility to receive care and treatment [29].
Much of the research around linkage to care has occurred in resource-rich settings, such as the United States and Europe, where delayed uptake of care is associated with long wait times for initial appointments [30], testing for the first time [19, 31], rural residence [32], or being diagnosed at an early stage of disease while still feeling well [33]. Few sub-Saharan Africa programs routinely assess the proportion of HIV-diagnosed patients who successfully link to care or treatment. Those that have measured linkage achieve strikingly poor results: between 30–62% of persons receiving HIV positive test results successfully link to care [34–39]. A recent systematic review estimates that if studies were to track patients from HIV testing to CD4 count results, clinic enrollment, and initiation of ART, between 17–33% of patients would complete all three linkage steps [40]. The dearth of studies identified by the systematic review highlights the urgent need for better research on linkage to care.
Of the few recent studies that examine linkage to care in sub-Saharan Africa, only one [39] has studied linkage from community-based HCT. This gap in the literature is crucial to fill, because a significant portion of HIV testing in the coming years will likely be community- and home-based strategies, particularly in light of recent findings that community-based HCT detects almost four times more HIV cases than standard clinic-based testing [41].
All existing studies, to our knowledge, examine clinical and demographic predictors of linkage. However, in resource-constrained settings, issues beyond demographics are likely to shape linkage. For example, research shows that broader gender dynamics [35, 42], entrenched poverty [43–45], and HIV-related stigma [46–49] inhibit uptake of HIV care and treatment, even as ART has become more available. This research adds to the knowledge base by exploring the interpersonal and institutional factors that determine linkage to care.
Methods
This paper presents a cross-sectional study of linkage to care following a community-based HCT campaign conducted in Nyanza Province, Kenya during August–September, 2009. Described fully elsewhere [26], the Integrated Prevention Demonstration (IPD) model combines community-based HIV testing with distribution of long-lasting insecticidal nets and point-of-use water purifiers, and was implemented by Vestergaard Frandsen in partnership with the Kenyan Ministry of Health. During the three-day campaigns, mobile tents were established in six community sites outside Kisumu, an urban center, and near Kisii, a hillside town.
HCT was offered in accordance with the Kenyan national guidelines and adhered to key principles of informed consent, confidentiality and privacy. Each client was registered and given a unique client number delinked from identifiers. A nationally certified HCT counselor provided individual pre-test counseling and obtained verbal informed consent. Finger prick blood samples were screened for HIV infection using serial rapid ELISA testing, as per national guidelines [50].
Clients testing HIV positive were provided with a referral to care and were offered a rapid CD4 count using Guava AutoCD4 system (Guava technologies, Massachusetts, USA). In most cases, patients received their CD4 count results within 3 h, with about 30% of the population asked to return the following day for results. Newly diagnosed clients were also invited to receive a follow-up home visit by a trained PLHA navigator. Those who consented were guided through completion of the locator form. Following the campaign, PLHA navigators attempted to conduct home visits with all persons providing locator information, in order to offer support for enrolling into HIV care.
Data Collection
Data presented here were collected by a separate team of trained researchers who conducted follow-up interviews 10 months after the HCT campaign. These interviews were separate from the home visits conducted by PLHA navigators, but relied on the same locator form information collected at the campaign by consenting participants. To conduct the 10-month follow-up interview, researchers liaised with PHLA navigators to invite eligible clients to participate via personal visit or cell phone. The researchers then used locator information to trace and consent interested clients. Individuals were eligible for our study if they were more than 18 years of age, had tested positive during the HCT campaign, were not previously enrolled in HIV care or treatment at the time of HCT campaign, and consented to be traced at their home. Persons who were not located successfully at the first visit received one additional visit by research staff.
Measurements
There were two primary outcomes for this study. The first outcome, linkage to care, was based on participant self-report of clinic enrollment up to the time of interview 10 months after the HCT campaign (Y/N). The second outcome, time to linkage, was measured in months from the first day of the month diagnosed at the HCT campaign (August or September) to the first day of the month participants reported that they enrolled in care.
Based on an extensive review of the literature, we used a social-ecological framework [51–53] to identify covariates of linkage to care at individual, interpersonal, and institutional levels. At the individual level, a number of socio-demographics were collected (sex, age, education, ethnicity, religion, marital status, and occupation). Health status was measured using one item from the SF-36 health survey that is highly predictive of many health behaviors and outcomes: “In general, would you say your health is excellent, good, fair or poor?” [54]. To measure mental health, we used the PHQ-9 clinical depression scale, a measure that has been validated in Kenya [55] and has internal consistency in this sample (Cronbach alpha (α) = 0.86). Substance use was measured through self-report of frequency of alcohol and drug use.
At the interpersonal level, we assessed PLHA Navigator home visits by asking whether the client received a home visit and number of visits received. We asked clients about disclosure of HIV status to friends, family, or healthcare workers. To understand gender dynamics, we used three items from the power in relationships scale concerning household decision-making [56]. We measured participant perceptions of HIV-related stigma using measures that have been validated and used in the study setting [57]. Anticipated stigma (α = 0.78) was measured using a 9-item scale of a person’s anticipation of experiencing stigma because of enrolling in HIV care [58] Community stigma (α = 0.84) was measured using the mean of 7 items from the ‘perceived discrimination’ sub-scale of a measure developed by NIMH Project Accept [59]. Self-stigma (α = 0.80) was measured using the ‘self-stigma’ sub-scale from the HASI-P stigma instrument [60]. Consistent with other studies in HIV-positive populations [61], we asked about three dimensions of social support: (1) having a confidante; (2) having people to depend on (social network); (3) having instrumental support (financial help, a place to stay, or assistance visiting the doctor). Social support variables were converted to binary (agree vs. disagree) for analysis.
At the institutional level, we asked questions about the logistics of linkage to care. Transportation was assessed by asking the distance (in kilometers), time (in minutes), and cost (in Kenya shillings) of travel by public transportation to the clinic. Associated costs were explored by asking the total amount spent monthly (in Kenya shillings) on medication, clinic costs, or missed income [62]. Questions around knowledge of ART and ART availability were drawn from the Kenyan ARTIS study [63] and those who responded “don’t know” to these items were considered to have uncertainty around ART knowledge/availability. Participants were asked about the type of ART medications they used, if any, and these were listed by prompting participants to gather the medication bottles and show them to the researcher.
Statistical Analysis
All analyses are presented separately for men and women, as we hypothesized that the factors associated with enrollment into care would differ by sex. We compared descriptive characteristics of men and women using χ2 tests for categorical variables. Bivariate analyses assessed differences in time to enrollment for each predictor variable using Cox proportional hazard models for time-to-event data with censoring. Variables were selected for inclusion in bivariate analysis provided there was enough variability in responses (>5%/binary response option) separately among male and female respondents. For inclusion in the multivariate model, variables had to be associated with linkage to care (P < 0.10) in the bivariate Cox regression models. All models accounted for the clustering of data within site.
Cox regression assumes that each predictor has an equal effect throughout the time of observation. We observed several violations of this assumption in bivariate analyses. We created interaction terms between predictors and the natural log of time to enrollment to address violations in multivariate models. Final models did not violate the proportionality assumption. All statistical analyses were performed using Stata 10.0 for Windows (StataCorp, Texas).
Ethical Review
Ethical approval was obtained from the Kenya Medical Research Institute Ethical Review Committee (SSC#1776) and the University of California, San Francisco Committee on Human Research (CHR#10035623).
Results
Out of 10,203 persons tested in the community-based HCT campaign, 808 persons were over 18 years of age, tested HIV-positive during the HCT campaign, and were not already enrolled in HIV care at time of HIV testing. A total of 702 (86.8%) HIV-infected persons had a CD4 test conducted, among whom 603 (85.9%) had CD4 counts above 250 cells/μl (89.4% of women and 75% of men; Pearson χ2 = 18.59 for high CD4 by sex, P < 0.001).
Of the eligible population, 684 (85%) provided locator information. A total of 547 (80.0%) were located 10 months later at time of follow-up interview. Of those who were traced, a total of 158 clients in Kisii consented to the interview (7.6% refusal rate), as did 342 in Kisumu (9.0% refusal rate). Of the 500 completed surveys, 17 individuals were subsequently excluded from analysis as they reported that they enrolled in care prior to testing HIV-positive at the IPD campaign.
Similar to the overall population of campaign attendees, the 483 individuals in our study constituted a sample (Table 1) with more women than men (73.7 vs. 26.3%) and more residents of Kisumu than Kisii (68.7 vs. 31.3%). Less than one-third were educated beyond primary school, though two-thirds could read a local language newspaper. Two-thirds of respondents owned either a cell phone or a radio (half had both). Although many characteristics were similar across sexes, women were more likely to be widowed, to have no formal education, and to state housework as their primary occupation than men. While less than 10% of men reported being polygamous, nearly 30% of women reported that they were married to a polygamous man.
Table 1.
Respondent characteristics compared by sex
| Characteristic | Men
|
Women
|
||
|---|---|---|---|---|
| Number | Valid percent | Number | Valid percent | |
| Sex (n = 483) | 127 | 356 | ||
| Age (n = 463)† | ||||
| 25 and under | 14 | 11.2 | 75 | 22.2 |
| 26–35 | 41 | 32.8 | 146 | 43.2 |
| 36–45 | 45 | 36.0 | 81 | 24.0 |
| 46 and over | 25 | 20.0 | 36 | 10.7 |
| Marital status | ||||
| Ever married (n = 478) | 116 | 91.3 | 334 | 95.2 |
| Current marital status (n = 475)† | ||||
| Single | 12 | 9.6 | 27 | 7.7 |
| Married/partnered | 99 | 79.2 | 217 | 62.0 |
| Separated | 6 | 4.8 | 24 | 6.9 |
| Widowed | 8 | 6.4 | 82 | 23.4 |
| Polygamy: men with multiple wives, women whose husbands have multiple wives (n = 478)† | 11 | 8.6 | 100 | 28.5 |
| Have children with current partner (n = 479) | 104 | 81.9 | 263 | 74.7 |
| Ethnic group (n = 480) | ||||
| Luo | 85 | 67.5 | 242 | 68.4 |
| Kikuyu | 0 | 0.0 | 1 | 0.3 |
| Kisii | 40 | 31.8 | 108 | 30.5 |
| Luhya | 1 | 0.8 | 3 | 0.9 |
| District (n = 483) | ||||
| Kisumu | 87 | 68.5 | 245 | 68.8 |
| Kisii | 40 | 31.5 | 111 | 31.2 |
| Education status (n = 481)† | ||||
| Never attended school | 0 | 0.0 | 22 | 6.2 |
| Primary school | 82 | 65.1 | 250 | 70.4 |
| Secondary school | 29 | 23.0 | 68 | 19.2 |
| Post-primary/Vocational | 12 | 9.5 | 9 | 2.5 |
| College/University | 3 | 2.4 | 6 | 1.7 |
| Local language reading (n = 458)* | ||||
| Not at all | 6 | 4.9 | 24 | 7.1 |
| Can read with difficulty | 22 | 18.0 | 94 | 28.0 |
| Can read easily | 94 | 77.0 | 218 | 64.9 |
| Religion (n = 245)a | ||||
| African independent churches | 28 | 45.9 | 71 | 38.6 |
| Protestant | 11 | 18.0 | 49 | 26.6 |
| Roman catholic | 15 | 24.6 | 39 | 21.2 |
| Other | 7 | 11.5 | 25 | 13.6 |
| Type of employment (n = 467)b | ||||
| Housework† | 1 | 0.8 | 52 | 15.3 |
| Selling things† | 16 | 12.7 | 111 | 32.6 |
| Farming or horticulture | 44 | 34.9 | 149 | 43.7 |
| Manual labor† | 32 | 25.4 | 24 | 7.0 |
| Fishing† | 4 | 3.2 | 1 | 0.3 |
| Teacher or health worker | 1 | 0.8 | 6 | 1.8 |
| Driver† | 8 | 6.4 | 0 | 0.0 |
| Other† | 33 | 26.2 | 25 | 7.3 |
P < 0.05 for test of hypothesis that male and female subgroups are from the same population (χ2 tests)
P < 0.01 for test of hypothesis that male and female subgroups are from the same population (χ2 tests)
Response rates to this question were low because original list of possible religions was not accurate
Employment percentages do not add to 100% across categories because multiple responses permitted
Cumulative linkage to HIV care and treatment was high: 63.2% enrolled within 3 months of the campaign; 81.4% enrolled by time of follow-up interview 10 months later (83.4% of women and 75.6% of men; Pearson χ2 = 3.79 for linkage by sex, P = 0.052). Median time to enrollment was one month and interquartile range was 0–6 months. A total of 72 men (56.7%) and 217 women (61.0%) received PLHA visits during the 10-month period. Neither proportion nor frequency of PLHA visits differed significantly between men and women.
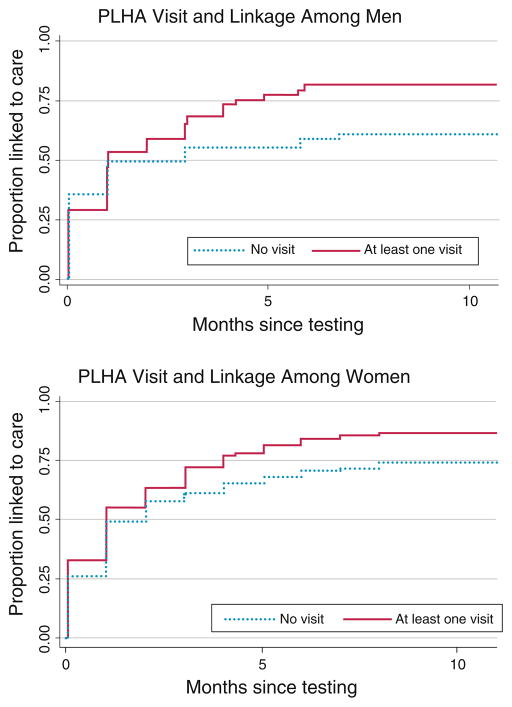
The Kaplan–Meier survival curves (Fig. 1) illustrate the significant relationship over time between receiving a PLHA navigator visit and linkage to care for men and women. In bivariate analysis, factors significantly associated with time to linkage (Table 2) for both men and women included: older age, being widowed, greater knowledge around ART, disclosure to family, and receiving a PLHA navigator home visit. For men, additional factors were occupation, marital status, alcohol use, self-stigma, disclosure to spouse, and having a confidante. For women, additional factors were education, physical health, occupation, and anticipating a negative reaction from a partner in the form of relationship breakup or physical violence.
Fig. 1.
Time to linkage following HIV testing for those receiving and not receiving a PLHA navigator home visit, by sex
Table 2.
Bivariate associations of demographics, health status, social factors, and PLHA visits with time to linkage for men and women
| Variable | Men
|
Women
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Enrolled within 3 months
|
HR for time to enroll (95% CI)a | Z | P value | N | Enrolled within 3 months
|
HR for time to enroll (95% CI)a | Z | P value | |||
| No. | % | No. | % | |||||||||
| Socio-demographics | ||||||||||||
| Age (standardized to mean 34.7 ± 9.2) | 120 | 1.64 (1.23–2.19) | 3.36 | 0.001 | 318 | 1.20 (1.05–1.37) | 2.67 | 0.008 | ||||
| Education level | 122 | 333 | ||||||||||
| None or primary school | 80 | 51 | 63.8 | 1 | 256 | 164 | 64.1 | 1 | ||||
| Secondary and above | 42 | 27 | 64.3 | 1.11 (0.72–1.73) | 0.48 | 0.631 | 77 | 63 | 81.8 | 1.63 (1.18–2.25) | 0.003 | |
| Marital status | 120 | 327 | ||||||||||
| Married | 95 | 64 | 67.4 | 1 | 202 | 126 | 62.4 | 1 | ||||
| Single | 11 | 2 | 18.2 | 0.15 (0.04–0.51)b | −3.00 | 0.003 | 27 | 19 | 70.4 | 1.09 (0.84–1.41) | 0.63 | 0.529 |
| Widowed or separated | 14 | 12 | 85.7 | 1.57 (0.99–2.50) | 1.91 | 0.056 | 98 | 78 | 79.6 | 1.59 (1.47–1.72) | 11.40 | <0.001 |
| Farming | 121 | 318 | ||||||||||
| No | 81 | 45 | 55.6 | 1 | 178 | 110 | 61.8 | 1 | ||||
| Yes | 40 | 32 | 80.0 | 1.95 (1.51–2.52)b | 5.10 | <0.001 | 140 | 106 | 75.7 | 1.29 (0.95–1.75) | 1.64 | 0.100 |
| Housework only | 0 | 318 | ||||||||||
| No | 276 | 192 | 69.6 | |||||||||
| Yes | 42 | 24 | 57.1 | 0.67 (0.60–0.76) | −6.23 | <0.001 | ||||||
| Manual labor | 121 | 318 | ||||||||||
| No | 89 | 60 | 67.4 | 1 | 295 | 201 | 68.1 | |||||
| Yes | 32 | 17 | 53.1 | 0.69 (0.62–0.77) | −6.87 | <0.001 | 23 | 15 | 65.2 | 0.96 (0.56–1.65) | −0.15 | 0.879 |
| Religionc | 61 | 172 | ||||||||||
| Protestant | 10 | 7 | 70.0 | 1 | 46 | 32 | 69.6 | 1 | ||||
| African independent | 28 | 14 | 50.0 | 0.45 (0.36–0.56)b | −7.23 | <0.001 | 62 | 33 | 53.2 | 0.65 (0.45–0.93) | −2.32 | 0.020 |
| Roman catholic | 14 | 10 | 71.4 | 0.74 (0.35–1.57) | −0.78 | 0.433 | 39 | 30 | 76.9 | 1.13 (0.72–1.77) | 0.54 | 0.591 |
| Other | 7 | 6 | 85.7 | 1.53 (0.62–3.77) | 0.93 | 0.353 | 25 | 20 | 80.0 | 1.19 (0.78–1.81)b | 0.79 | 0.427 |
| Health status | 122 | 330 | ||||||||||
| Self-evaluation of overall health | ||||||||||||
| Poor-fair | 35 | 20 | 57.1 | 1 | 112 | 70 | 62.5 | 1 | ||||
| Good–excellent | 87 | 58 | 66.7 | 1.23 (0.86–1.76) | 1.14 | 0.256 | 218 | 156 | 71.6 | 1.14 (0.98–1.33) | 1.68 | 0.093 |
| Alcohol use in past 4 weeks | 122 | 333 | ||||||||||
| No | 74 | 59 | 79.7 | 1 | 323 | 220 | 68.1 | 1 | ||||
| Yes | 48 | 19 | 39.6 | 0.43 (0.26–0.71) | −3.34 | 0.001 | 10 | 7 | 70.0 | 1.35 (1.02–1.80) | 2.09 | 0.037 |
| Social factors | ||||||||||||
| Has a Confidante | 122 | 331 | ||||||||||
| No | 29 | 12 | 41.4 | 1 | 98 | 56 | 57.1 | 1 | ||||
| Yes | 93 | 66 | 71.0 | 1.94 (1.09–3.44) | 2.25 | 0.025 | 233 | 170 | 73.0 | 1.64 (0.89–3.02)b | 1.60 | 0.11 |
| Total anticipated stigma | 121 | 0.87 (0.22–3.43)b | 0.846 | 317 | 0.45 (0.20–1.00)b | 0.049 | ||||||
| Anticipated stigma from family | 121 | 327 | ||||||||||
| No | 101 | 66 | 65.4 | 1 | 247 | 174 | 70.5 | 1 | ||||
| Yes | 20 | 12 | 60.0 | 0.81 (0.45–1.45) | −0.19 | 0.484 | 80 | 47 | 58.8 | 0.68 (0.43–1.07)b | −1.97 | 0.095 |
| Anticipated negative reaction from partner | 122 | 333 | ||||||||||
| No | 83 | 55 | 66.3 | 1 | 171 | 119 | 69.6 | 1 | ||||
| Yes | 14 | 10 | 71.4 | 0.98 (0.58–1.69)b | −0.04 | 0.967 | 71 | 46 | 64.8 | 0.70 (0.50–0.99) | −2.04 | 0.041 |
| Missing (includes N/A) | 25 | 13 | 52.0 | 0.69 (0.41–1.18)b | − 1.35 | 0.178 | 91 | 62 | 68.10 | 0.92 (0.68–1.24) | −0.54 | 0.592 |
| Self-stigma scale | 122 | 0.71 (0.51–1.01) | −1.93 | 0.054 | 330 | 0.93 (0.84–1.02) | −1.60 | 0.110 | ||||
| Disclosure to spouse | 122 | 333 | ||||||||||
| No | 48 | 24 | 50.0 | 1 | 176 | 125 | 71.0 | 1 | ||||
| Yes | 74 | 54 | 73.0 | 1.86 (1.28–2.69)b | 1.86 | 0.001 | 157 | 102 | 65.0 | 0.96 (0.70–1.30) | −0.29 | 0.775 |
| Disclosure to family | 122 | 333 | ||||||||||
| No | 77 | 40 | 52.0 | 1 | 193 | 120 | 62.2 | 1 | ||||
| Yes | 45 | 38 | 84.4 | 2.02 (1.28–3.17) | 3.02 | 0.002 | 140 | 107 | 76.4 | 1.45 (1.11–1.90)b | 2.71 | 0.007 |
| Perceived discrimination scale | 122 | 1.29 (0.95–1.77) | 1.61 | 0.107 | 330 | 0.87 (0.57–1.32) | −0.64 | 0.520 | ||||
| ART beliefs | ||||||||||||
| Uncertainty around ART knowledge | 120 | 0.11 (0.03–0.40)b | −3.40 | 0.001 | 329 | 0.26 (0.09–0.71) | −2.61 | 0.009 | ||||
| Uncertainty around ART availability | 114 | 0.046 (0.01–0.27) | −3.42 | 0.001 | 291 | 0.221 (0.09–0.56) | −3.16 | 0.002 | ||||
| PLHA navigator | ||||||||||||
| Received a visit | 122 | 333 | ||||||||||
| No | 50 | 28 | 56.0 | 1 | 116 | 71 | 61.2 | 1 | ||||
| Yes | 72 | 50 | 69.4 | 1.48 (1.08–2.04)b | 2.41 | 0.016 | 217 | 156 | 71.9 | 1.34 (1.10–1.63) | 2.87 | 0.004 |
Table includes hazard ratios (HR) for all variables with P value <0.10 for at least one of the groups (men or women) and is adjusted for clustering by site, otherwise unadjusted
Association is not proportional
Low response rate to this question due to inaccurate initial categories
For several variables analyzed, the association with linkage to care was not consistent over time. Much of this effect in men was due to a subset of men (aged 18–25 years) who were unlikely to enroll in care. Only 2 of 14 (14.3%) young men linked to care, and differed from the larger sample in several ways: they were more likely to be single (66.7 vs. 3.5% of older men) and use alcohol (78.6 vs. 33.6%), and less likely to disclose their HIV status (14.3 vs. 84.1%). Because of these differences, the subsequent multivariate survival analysis was restricted to men over 25 years of age (n = 104).
Multivariate analyses for men and women produced adjusted associations of predictors of linkage to care (Table 3). The model for men included all eligible males over 25 years with complete data (80.3% of total sample, 92.0% of men over 25 years). The multivariate model suggests that greater age was associated with higher likelihood of linkage to care, while uncertainty around ART knowledge was associated with lower likelihood. Higher perceived discrimination was associated with a higher rate of linkage over time. Disclosure of HIV status to spouse was associated with higher likelihood of linkage. This association increased over time, as indicated by the interaction term, to 2.41 at 5 months (95% CI 1.74–3.35), and 2.83 at 10 months (95% CI 1.77–4.52). As with the unadjusted Kaplan–Meier curves, receiving a PLHA visit remained associated with linkage in the multivariate model, and the association likewise increased over time to 1.77 at 5 months (95% CI 1.47–2.13) and 1.99 at 10 months (95% CI 1.52–2.60).
Table 3.
Multivariate associations among demographics, health status, social factors, PLHA visits, and time to linkage among men (> 25 years) and women
| AHRa (95% CI) | Z | P value | |
|---|---|---|---|
| A: Multivariate Cox regression for men over 25 years (n = 104) | |||
| Age (standardized values) | 1.30 (1.09–1.55) | 2.97 | 0.003 |
| Education greater than primary school | 0.86 (0.55–1.33) | −0.69 | 0.487 |
| Uncertainty around ART knowledge | 0.23 (0.10–0.54) | −3.39 | 0.001 |
| Perceived discrimination | 1.29 (1.11–1.51) | 3.22 | 0.001 |
| Disclosure to spouse | 1.67 (1.07–2.61) | 2.24 | 0.025 |
| Interaction of disclosure and time | 1.26 (1.03–1.54) | 2.21 | 0.027 |
| PLHA visit | 1.35 (0.97–1.87) | 1.81 | 0.071 |
| Interaction of PLHA visit and time | 1.18 (1.05–1.33) | 2.86 | 0.004 |
|
| |||
| AHR (95% CI)b | Z | P value | |
|
| |||
| B: Multivariate Cox regression for women (n = 293) | |||
| Age (standardized values) | 1.08 (1.05–1.15) | 4.98 | <0.001 |
| Education above primary school | 1.54 (1.11–2.14) | 2.58 | 0.010 |
| Uncertainty around ART knowledge | 0.36 (0.19–0.70) | −3.04 | 0.002 |
| Health status of good or excellent | 1.43 (1.19–1.72) | 3.82 | <0.001 |
| Interaction of health status and time | 1.08 (1.05–1.11) | 5.46 | <0.001 |
| Marital status: widowed or separated | 1.36 (1.19–1.56) | 4.53 | <0.001 |
| Anticipated negative partner response (break-up or intimate partner violence) | 0.64 (0.51–0.79) | −4.08 | <0.001 |
| Disclosure to family | 1.33 (1.10–1.61) | 2.89 | 0.004 |
| Interaction of disclosure and time | 1.07 (1.02–1.12) | 3.00 | 0.003 |
| Distance to HIV care facility (in hours) | 1.26 (1.04–1.54) | 2.32 | 0.021 |
| PLHA visit | 1.20 (1.00–1.43) | 1.99 | 0.047 |
AHR adjusted hazard ratio, CI confidence interval. Schöenfeld test for final model for men = 2.12; degree of freedom = 5; P = 0.833
In addition to variables listed, model is adjusted for missingness in anticipated partner response. Schöenfeld test for final model for women = 3.92; degree of freedom = 5; P = 0.561
The multivariate model for women included all women with complete data (82.3% of total sample). The model suggests greater education, being older, and being widowed/single were associated with higher rates of linkage per month, while lack of HIV knowledge was associated with lower rates. Women who anticipated a negative response from their partner, in the form of breakup or violence, were less likely to link to care. Similar to men, receiving a PLHA visit was associated with a higher rate of linkage among women. Women who had disclosed to family had rates of linkage that were 1.33 (95% CI 1.10–1.61), 1.47 (95% CI 1.38–1.58), and 1.54 (95% CI 1.40–1.70) times as high as non-disclosers at 1, 5, and 10 months, respectively. The association between better health and enrolling in care strengthened over time to 5 months (AHR = 1.62; 95% CI 1.55–1.69) and 10 months (AHR = 1.71; 95% CI 1.60–1.82). There was also a positive association between travel time to a clinic of choice and enrollment (AHR = 1.26; 95% CI 1.04–1.54), meaning women who reported a farther distance to the clinic were more likely to enroll over time.
Discussion
The cumulative proportions of patients in our sample linked to care by three months (63.2%) and by the time of the interview (81.4%) were high, matching linkage rates of cohorts in resource-rich settings [29]. One challenge of mobile testing is that it targets people at an earlier stage of HIV infection [64–67], potentially inhibiting linkage for those who wait for signs of illness to emerge before seeking care [68–70]. Though selection bias in our study may have played a role, the high enrollment rates suggest that aspects of the HCT campaign were successful in encouraging linkage even among early-diagnosis patients.
At the time of the HIV testing campaign, Kenyan National Guidelines recommended ART for patients with a CD4 count <250 cells/μl (current guidelines use a cutoff of <350 cells/μl). Knowledge of a low CD4 count may have motivated patients to go earlier for HIV care than patients with higher CD4 counts. Since we were unable to link CD4 testing data to follow-up interviews, we cannot ascertain precisely how CD4 counts influenced time to linkage in our sample. This will be an essential component of future studies on linkage to care as ART is scaled-up.
Home visits by PLHA navigators seem to be a successful linkage strategy in sub-Saharan Africa, where research has already shown that lay community health workers (CHW) can increase HIV testing uptake [71], reduce linkage delays [64], improve ART adherence [72, 73], and improve clinical indicators such as CD4 cell count [74]. Called a ‘navigation model’, similar methods are being employed widely in the United States [75–77]. The PLHA navigator strategy deserves further attention to HIV care and treatment programs in resource-constrained settings.
Contrary to some studies [6, 7, 78, 79], but aligned with others [39], older clients in our sample were more likely to link to care each month. In particular, young men were highly unlikely to ever enroll in care, and exhibited a range of traits (single marital status, low disclosure, higher prevalence of alcohol use) that differed strongly from the rest of the men interviewed. Though it is difficult to generalize from this small sample of young men (n = 14), this study suggests that PLHA visits were not a successful strategy for this group and that alternative approaches should be considered for recruiting young, HIV-positive men into care. This finding is consistent with other studies that suggest men enroll at a slower rate than female counterparts [42, 79]. However, studies in Kenya have found healthcare utilization around HIV testing and treatment of sexually transmitted infections slower amongst women than male counterparts [80–82]. These discrepancies underscore the importance of disaggregating data by sex and age in future studies on uptake of HIV care.
Similar to other studies [78, 83–85], women with less formal education were less likely to link to care, as were both men and women with less HIV knowledge. Lack of information about ART is associated with delayed care [49], high-risk HIV sexual behaviors among both men and women [86], and HIV seroprevalence among women [63]. It may be challenging for newly diagnosed clients to take in nuanced information about ART treatment at the time of HIV testing [87–89]. Employing CHWs or PHLA navigators to distill information, answer questions, and support treatment literacy following HIV testing may address the information gap.
Women were less likely to link to care over time when they anticipated a negative partner reaction, in the form of intimate partner violence or breakup of a relationship. Disclosure was associated with greater linkage among both men and women, although for men the main association derived from disclosure to a spouse, while for women it was disclosure to family. Non-disclosure to partners has been recognized as an impediment to enrollment in other studies in Africa, in terms of timely presentation to HIV care [79], initiation of ART [35, 46], and treatment adherence [90]. Women, in particular, often choose not to disclose their HIV status for fear their male partner will react with abuse or abandonment [91–95], and partner disclosure has been shown to lead to a loss of economic support, blame, stigma, and violence [96–99].
As in other sub-Saharan African settings [46, 47, 68, 100–103], HIV-related stigma shaped decisions regarding uptake of care and treatment. The surprising finding that higher perceived discrimination was associated with higher rates of enrollment among men could be a result of men encountering additional discrimination following the decision to enroll, or could have resulted from other unmeasured associations. Likewise, we found that longer travel time was associated with higher rates of enrollment into care among women—perhaps a consequence of the fact that the greater the distance to a facility, the less likely a client was to risk a potentially stigmatizing encounter with a neighbor or relative. Distance and transportation costs associated with accessing care have been shown to be a determinant of healthcare utilization in other studies [46, 48, 104]. Findings from this study suggest that motivation to engage in care may overcome logistical barriers, at least at the beginning of care and treatment, but it is unclear whether this remains the case as individuals confront the cost of care over time.
Study Limitations
Findings from our study should be reviewed in light of study design limitations. We followed HIV-positive persons prospectively from time of diagnosis, but had access to only a minimal amount of data collected by the HCT campaign at the time of testing. The lack of exact date of diagnoses and enrollment decreased the precision of our measure of time to enrollment. Many individuals testing HIV-positive at the campaign did not participate in the study because they were ineligible (e.g. already on ART), were untraceable (e.g. chose not to provide locator information), or refused the interview. The latter two subsets may be at particular risk of not linking to care, since stigma or other social factors may have caused them to decline the opportunity to be followed at home. This sampling bias may overestimate enrollment rates and potentially underestimate the extent to which the various social and interpersonal predictors inhibited enrollment.
The data reported here are comprised of follow-up interviews conducted 10 months after testing, when clients may have had difficulty recalling their exact date of linkage to care. We were unable to systematically triangulate findings from our data with clinic enrollment data. Social desirability bias may have influenced self-reports on the part of clients. In addition, the retrospective nature of data collection makes it difficult to ascertain the direction of causality for the associations observed. Because this study took place within a single cohort in two sites, the findings may not be generalizable to other settings.
Recommendations
There is an urgent need to better understand the reasons for delays in linkage to care, particularly in sub-Saharan Africa, where only a select few studies have examined linkage from HIV testing to clinic enrollment [64, 105–107]. The social-ecological approach used in this study may provide a useful theoretical framework for future research and interventions. In exploring social-ecological drivers of linkage, we found that interpersonal dynamics strongly informed time to linkage for both men and women. In future studies, interpersonal issues such as spousal disclosure, fear of relationship break-up or violence, and access to social support should be included. We posit that expanding beyond individual level clinical characteristics in HIV linkage research will be a necessary step for designing effective interventions.
High enrollment rates among our sample suggest that a ‘navigator model’ may be supportive of early linkage to care following community-based HCT campaigns. The population attending this HCT campaign had relatively high CD4 counts, suggesting that this HCT approach could be used to identify PLHA earlier in disease progression. Significant gains in prevention of morbidity and vertical transmission can be expected when patients initiate ART earlier [108], highlighting the urgent need for interventions, like the navigator model, that speed linkage to care.
Beyond the navigator model, two further recommendations should be considered by future programs. First, partner dynamics should form a central part of future HIV testing campaigns, especially since less than 10% of PLHA globally know the HIV status of their partners [109]. Couple-centered testing is a promising strategy for improving disclosure to partners [110] and increasing uptake of HIV treatment [111]. At a minimum, HIV testing campaigns should address real concerns about HIV status disclosure, risk of intimate partner violence, and social support. Second, campaigns need to build strategies for reaching and linking young, single individuals to HIV care, since these are populations that may be at greatest risk of transmitting HIV and are least likely to enroll in care.
Acknowledgments
A grant from Vestergaard Frandsen.
Footnotes
Conflicts of interest We wish to declare a potential perceived conflict of interest and the measures taken to ensure this has not influenced our findings. Funding for this study was provided by the HIV counseling and testing (HCT) campaign implementer, Vestergaard Frandsen. The sponsor did not have any role in the study design, analysis or interpretation of the data. However, as is good practice in implementation science, two authors from Vestergaard Frandsen (L.K. and A.D) were included in the final review of the manuscript to ensure accurate presentation of the HCT campaign and the study setting.
Contributor Information
Abigail M. Hatcher, Email: HatcherA@globalhealth.ucsf.edu, Department of Obstetrics, Gynecology and Reproductive Sciences, University of California San Francisco, 50 Beale Street, Suite 1200, San Francisco, CA 94105, USA. Center for AIDS Prevention Studies, University of California San Francisco, San Francisco, USA
Janet M. Turan, Department of Obstetrics, Gynecology and Reproductive Sciences, University of California San Francisco, 50 Beale Street, Suite 1200, San Francisco, CA 94105, USA. Department of Health Care Organization and Policy, School of Public Health, University of Alabama at Birmingham, Birmingham, USA
Hannah H. Leslie, Prevention and Public Health Group, University of California San Francisco, San Francisco, USA
Lucy W. Kanya, Vestergaard Frandsen, Nairobi, Kenya
Zachary Kwena, Center for Microbiology Research, Kenya Medical Research Institute, Nairobi, Kenya.
Malory O. Johnson, Center for AIDS Prevention Studies, University of California San Francisco, San Francisco, USA
Starley B. Shade, Center for AIDS Prevention Studies, University of California San Francisco, San Francisco, USA
Elizabeth A. Bukusi, Department of Obstetrics, Gynecology and Reproductive Sciences, University of California San Francisco, 50 Beale Street, Suite 1200, San Francisco, CA 94105, USA. Center for Microbiology Research, Kenya Medical Research Institute, Nairobi, Kenya
Alexandre Doyen, Vestergaard Frandsen, Nairobi, Kenya.
Craig R. Cohen, Email: CCohen@globalhealth.ucsf.edu, Department of Obstetrics, Gynecology and Reproductive Sciences, University of California San Francisco, 50 Beale Street, Suite 1200, San Francisco, CA 94105, USA
References
- 1.Jahn A, Floyd S, Crampin AC, Mwaungulu F, Mvula H, Munthali F, et al. Population-level effect of HIV on adult mortality and early evidence of reversal after introduction of antiretroviral therapy in Malawi. Lancet. 2008;10(371(9624)):1603–11. doi: 10.1016/S0140-6736(08)60693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;26(9635):293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV outpatient study investigators. N Engl J Med. 1998;26(338(13)):853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;11(367(9513)):817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 5.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;1(22(15)):1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krentz HB, Auld MC, Gill MJ. The high cost of medical care for patients who present late (CD4 < 200 cells/microL) with HIV infection. HIV Med. 2004;5(2):93–8. doi: 10.1111/j.1468-1293.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 7.Samet JH, Freedberg KA, Savetsky JB, Sullivan LM, Stein MD. Understanding delay to medical care for HIV infection: the long-term non-presenter. AIDS. 2001;5(15(1)):77–85. doi: 10.1097/00002030-200101050-00012. [DOI] [PubMed] [Google Scholar]
- 8.Brinkhof MW, Dabis F, Myer L, Bangsberg DR, Boulle A, Nash D, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86(7):559–67. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;9(18(6)):887–95. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 10.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10, 000 adults in Cote d’Ivoire: 2-year outcomes and determinants. AIDS. 2008;23(22(7)):873–82. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox MP, Sanne IM, Conradie F, Zeinecker J, Orrell C, Ive P, et al. Initiating patients on antiretroviral therapy at CD4 cell counts above 200 cells/microL is associated with improved treatment outcomes in South Africa. AIDS. 2010;24(24(13)):2041–50. doi: 10.1097/QAD.0b013e32833c703e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stangl AL, Wamai N, Mermin J, Awor AC, Bunnell RE. Trends and predictors of quality of life among HIV-infected adults taking highly active antiretroviral therapy in rural Uganda. AIDS Care. 2007;19(5):626–36. doi: 10.1080/09540120701203915. [DOI] [PubMed] [Google Scholar]
- 13.Ford N, Kranzer K, Hilderbrand K, Jouquet G, Goemaere E, Vlahakis N, et al. Early initiation of antiretroviral therapy and associated reduction in mortality, morbidity and defaulting in a nurse-managed, community cohort in Lesotho. AIDS. 2010;24(17):2645. doi: 10.1097/QAD.0b013e32833ec5b2. [DOI] [PubMed] [Google Scholar]
- 14.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;30(360(18)):1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kigozi BK, Sumba S, Mudyope P, Namuddu B, Kalyango J, Karamagi C, et al. The effect of AIDS defining conditions on immunological recovery among patients initiating antiretroviral therapy at Joint. Clinical Research Centre, Uganda. AIDS Res Ther. 2009;6:17. doi: 10.1186/1742-6405-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy RA, Sunpath H, Taha B, Kappagoda S, Maphasa KT, Kuritzkes DR, et al. Low uptake of antiretroviral therapy after admission with human immunodeficiency virus and tuberculosis in KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis. 2010;14(7):903–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Bunnell R, Mermin J, De Cock KM. HIV prevention for a threatened continent: implementing positive prevention in Africa. JAMA. 2006;16(296(7)):855–8. doi: 10.1001/jama.296.7.855. [DOI] [PubMed] [Google Scholar]
- 18.Castilla J, Del Romero J, Hernando V, Marincovich B, Garcia S, Rodriguez C. Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. J Acquir Immune Defic Syndr. 2005;1(40(1)):96–101. doi: 10.1097/01.qai.0000157389.78374.45. [DOI] [PubMed] [Google Scholar]
- 19.Girardi E, Sabin CA, Monforte AD. Late diagnosis of HIV infection: epidemiological features, consequences and strategies to encourage earlier testing. J Acquir Immune Defic Syndr. 2007;46(1):S3–8. doi: 10.1097/01.qai.0000286597.57066.2b. [DOI] [PubMed] [Google Scholar]
- 20.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;17(23(11)):1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 21.Vernazza P, Hirschel B, Bernasconi E, Flepp M. HIV transmission under highly active antiretroviral therapy. Lancet. 2008;22(372(9652)):1806–7. doi: 10.1016/S0140-6736(08)61753-5. author reply 1807. [DOI] [PubMed] [Google Scholar]
- 22.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 23.Menzies N, Abang B, Wanyenze R, Nuwaha F, Mugisha B, Coutinho A, et al. The costs and effectiveness of four HIV counseling and testing strategies in Uganda. AIDS. 2009;28(23(3)):395–401. doi: 10.1097/QAD.0b013e328321e40b. [DOI] [PubMed] [Google Scholar]
- 24.Negin J, Wariero J, Mutuo P, Jan S, Pronyk P. Feasibility, acceptability and cost of home-based HIV testing in rural Kenya. Trop Med Int Health. 2009;14(8):849–55. doi: 10.1111/j.1365-3156.2009.02304.x. [DOI] [PubMed] [Google Scholar]
- 25.Morin SF, Khumalo-Sakutukwa G, Charlebois ED, Routh J, Fritz K, Lane T, et al. Removing barriers to knowing HIV status: same-day mobile HIV testing in Zimbabwe. J Acquir Immune Defic Syndr. 2006;1(41(2)):218–24. doi: 10.1097/01.qai.0000179455.01068.ab. [DOI] [PubMed] [Google Scholar]
- 26.Lugada E, Millar D, Haskew J, Grabowsky M, Garg N, Vestergaard M, et al. Rapid implementation of an integrated large-scale HIV counseling and testing, malaria, and diarrhea prevention campaign in rural Kenya. PLoS One. 2010;5(8):e12435. doi: 10.1371/journal.pone.0012435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabbe KL, Menzies N, Taegtmeyer M, Emukule G, Angala P, Mwega I, et al. Increasing access to HIV counseling and testing through mobile services in Kenya: strategies, utilization, and cost-effectiveness. J Acquir Immune Defic Syndr. 2010;1(54(3)):317–23. doi: 10.1097/QAI.0b013e3181ced126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tumwesigye E, Wana G, Kasasa S, Muganzi E, Nuwaha F. High uptake of home-based, district-wide, HIV counseling and testing in Uganda. AIDS Patient Care STDS. 2010;24(11):735–41. doi: 10.1089/apc.2010.0096. [DOI] [PubMed] [Google Scholar]
- 29.Torian LV, Wiewel EW, Liu KL, Sackoff JE, Frieden TR. Risk factors for delayed initiation of medical care after diagnosis of human immunodeficiency virus. Arch Intern Med. 2008;9168(11):1181–7. doi: 10.1001/archinte.168.11.1181. [DOI] [PubMed] [Google Scholar]
- 30.Mugavero M, Lin H, Allison J, Willig J, Chang P, Marler M, et al. Failure to establish HIV care: characterizing the “no show” phenomenon. Clin Infect Dis. 2007;45:127–30. doi: 10.1086/518587. [DOI] [PubMed] [Google Scholar]
- 31.Reed JB, Hanson D, McNaghten AD, Bertolli J, Teshale E, Gardner L, et al. HIV testing factors associated with delayed entry into HIV medical care among HIV-infected persons from eighteen states, United States, 2000–2004. AIDS Patient Care STDS. 2009;23(9):765–73. doi: 10.1089/apc.2008.0213. [DOI] [PubMed] [Google Scholar]
- 32.Ohl M, Tate J, Duggal M, Skanderson M, Scotch M, Kaboli P, et al. Rural residence is associated with delayed care entry and increased mortality among veterans with human immunodeficiency virus infection. Med Care. 2010;48(12):1064–70. doi: 10.1097/MLR.0b013e3181ef60c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tobias CR, Cunningham W, Cabral HD, Cunningham CO, Eldred L, Naar-King S, et al. Living with HIV but without medical care: barriers to engagement. AIDS Patient Care STDS. 2007;21(6):426–34. doi: 10.1089/apc.2006.0138. [DOI] [PubMed] [Google Scholar]
- 34.Decroo T, Panunzi I, das Dores C, Maldonado F, Biot M, Ford N, et al. Lessons learned during down referral of antiretroviral treatment in Tete, Mozambique. J Int AIDS Soc. 2009;12(1):6. doi: 10.1186/1758-2652-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bassett IV, Regan S, Chetty S, Giddy J, Uhler LM, Holst H, et al. Who starts antiretroviral therapy in Durban, South Africa? not everyone who should. AIDS. 2010;24(Suppl 1):S37–44. doi: 10.1097/01.aids.0000366081.91192.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unge C, Johansson A, Zachariah R, Some D, Van Engelgem I, Ekstrom AM. Reasons for unsatisfactory acceptance of antiretroviral treatment in the urban Kibera slum, Kenya. AIDS Care. 2008;20(2):146–9. doi: 10.1080/09540120701513677. [DOI] [PubMed] [Google Scholar]
- 37.Wanyenze RK, Hahn JA, Liechty CA, Ragland K, Ronald A, Mayanja-Kizza H, et al. Linkage to HIV Care and survival following inpatient HIV counseling and testing. AIDS Behav. 2011;15(4):751–60. doi: 10.1007/s10461-010-9704-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kranzer K, Zeinecker J, Ginsberg P, Orrell C, Kalawe NN, Lawn SD, et al. Linkage to HIV care and antiretroviral therapy in Cape Town, South Africa. PLoS One. 2010;5(11):e13801. doi: 10.1371/journal.pone.0013801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakigozi G, Makumbi F, Reynolds S, Galiwango R, Kagaayi J, Nalugoda F, et al. Non-enrollment for free community HIV care: findings from a population-based study in Rakai, Uganda. AIDS Care. 2011;23(6):764–70. doi: 10.1080/09540121.2010.525614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosen S, Fox MP. Retention in HIV Care between testing and treatment in Sub-Saharan Africa: a systematic review. PLoS Med. 2011;8(7):e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sweat M, Morin S, Celentano D, Mulawa M, Singh B, Mbwambo J, et al. Community-based intervention to increase HIV testing and case detection in people aged 16–32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): a randomised study. Lancet Infect Dis. 2011;11(7):525–32. doi: 10.1016/S1473-3099(11)70060-3. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braitstein P, Boulle A, Nash D, Brinkhof MW, Dabis F, Laurent C, et al. Gender and the use of antiretroviral treatment in resource-constrained settings: findings from a multicenter collaboration. J Womens Health. 2008;17(1):47–55. doi: 10.1089/jwh.2007.0353. [DOI] [PubMed] [Google Scholar]
- 43.Amuyunzu-Nyamongo M, Okeng’o L, Wagura A, Mwenzwa E. Putting on a brave face: the experiences of women living with HIV and AIDS in informal settlements of Nairobi, Kenya. AIDS Care. 2007;19:S25–34. doi: 10.1080/09540120601114618. [DOI] [PubMed] [Google Scholar]
- 44.Bond V. AIDS, Poverty, and Hunger. Washington: International Food Policy Research Insitute; 2006. Stigma when there is no other option: understanding how poverty fuels discrimination toward people living with HIV in Zambia; pp. 181–197. [Google Scholar]
- 45.Kiwanuka SN, Ekirapa EK, Peterson S, Okui O, Rahman MH, Peters D, et al. Access to and utilisation of health services for the poor in Uganda: a systematic review of available evidence. Trans R Soc Trop Med Hyg. 2008;102(11):1067–74. doi: 10.1016/j.trstmh.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 46.Mshana GH, Wamoyi J, Busza J, Zaba B, Changalucha J, Kaluvya S, et al. Barriers to accessing antiretroviral therapy in Kisesa, Tanzania: a qualitative study of early rural referrals to the national program. AIDS Patient Care STDS. 2006;20(9):649–57. doi: 10.1089/apc.2006.20.649. [DOI] [PubMed] [Google Scholar]
- 47.Murray LK, Semrau K, McCurley E, Thea DM, Scott N, Mwiya M, et al. Barriers to acceptance and adherence of antiretroviral therapy in urban Zambian women: a qualitative study. AIDS Care. 2009;21(1):78–86. doi: 10.1080/09540120802032643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Otieno PA, Kohler PK, Bosire RK, Brown ER, Macharia SW, John-Stewart GC. Determinants of failure to access care in mothers referred to HIV treatment programs in Nairobi, Kenya. AIDS Care. 2010;22(6):729–36. doi: 10.1080/09540120903373565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Posse M, Meheus F, van Asten H, van der Ven A, Baltussen R. Barriers to access to antiretroviral treatment in developing countries: a review. Trop Med Int Health. 2008;13(7):904–13. doi: 10.1111/j.1365-3156.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 50.Marum E, Taegtmeyer M, Chebet K. Scale-up of voluntary HIV counseling and testing in Kenya. JAMA. 2006;16(296(7)):859–62. doi: 10.1001/jama.296.7.859. [DOI] [PubMed] [Google Scholar]
- 51.Mugavero MJ, Norton W, Saag Health care system and policy factors influencing engagement in HIV medical care: piecing together the fragmanets of a fractured health care delivery system. Clin Infect Dis. 2011;52(S2):S238–46. doi: 10.1093/cid/ciq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DiClemente RJ, Salazar LF, Crosby RA. A review of STD/HIV preventive interventions for adolescents: sustaining effects using an ecological approach. J Pediatr Psychol. 2007;32(8):888–906. doi: 10.1093/jpepsy/jsm056. [DOI] [PubMed] [Google Scholar]
- 53.Latkin CA, Knowlton AR. Micro-social structural approaches to HIV prevention: a social ecological perspective. AIDS Care. 2005;17(1):S102–13. doi: 10.1080/09540120500121185. [DOI] [PubMed] [Google Scholar]
- 54.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item short-form health survey (SF-36) II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Monahan PO, Shacham E, Reece M, Kroenke K, Ong’or WO, Omollo O, et al. Validity/reliability of PHQ-9 and PHQ-2 depression scales among adults living with HIV/AIDS in Western Kenya. J Gen Intern Med. 2009;24(2):189–97. doi: 10.1007/s11606-008-0846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harrison A, O’Sullivan LF, Hoffman S, Dolezal C, Morrell R. Gender role and relationship norms among young adults in South Africa: measuring the context of masculinity and HIV risk. J Urban Health. 2006;83(4):709–22. doi: 10.1007/s11524-006-9077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turan JM, Bukusi EA, Onono M, Holzemer WL, Miller S, Cohen CR. HIV/AIDS stigma and refusal of HIV testing among pregnant women in rural Kenya: results from the MAMAS Study. AIDS Behav. 2011;15(6):1111–20. doi: 10.1007/s10461-010-9798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiser S, Heisler M, Leiter K, Percyde Korte F, Tlou S, DeMonner S. Routine HIV testing in Botswana: a population based study on attitudes, practices, and human rights concerns. PLoS Med. 2006;3:e261. doi: 10.1371/journal.pmed.0030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Genberg BL, Kawichai S, Chingono A, Sendah M, Chariyalertsak S, Konda KA, et al. Assessing HIV/AIDS stigma and discrimination in developing countries. AIDS Behav. 2008;12(5):772–80. doi: 10.1007/s10461-007-9340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holzemer WL, Uys L, Makoae L, Stewart A, Phetlhu R, Dlamini PS, et al. A conceptual model of HIV/AIDS stigma from five African countries. J Adv Nurs. 2007;58(6):541–51. doi: 10.1111/j.1365-2648.2007.04244.x. [DOI] [PubMed] [Google Scholar]
- 61.Gielen AC, McDonnell KA, Wu AW, O’Campo P, Faden R. Quality of life among women living with HIV: the importance violence, social support, and self care behaviors. Soc Sci Med. 2001;52(2):315–22. doi: 10.1016/s0277-9536(00)00135-0. [DOI] [PubMed] [Google Scholar]
- 62.Hardon AP, Akurut D, Comoro C, Ekezie C, Irunde HF, Gerrits T, et al. Hunger, waiting time and transport costs: time to confront challenges to ART adherence in Africa. AIDS Care. 2007;19(5):658–65. doi: 10.1080/09540120701244943. [DOI] [PubMed] [Google Scholar]
- 63.Cohen CR, Montandon M, Carrico AW, Shiboski S, Bostrom A, Obure A, et al. Association of attitudes and beliefs towards antiretroviral therapy with HIV-seroprevalence in the general population of Kisumu, Kenya. PLoS One. 2009;4(3):e4573. doi: 10.1371/journal.pone.0004573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nsigaye R, Wringe A, Roura M, Kalluvya S, Urassa M, Busza J, et al. From HIV diagnosis to treatment: evaluation of a referral system to promote and monitor access to antiretroviral therapy in rural Tanzania. J Int AIDS Soc. 2009;12(1):31. doi: 10.1186/1758-2652-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anyango B. personal communication. Kisumu: Tuungane Health Center; 2009. [Google Scholar]
- 66.Menya P. Personal communication. Kisumu: Liverpool VCT; 2009. [Google Scholar]
- 67.Musili P. Personal communication. Eldoret: AMPATH; 2009. [Google Scholar]
- 68.Adeneye AK, Adewole TA, Musa AZ, Onwujekwe D, Odunukwe NN, Araoyinbo ID, et al. Limitations to access and use of antiretroviral therapy (ART) among HIV positive persons in Lagos, Nigeria. World Health Popul. 2006;8(2):46–56. doi: 10.12927/whp.2006.18134. [DOI] [PubMed] [Google Scholar]
- 69.Nyanzi-Wakholi B, Lara AM, Watera C, Munderi P, Gilks C, Grosskurth H. The role of HIV testing, counselling, and treatment in coping with HIV/AIDS in Uganda: a qualitative analysis. AIDS Care. 2009;21(7):903–8. doi: 10.1080/09540120802657498. [DOI] [PubMed] [Google Scholar]
- 70.Lertpiriyasuwat C, Yachompoo C, Yuktanon P, Thanprasertsuk S, Leusaree T, Khemngern P, et al., editors. Access to antiretroviral drugs in northern Thailand: perspectives of persons living with HIV/AIDS; XV International AIDS Conference; Bangkok: 2004. [Google Scholar]
- 71.Efuntoye M, Okonkwo O, Omorgeie G, Musa G. Increasing access to HIV counselling and testing: a demonstration project trialling the greater involvement of people living with HIV/AIDS (GIPA) in the workplace; XVII International AIDS Conference; Mexico: 2008. p. Abstract TUZX0405. [Google Scholar]
- 72.Chang LW, Kagaayi J, Nakigozi G, Ssempijja V, Packer AH, Serwadda D, et al. Effect of peer health workers on AIDS care in Rakai, Uganda: a cluster-randomized trial. PLoS One. 2010;5(6):e10923. doi: 10.1371/journal.pone.0010923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mukherjee J, Leandre F, Lambert W, editors. Excellent outcomes, high retention in treatment, and low rate of switch to second line ART in community based HIV treatment program in Haiti; XVII International AIDS conference Mexico; Mexico: 2008. [Google Scholar]
- 74.Kabore I, Bloem J, Etheredge G, Obiero W, Wanless S, Doykos P, et al. The effect of community-based support services on clinical efficacy and health-related quality of life in HIV/AIDS patients in resource-limited set tings in sub-Saharan Africa. AIDS Patient Care STDS. 2010;24(9):581–94. doi: 10.1089/apc.2009.0307. [DOI] [PubMed] [Google Scholar]
- 75.Cabral HJ, Tobias C, Rajabiun S, Sohler N, Cunningham C, Wong M, et al. Outreach program contacts: do they increase the likelihood of engagement and retention in HIV primary care for hard-to-reach patients? AIDS Patient Care STDS. 2007;21(1):S59–67. doi: 10.1089/apc.2007.9986. [DOI] [PubMed] [Google Scholar]
- 76.Gardner LI, Metsch LR, Anderson-Mahoney P, Loughlin AM, del Rio C, Strathdee S, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;4(19(4)):423–31. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 77.Bradford JB, Coleman S, Cunningham W. HIV system navigation: an emerging model to improve HIV care access. AIDS Patient Care STDS. 2007;21(1):S49–58. doi: 10.1089/apc.2007.9987. [DOI] [PubMed] [Google Scholar]
- 78.Louis C, Ivers LC, Smith Fawzi MC, Freedberg KA, Castro A. Late presentation for HIV care in central Haiti: factors limiting access to care. AIDS Care. 2007;19(4):487–91. doi: 10.1080/09540120701203246. [DOI] [PubMed] [Google Scholar]
- 79.Kigozi IM, Dobkin LM, Martin JN, Geng EH, Muyindike W, Emenyonu NI, et al. Late-disease stage at presentation to an HIV clinic in the era of free antiretroviral therapy in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2009;1(52(2)):280–9. doi: 10.1097/QAI.0b013e3181ab6eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fonck K, Mwai C, Ndinya-Achola J, Bwayo J, Temmerman M. Health-seeking and sexual behaviors among primary healthcare patients in Nairobi, Kenya. Sex Transm Dis. 2002;29(2):106–11. doi: 10.1097/00007435-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 81.Taegtmeyer M, Kilonzo N, Mung’ala L, Morgan G, Theobald S. Using gender analysis to build voluntary counselling and testing responses in Kenya. Trans R Soc Trop Med Hyg. 2006;100(4):305–11. doi: 10.1016/j.trstmh.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 82.Voeten HA, O’Hara HB, Kusimba J, Otido JM, Ndinya-Achola JO, Bwayo JJ, et al. Gender differences in health care-seeking behavior for sexually transmitted diseases: a population-based study in Nairobi, Kenya. Sex Transm Dis. 2004;31(5):265–72. doi: 10.1097/01.olq.0000124610.65396.52. [DOI] [PubMed] [Google Scholar]
- 83.Luseno WK, Wechsberg WM, Kline TL, Ellerson RM. Health services utilization among South African women living with HIV and reporting sexual and substance-use risk behaviors. AIDS Patient Care STDS. 2010;24(4):257–64. doi: 10.1089/apc.2009.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boyer S, Marcellin F, Ongolo-Zogo P, Abega SC, Nantchouang R, Spire B, et al. Financial barriers to HIV treatment in Yaounde, Cameroon: first results of a national cross-sectional survey. Bull World Health Organ. 2009;87(4):279–87. doi: 10.2471/BLT.07.049643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karcher H, Omondi A, Odera J, Kunz A, Harms G. Risk factors for treatment denial and loss to follow-up in an antiretroviral treatment cohort in Kenya. Trop Med Int Health. 2007;12(5):687–94. doi: 10.1111/j.1365-3156.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 86.Smith R, Carrico A, Montadon M, Bailey R, Kwena Z, Bukusi E, et al. Attitudes and beliefs about antiretroviral therapy are associated with risky sexual behavior in Kisumu, Kenya. 6th annual Conference on Retroviruses and Opportunistic Infectional; Montreal. 2009;Feb. 8–10. [Google Scholar]
- 87.Unge C, Johansson A, Zachariah R, Some D, Van Engelgem I, Ekstrom AM. Reasons for unsatisfactory acceptance of antiretroviral treatment in the urban Kibera slum, Kenya. AIDS Care. 2008;20(2):146–9. doi: 10.1080/09540120701513677. [DOI] [PubMed] [Google Scholar]
- 88.Stevens W, Kaye S, Corrah T. Antiretroviral therapy in Africa. BMJ. 2004;31(328(7434)):280–2. doi: 10.1136/bmj.328.7434.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Micek MA, Gimbel-Sherr K, Baptista AJ, Matediana E, Montoya P, Pfeiffer J, et al. Loss to follow-up of adults in public HIV care systems in central Mozambique: identifying obstacles to treatment. J Acquir Immune Defic Syndr. 2009;1(52(3)):397–405. doi: 10.1097/QAI.0b013e3181ab73e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Olley BO, Seedat S, Stein DJ. Self-disclosure of HIV serostatus in recently diagnosed patients with HIV in South Africa. Afr J Reprod Health. 2004;8(2):71–6. [PubMed] [Google Scholar]
- 91.Maman S, Mbwambo J, Hogan NM, Kilonzo GP, Sweat M. Women’s barriers to HIV-1 testing and disclosure: challenges for HIV-1 voluntary counselling and testing. AIDS Care. 2001;13(5):595–603. doi: 10.1080/09540120120063223. [DOI] [PubMed] [Google Scholar]
- 92.Emusu D, Ivankova N, Jolly P, Kirby R, Foushee H, Wabwire-Mangen F, et al. Experience of sexual violence among women in HIV discordant unions after voluntary HIV counselling and testing: a qualitative critical incident study in Uganda. AIDS Care. 2009;21(11):1363–70. doi: 10.1080/09540120902883077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mlay R, Lugina H, Becker S. Couple counselling and testing for HIV at antenatal clinics: views from men, women and counsellors. AIDS Care. 2008;20(3):356–60. doi: 10.1080/09540120701561304. [DOI] [PubMed] [Google Scholar]
- 94.Martin-Herz SP, Shetty AK, Bassett MT, Ley C, Mhazo M, Moyo S, et al. Perceived risks and benefits of HIV testing, and predictors of acceptance of HIV counselling and testing among pregnant women in Zimbabwe. Int J STD AIDS. 2006;17(12):835–41. doi: 10.1258/095646206779307630. [DOI] [PubMed] [Google Scholar]
- 95.Pool R, Nyanzi S, Whitworth JA. Attitudes to voluntary counselling and testing for HIV among pregnant women in rural south-west Uganda. AIDS Care. 2001;13(5):605–15. doi: 10.1080/09540120120063232. [DOI] [PubMed] [Google Scholar]
- 96.Gielen AC, McDonnell KA, Burke JG, O’Campo P. Women’s lives after an HIV-positive diagnosis: disclosure and violence. Matern Child Health J. 2000;4(2):111–20. doi: 10.1023/a:1009522321240. [DOI] [PubMed] [Google Scholar]
- 97.Grinstead OA, Gregorich SE, Choi KH, Coates T. Positive and negative life events after counselling and testing: the Voluntary HIV-1 counselling and testing efficacy study. AIDS. 2001;25 (15(8)):1045–52. doi: 10.1097/00002030-200105250-00013. [DOI] [PubMed] [Google Scholar]
- 98.Kilewo C, Massawe A, Lyamuya E, Semali I, Kalokola F, Urassa E, et al. HIV counseling and testing of pregnant women in sub-Saharan Africa: experiences from a study on prevention of mother-to-child HIV-1 transmission in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr. 2001;15(28(5)):458–62. doi: 10.1097/00042560-200112150-00009. [DOI] [PubMed] [Google Scholar]
- 99.Maman S, Mbwambo JK, Hogan NM, Kilonzo GP, Campbell JC, Weiss E, et al. HIV-positive women report more lifetime partner violence: findings from a voluntary counseling and testing clinic in Dar es Salaam, Tanzania. Am J Public Health. 2002;92(8):1331–7. doi: 10.2105/ajph.92.8.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Makoae LN, Greeff M, Phetlhu RD, Uys LR, Naidoo JR, Kohi TW, et al. Coping with HIV-related stigma in five African countries. J Assoc Nurses AIDS Care. 2008;19(2):137–46. doi: 10.1016/j.jana.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nam SL, Fielding K, Avalos A, Dickinson D, Gaolathe T, Geissler PW. The relationship of acceptance or denial of HIV-status to antiretroviral adherence among adult HIV patients in urban Botswana. Soc Sci Med. 2008;67(2):301–10. doi: 10.1016/j.socscimed.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 102.Ogden J, Nyblade L. Common at its core: HIV-related stigma across contexts. Washington: International Center for Research on Women; 2005. [Google Scholar]
- 103.Reis C, Heisler M, Amowitz L. Discriminatory attitudes and practices by health workers toward patients with HIV/AIDS in Nigeria. PLoS Med. 2005;2(8):e246. doi: 10.1371/journal.pmed.0020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in Southwestern Uganda: a qualitative study. AIDS Behav. 2009;14(4):778–84. doi: 10.1007/s10461-009-9533-2. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Assefa Y, Van Damme W, Mariam DH, Kloos H. Toward universal access to HIV counseling and testing and antiretroviral treatment in Ethiopia: looking beyond HIV testing and ART initiation. AIDS Patient Care STDS. 2010;24(8):521–5. doi: 10.1089/apc.2009.0286. [DOI] [PubMed] [Google Scholar]
- 106.Larson BA, Brennan A, McNamara L, Long L, Rosen S, Sanne I, et al. Early loss to follow up after enrolment in pre-ART care at a large public clinic in Johannesburg, South Africa. Trop Med Int Health. 2010;15(1):43–7. doi: 10.1111/j.1365-3156.2010.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Amuron B, Namara G, Birungi J, Nabiryo C, Levin J, Grosskurth H, et al. Mortality and loss-to-follow-up during the pre-treatment period in an antiretroviral therapy programme under normal health service conditions in Uganda. BMC Public Health. 2009;9:290. doi: 10.1186/1471-2458-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hoffman RM, Black V, Technau K, van der Merwe KJ, Currier J, Coovadia A, et al. Effects of highly active antiretroviral therapy duration and regimen on risk for mother-to-child transmission of HIV in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2010;1(54(1)):35–41. doi: 10.1097/QAI.0b013e3181cf9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.WHO. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Geneva: World Health Organization; 2008. [Google Scholar]
- 110.Kairania R, Gray RH, Kiwanuka N, Makumbi F, Sewankambo NK, Serwadda D, et al. Disclosure of HIV results among discordant couples in Rakai, Uganda: a facilitated couple counselling approach. AIDS Care. 2010;22(9):1041–51. doi: 10.1080/09540121003602226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Farquhar C, Kiarie JN, Richardson BA, Kabura MN, John FN, Nduati RW, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J Acquir Immune Defic Syndr. 2004;15(375):1620–6. doi: 10.1097/00126334-200412150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]