Abstract
Background
Type 2 diabetes is associated with low-grade systemic inflammation, increasing the risk for various adverse health outcomes.
Purpose
Our objective was to investigate the association between C-reactive protein (CRP), a marker for systemic inflammation, and lifestyle factors in a national sample of people with type 2 diabetes.
Methods
This study analyzed data from 1086 men and women with diabetes, who completed the 1999-2004 NHANES. Lifestyle factors included diet quality, body mass index (BMI), smoking, and physical activity.
Results
Stratified logistic regression showed that for both men and women, BMI was a strong predictor of elevated CRP after adjusting for age, energy intake, race/ethnicity, medications, diabetes duration, and glycosylated hemoglobin. However, among men, but not among women, the likelihood of elevated CRP increased with lower diet quality and physical inactivity.
Conclusions
Among people with type 2 diabetes, higher levels of CRP were associated with lower diet quality and physical inactivity among men, and with obesity among both men and women.
Keywords: type 2 diabetes, C-reactive protein, lifestyle, inflammation
Introduction
Type 2 diabetes is among the most common chronic diseases in the United States, affecting about 8% of the population [1]. People with type 2 diabetes are at increased risk for many diseases and conditions; for example, they are up to 4 times more likely to develop cardiovascular disease than people without diabetes [2]. Diabetes is associated with low-grade systemic inflammation [3], which is suggested to play a role in pathogenesis of cardiovascular disease, and thus may be responsible for the increased cardiovascular risk among diabetics [4].
A healthy lifestyle may reduce systemic inflammation, and thus decrease the risk of cardiovascular disease among the general population [5]. In particular, elevated C-reactive protein (CRP), a marker for systemic inflammation, has been associated with lifestyle risk factors for cardiovascular disease, such as obesity [6], physical inactivity [7], cigarette smoking [8], and high intake of saturated fat [9] and low intake of fruit and vegetables [10], and whole grains [11]. However, little is known about the association between CRP and modifiable lifestyle factors among people with diabetes who already have increased CRP levels [12]. In women with type 2 diabetes, the Women's Health Study demonstrated that consumption of whole grains and a low glycemic diet was associated with lower levels of CRP [13]. Nonetheless, the overall impact of diet, particularly diet quality, has not yet been tested in people with diabetes. In addition, the evidence for an association between physical activity and CRP in type 2 diabetes has been mainly restricted to a few experimental studies that yielded inconsistent results [14, 15]. Since women with diabetes have higher CRP levels [16] and exhibit a higher risk of cardiovascular disease than men with diabetes [17], lifestyle factors also might differentially vary between the sexes in their associations with CRP. However, the association between lifestyle factors and CRP among people with diabetes has not been examined separately by gender. This study evaluated the associations of diet quality and other lifestyle factors with CRP separately in men and in women with type 2 diabetes, using data from the 1999-2004 National Health and Nutrition Examination Survey (NHANES).
Methods
Participants
NHANES collects cross-sectional data from a nationally representative sample of the non-institutionalized civilian U.S. population [18]. The survey included household interviews and physical examinations, including phlebotomy, conducted in mobile examination centers. Respondents were determined to have diabetes if they gave a positive response to the question, “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Respondents younger than 30 years of age and those with the first diagnosis of diabetes or insulin therapy before age 30 were excluded, in order to minimize the likelihood of including people with type 1 diabetes.
C-reactive protein
In NHANES, standard phlebotomy techniques were used to obtain serum specimens. The samples were frozen to -20 C before laboratory analysis. CRP was analyzed with a highly sensitive assay technique, using latex-enhanced nephelometry. Details about the laboratory procedures are reported elsewhere [19]. Elevated CRP was defined using the cut-off value of CRP > 3.0 mg/L, defined by the American Heart Association and the Centers for Disease Control and Prevention as a group at high risk for cardiovascular disease [20].
Diet quality: Healthy Eating Index-2005
Dietary data were collected via a 24-hour recall method. The interview was conducted by a trained dietary interviewer in the mobile examination center, using a computer-assisted dietary interview in NHANES 1999-2001 and a fully computerized recall method beginning with NHANES 2002. From the 24-hour recall data, NHANES estimated intakes of energy and nutrients using U.S. Department of Agriculture food composition data [21].
Diet quality was measured with the Healthy Eating Index-2005 (HEI-2005) [22], which was developed by the National Cancer Institute and the U.S. Department of Agriculture to measure compliance with Dietary Guidelines for Americans, 2005 [23]. The HEI-2005 score is the sum of scores for 12 components including: total fruit, whole fruit, total vegetables, dark green and orange vegetables and legumes, total grains, whole grains, milk, meat and beans, oils, saturated fat, sodium, and calories from solid fats, alcoholic beverages, and added sugars (SoFAAS). The score for each of the 12 components ranges from 0 to 5 for total fruit, whole fruit, total vegetables, dark green and orange vegetables and legumes, total grains, whole grains, from 0 to 10 for milk, meat and beans, oils, saturated fat, sodium, and from 0 to 20 for the calories from SoFAAS. The total possible score ranges from 0 to 100, with higher scores indicating greater compliance with the guidelines, and a score above 80 indicating a “good” diet, as defined for the original HEI [24]. The score for each component and the total HEI-2005 were calculated from the 24-hour dietary recall information, using SAS code released by the U.S. Department of Agriculture [25].
Total HEI-2005 scores were categorized by quartile (Q), with Q1 indicating least compliant (poorest-quality diet) and Q4 indicating most compliant with dietary guidelines (healthiest-quality diet) in order to examine nonlinear effects. In addition, to compare the components of the HEI-2005 between men and women and also between the poorest- and healthiest-quality diets, the percentage of maximum possible score was calculated for each component by taking the ratio of the participant's score to the maximum possible score, and multiplying the value by 100.
Other lifestyle factors
Physical activity was measured by a standard leisure-time activity questionnaire. For respondents who reported engaging in moderate/vigorous-intensity activity, metabolic equivalent of task (MET) scores were assigned, based on the reported frequency, duration and intensity of the activity [18]. A score of 0 was assigned if moderate/vigorous-intensity activity was not reported. MET-hours per week spent in each activity were summed to get the total leisure-time physical activity score for each respondent. Three categories of physical activity were then defined, similar to a previous study [26], based on MET-hours/week scores as: inactive (0), somewhat active (> 0 to ≥ 9) and active (≥ 9), according to the recommended 150 minutes/week of moderate-intensity physical activity which equals about 9 MET-hours/week [27]. Body mass index (BMI), calculated from measured weight and height, was categorized as underweight (< 18.5), normal range (18.5-24.9), pre-obese (25.0-29.9), obese class I (30.0-34.9), obese class II (35.0-39.9), and obese class III, also called very severe obesity, (≥ 40.0) [28]. In regression analyses, underweight and normal range groups were combined because of the small numbers of underweight individuals. Self-reported smoking status was categorized as current, past, and never smokers.
Covariates
Age and race/ethnicity were assessed by self-report. Poverty status was assessed by Poverty Income Ratio (PIR), calculated by NHANES based on self-reported family income and family size, using tables published yearly by the U.S. Census Bureau. Poverty status was categorized as below (PIR < 1) or above (PIR ≥ 1) the poverty level. Use of prescription and non-prescription medications in the past month was collected by self report and verified by the interviewer's inspection of the medications containers. Specifically, regular use of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) and lipid-lowering drugs were included in the analysis because of their potential beneficial effects on CRP [29, 30]. Self-reported current use of estrogen replacement therapy was included for women. Duration of diabetes was calculated by subtracting age at diagnosis of diabetes from current age, and was categorized as: < 5, 5–10, and >10 years [31]. Reported energy intake (kcal) from the dietary information was used as a continuous variable. Glycosylated hemoglobin (HbA1c), an indicator of metabolic control of diabetes that is associated with elevated CRP in people with diabetes [32], was included as a covariate in the analysis; HbA1c was measured in NHANES using a high performance liquid chromatography (HPLC) system. Self-reported current treatment with insulin and/or oral hypoglycemic agents was identified for descriptive purposes.
Statistical analysis
All statistical analyses were performed using SAS 9.2 proc survey procedures (SAS Institute, Cary, NC). Data from NHANES cycles 1999-2000, 2001-2002 and 2003-2004 were combined and weighted using sampling weights to account for the complex sampling design in NHANES. The domain option in SAS was used for sub-sample analysis. Descriptive statistics were performed to compare men and women, using chi-square tests and one-way analyses of variance (ANOVAs). In addition, one-way ANOVAs were performed to compare the percentages of respondents with maximum possible scores on the HEI-2005 components between men and women as well as between respondents with the poorest- and healthiest-quality diets. Sex-specific logistic regression models were constructed to estimate the odds ratio (OR) and 95% confidence intervals (95% CI) of dietary and other potential lifestyle determinants of elevated CRP. In addition to the total dietary score, we examined the impact of HEI-2005 components by including each component, individually, in the logistic regression models.
We also developed a behavioral/lifestyle variable to examine the combined effect of BMI, physical activity and diet on the risk of elevated CRP. Respondents were classified into three behavioral/lifestyle groups: the poorest behavior (BMI ≥ 30, Q1 diet quality, and being inactive); a relatively healthy behavior (BMI < 30, Q2-Q4 diet quality, and being either active or somewhat active); and any other combination of BMI, physical activity and diet. All logistic regression models controlled for age, race/ethnicity, poverty status, medications (NSAIDs and lipid lowering), energy intake, duration of diabetes, use of estrogen replacement therapy (for women), and HbA1c. For categorical covariates, a missing-data category was created, as needed, to include as many respondents in the analysis as possible.
Because the results of logistic regression could be affected by the cut-off value for CRP (CRP > 3.0 mg/L indicating high cardiovascular disease risk [20]), separate multiple linear regression models for men and for women also were fitted with log CRP as the dependent variable, including the same independent variables as used in the logistic regression model. The direction of the associations observed in the linear and logistic regression models were compared to assess if any differences observed in the separate logistic regression models for men and women could be attributed to the CRP cut-off point.
Results
A total of 1576 participants reported having diabetes. Of these, 490 were excluded because of missing CRP (n = 233) or lifestyle variable (n = 119) data or because of other exclusion criteria (< 30 years of age [n = 54] or first diagnosis of diabetes or insulin therapy before age 30 [n = 84]). A higher proportion of NHANES respondents who were excluded were women compared with respondents who were included in the analysis (57% vs. 49%, p < 0.05). Among 1,086 adults that were included in this analysis, 533 were women (462 reported being postmenopausal; 85 were < 50 years of age), and 553 were men (79 were < 50 years) (Table 1). The median (interquartile range [IQR]) CRP was 4.5 (2.2-9.1) mg/L among women and 2.7 (1.1-5.4) mg/L among men. Two women and 3 men had CRP >10.0 mg/L, and since removal of these outliers did not affect the results, they were included in the analysis. A greater proportion of women (64%) than men (44%) had elevated CRP levels. Fifty-five percent of women and 41% of men were obese (BMI ≥ 30), and the majority of both men (51%) and women (62%) were physically inactive. Compared to men, women were significantly older (61.9 vs. 58.4 years) and less likely to be non-Hispanic White, to smoke, be above poverty level; women also had a higher mean HEI-2005 score and a lower mean daily energy intake compared to men (each p < 0.05). Hypoglycemic treatment included insulin alone (13%), oral agents alone (62%), both insulin and oral agents (9%), and no medication (16%), with a higher proportion of no medication reported by participants with BMI < 30 compared with participants with BMI ≥ 30 (19% vs. 12%, p < 0.05).
Table 1.
Baseline characteristics in individuals with diabetes (N = 1086), by gender
| Women | Men | p a | |
|---|---|---|---|
| N (%) | 533 (49) | 553 (51) | 0.22 |
| Inflammation | |||
| CRP levels, mg/L | < 0.01 | ||
| ≤ 3.0 | 191 (36) | 308 (56) | |
| > 3.0 | 342 (64) | 245 (44) | |
| Participants’ characteristics | |||
| Age, years, mean (SE) | 62 (0.63) | 58 (0.69) | < 0.01 |
| Race / ethnicity | 0.02 | ||
| Non-Hispanic Black | 133(25) | 121(22) | |
| Mexican American | 166 (31) | 161 (29) | |
| Non-Hispanic White | 191(36) | 235 (43) | |
| Other | 43(8) | 36 (6) | |
| Poverty status | < 0.01 | ||
| Below poverty level (PIR < 1) | 137 (26) | 91 (16) | |
| Above poverty level (PIR ≥ 1) | 346 (65) | 412 (75) | |
| missing | 50 (9) | 50 (9) | |
| Diabetes duration, years | 0.49 | ||
| < 5 | 121 (23) | 134 (24) | |
| 5-10 | 94 (18) | 115 (21) | |
| > 10 | 124 (23) | 118 (21) | |
| Missing | 194 (36) | 186 (34) | |
| Medications | |||
| NSAIDs (Yes vs. No) | 171 (32) | 186 (34) | 0.51 |
| Lipid lowering (Yes vs. No) | 148 (28) | 176 (32) | 0.63 |
| Glycemic control | |||
| HbA1c, mean (SE) | 7.40 (0.13) | 7.48 (0.12) | 0.62 |
| Estrogen replacement therapy | |||
| Current use (Yes vs. No) | 61 (11) | . | |
| Lifestyle factors | . | ||
| HEI-2005 score (0-100), mean (SE) | 59.87 (0.93) | 56.91 (0.83) | 0.03 |
| Energy/day (kcal), mean (SE) | 1585 (34) | 2162 (65) | <.0001 |
| Smoking status | <.0001 | ||
| Current | 77 (14) | 107 (19) | |
| Past | 131 (25) | 255 (46) | |
| Never | 325 (61) | 191 (35) | |
| BMI, kg/m2 | 0.10 | ||
| Obesity class III (≥ 40.0) | 70 (13) | 34 (6) | |
| Obesity class II (35.0-39.9) | 95 (18) | 63 (11) | |
| Obesity class I (30.0-34.9) | 128 (24) | 130 (24) | |
| Pre-obese (25.0-29.9) | 166 (31) | 224 (41) | |
| Normal range (18.5-24.9) | 73 (14) | 100 (18) | |
| Underweight (< 18.5) | 1 (0.2) | 2 (0.4) | |
| MET-hours/week of leisure-time physical activity | 0.13 | ||
| Inactive (0) | 330 (62) | 280 (51) | |
| Somewhat active (> 0 to < 9) | 148 (28) | 197 (35) | |
| Active ( ≥ 9) | 55 (10) | 76 (14) |
Data are n (%) unless otherwise stated
Chi-square test was used to compare frequencies and ANOVA was used to compare means between men and women.
Abbreviations: BMI, Body Mass Index; HEI-2005, Healthy Eating Index-2005; MET, Metabolic Equivalent Task; PIR: Poverty Income Ratio.
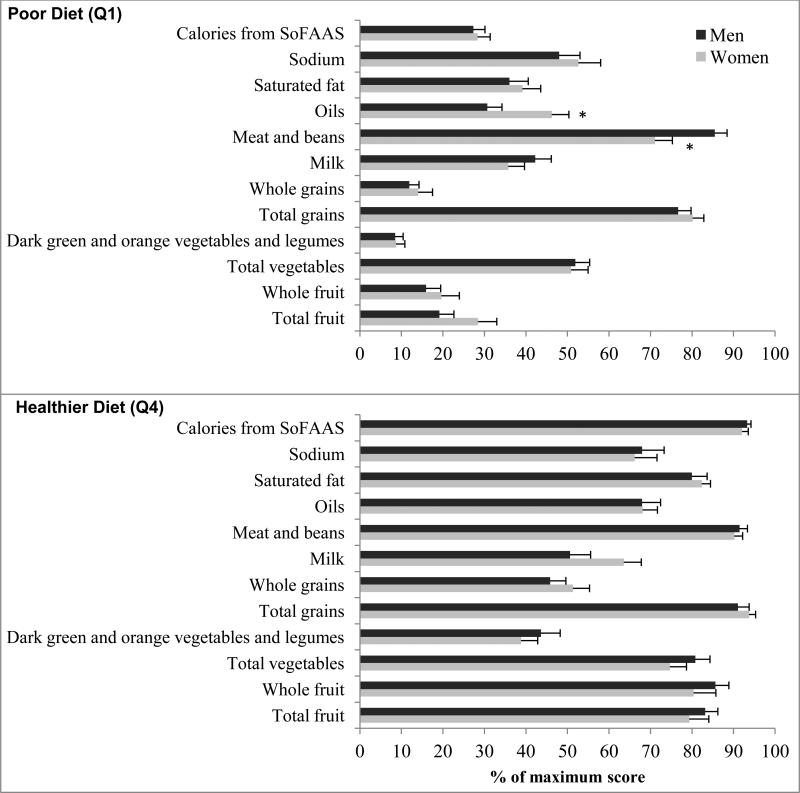
A “good” diet (i.e., total HEI-2005 score greater than 80) was found in 7% of women and 6% of men. Women had higher percentage scores for fruit (mean difference = 10.00, 95% CI: 4.12, 15.87), whole fruit (mean difference = 9.41, 95% CI: 1.76, 17.07) and a lower percentage score for meat and beans (mean difference = -6.59, 95% CI: -10.84, -2.34) than men. However, the differences between men and women varied by diet quality, i.e., Q1 and Q4 of HEI-2005 (Figure 1). With a poor diet, women had a higher percentage score on oils and a lower score on meat and beans, whereas there were no significant differences in the component scores between men and women with a healthier diet. Furthermore, with a poor diet, 11% of men and women met the dietary goal of < 7% of calories from saturated fat according to the American Diabetes Association guidelines [32], whereas with a healthier diet, 35% of men and 25% of women met the goal.
Figure 1.
Dietary score for each component of the Healthy Eating Index-2005 (HEI-2005) as a percentage of the maximum possible score for that component, in poor diet (Q1: upper panel) and healthier diet (Q4: lower panel). Error bars indicate standard error
SoFAAS: Calories from solid fats, alcoholic beverages, and added sugars.
*P < 0.01. ANOVA was used to test differences between men and women.
Both univariate and multivariate logistic regression analyses showed that obesity (class I, II and III) was strongly associated with elevated CRP in both women and men (Table 2). In addition, the poorest- versus healthiest-quality diet (HEI-2005 scores Q1 versus Q4 as reference) and being physically inactive versus active (as reference) were independently associated with greater likelihood of elevated CRP among men, but not women. Moreover, in both men and women, smoking status was not associated with elevated CRP in either univariate or multivariate models. Of the covariates included in the multivariate analyses, use of lipid-lowering medications was associated with lower CRP levels only among men. In addition, increased risk of elevated CRP was associated with higher HbA1C in both women (OR: 1.19, 95% CI: 1.05, 1.35, p < 0.01) and men (OR: 1.15, 95% CI: 1.02, 1.30, p < 0.05), but was associated with older age (OR: 1.03, 95% CI: 1.00, 1.06, p < 0.05), poverty (below vs. above poverty level OR: 1.94, 95% CI: 1.17, 3.22, p < 0.001), and shorter duration of diabetes (< 5 years vs. > 10 years as reference OR: 2.85, 95% CI: 1.38, 5.90, p < 0.01) only among men. In addition, use of estrogen replacement therapy in women was associated with elevated CRP (OR: 2.57, 95% CI: 1.04, 6.34, p < 0.05).
Table 2.
Univariate and multivariate logistic regression analyses of associations between lifestyle factors and elevated CRPa
| Women (n = 533) |
Men (n = 553) |
|||
|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |
| Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | |
| BMI, kg/m2 | ||||
| ≥ 40.0 | 16.50 (5.73, 47.53) | 15.86 (4.61, 54.58) | 10.02 (3.66, 27.42) | 18.27 (5.06, 65.89) |
| 35.0-39.9 | 11.82 (4.08, 34.21) | 16.79 (5.98, 47.14) | 3.32 (1.44, 7.67) | 4.65 (1.87, 11.54) |
| 30.0-34.9 | 3.28 (1.48, 7.26) | 3.57 (1.49, 8.55) | 4.44 (2.03, 9.72) | 5.79 (2.55, 13.14) |
| 25.0-29.9 | 2.09 (1.02, 4.30) | 2.12 (1.02, 4.43) | 2.03 (0.98, 4.17) | 2.08 (0.88, 4.92) |
| < 25.0 | 1.00 | 1.00 | 1.00 | 1.00 |
| HEI-2005 quartiles | ||||
| Q1 (20-50) | 1.22 (0.53, 2.82) | 0.63 (0.25, 1.59) | 1.94 (1.18, 3.21) | 1.95 (1.08, 3.54) |
| Q2 (50-60) | 1.34 (0.72, 2.49) | 1.32 (0.58, 2.98) | 2.04 (1.04, 4.00) | 1.95 (0.96, 3.95) |
| Q3 (60-69) | 1.36 (0.78, 2.37) | 1.02 (0.53, 1.96) | 0.91 (0.47, 1.76) | 0.90 (0.45, 1.79) |
| Q4 (69-94) | 1.00 | 1.00 | 1.00 | 1.00 |
| MET-hours/week of leisure-time physical activity | ||||
| Inactive (0) | 1.30 (0.56, 2.97) | 1.65 (0.69, 3.94) | 2.73 (1.24, 6.00) | 2.36 (1.09, 5.08) |
| Somewhat active (> 0 to < 9) | 0.94 (0.42, 2.12) | 1.09 (0.47, 2.54) | 1.94 (0.99, 3.81) | 1.84 (0.85, 3.96) |
| Active ( ≥ 9) | 1.00 | 1.00 | 1.00 | 1.00 |
| Smoking status | ||||
| Current | 1.27 (0.69, 2.34) | 1.23 (0.61, 2.48) | 1.30 (0.70, 2.39) | 1.42 (0.74, 2.74) |
| Past | 1.43 (0.85, 2.43) | 1.43 (0.77, 2.66) | 0.96 (0.60, 1.50) | 1.28 (0.74, 2.22) |
| Never | 1.00 | 1.00 | 1.00 | 1.00 |
Elevated CRP: CRP > 3.0 mg/L
Abbreviations: BMI, Body Mass Index; CI, Confidence Interval; CRP, C-Reactive Protein; HEI-2005, Healthy Eating Index-2005; MET, Metabolic Equivalent Task.
Multivariate analysis also adjusted for age (continuous), energy (kcal/day), poverty status (Poverty Income Ratio [PIR]: < 1; ≥ 1; missing), race/ethnicity (non-Hispanic Black; Mexican American; non-Hispanic White; other), NSAIDs (Yes; No), lipid-lowering medications (Yes; No), diabetes duration (< 5; 5-10; > 10 years; missing), current estrogen replacement therapy (Yes; No - for women), and HbA1c (continuous).
The effect of each HEI-2005 component was also examined in the multivariate models. Among women, higher scores on whole fruit (OR: 0.87, 95% CI: 0.76, 0.99, p < 0.05) and sodium (OR: 0.92, 95% CI: 0.85, 0.99, p < 0.05) were associated with lower CRP levels, while a higher score on oils (OR: 1.12, 95% CI: 1.04, 1.21, p < 0.01) was associated with higher CRP levels. Among men, better scores on total grains (OR: 0.59, 95% CI: 0.48, 0.74, p < 0.0001), oils (OR: 0.93, 95% CI: 0.86, 1.00, p < 0.05) and SoFAAS (OR: 0.95, 95% CI: 0.92, 0.98, p < 0.001) were associated with lower CRP levels.
In addition, analysis of the combination of lifestyle factors showed that respondents who had the poorest behaviors (i.e., the combination of BMI ≥ 30, Q1 diet quality, and being inactive) had an increased likelihood of elevated CRP with an odds ratio of 13.65 (95% CI: 4.17, 44.64) in men and 3.97 (95% CI: 1.20, 13.14) in women, compared with respondents with relatively healthy lifestyle factors (i.e., the combination of BMI < 30, Q2-Q4 diet quality, and being either active or somewhat active).
To assess whether using the same cut-off point for men and women influenced the associations in logistic regression, we used multiple linear regression models, which replicated the finding that higher CRP levels were associated with the poorest diet among men but not women. However, the association between higher CRP levels and inactivity was borderline significant both among men and among women (Table 3).
Table 3.
Estimated linear regression coefficients (β) and associated 95% confidence intervals for lifestyle factors, with log CRP as outcome
| Women | Men | |||
|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | |
| BMI, kg/m2 | ||||
| ≥ 40 | 1.49 (1.00, 1.98) | <.0001 | 1.20 (0.65, 1.75) | <0.0001 |
| 35-39.9 | 1.35 (0.90, 1.81) | <.0001 | 0.88 (0.43, 1.33) | 0.0003 |
| 30-34.9 | 0.65 (0.25, 1.04) | 0.002 | 0.82 (0.41, 1.24) | 0.0002 |
| 25-29.9 | 0.45 (0.01, 0.88) | 0.04 | 0.40 (-0.03, 0.83) | 0.07 |
| < 25 | Referent | Referent | ||
| HEI-2005 quartiles | ||||
| Q1 (20-50) | -0.18 (-0.51, 0.15) | 0.27 | 0.32 (0.06, 0.58) | 0.02 |
| Q2 (50-60) | 0.07 (-0.26, 0.40) | 0.69 | 0.16 (-0.16, 0.48) | 0.32 |
| Q3 (60-69) | -0.02 (-0.28, 0.25) | 0.89 | -0.05 (-0.30, 0.19) | 0.66 |
| Q4 (69-94) | Referent | Referent | ||
| MET-hours/week of leisure-time physical activity | ||||
| Inactive (0) | 0.34 (-0.02, 0.69) | 0.06 | 0.34 (-0.06, 0.73) | 0.09 |
| Somewhat active (> 0 to < 9) | 0.25 (-0.13, 0.64) | 0.19 | 0.28 (-0.05, 0.62) | 0.10 |
| Active ( ≥ 9) | Referent | Referent | ||
| Smoking | ||||
| Current | -0.06 (-0.35, 0.23) | 0.68 | 0.06 (-0.15, 0.28) | 0.54 |
| Past | 0.10 (-0.16, 0.37) | 0.43 | -0.05 (-0.28, 0.18) | 0.64 |
| Never | Referent | Referent |
Abbreviations: BMI, Body Mass Index; CRP, C-Reactive Protein; HEI-2005, Healthy Eating Index-2005, MET, Metabolic Equivalent Task.
Multivariate analysis also adjusted for age (continuous), energy (kcal/day), poverty status (Poverty Income Ratio [PIR]: < 1; ≥ 1; missing), race/ethnicity (non-Hispanic Black; Mexican American; non-Hispanic White; other), NSAIDs (Yes; No), lipid-lowering medications (Yes; No), diabetes duration (< 5; 5-10; > 10 years; missing), current estrogen replacement therapy (Yes; No - for women), and HbA1c (continuous).
Discussion
To our knowledge, this is the first study to use NHANES data to examine the association between systemic inflammation, measured by serum CRP, and lifestyle factors among men and women with type 2 diabetes. This study showed that the poorest diet quality (Q1) and physical inactivity were associated with elevated CRP levels among men, but not among women. The results remained significant when controlling for demographic and other potential risk factors. In addition, obesity was the only common lifestyle factor associated with elevated CRP levels among both men and women.
Previous research has shown an association between higher levels of CRP and development of type 2 diabetes [33]. Inflammatory markers also have been associated with lifestyle factors among people without diabetes [7, 34]. The present study provides evidence that among people with diabetes, the likelihood of elevated CRP increased with physical inactivity and the poorest diet quality among men and with obesity among both men and women. This underscores the importance of lifestyle modifications to reduce inflammation not only in primary prevention of diabetes but also potentially in secondary prevention of diabetes complications.
The proportion of people with a ‘good’ diet quality was similar in men (6%) and women (7%). However, there was no significant association between total diet quality score and CRP levels among women, as reported previously in overweight and obese postmenopausal women [35]. Nevertheless, higher HEI-2005 whole fruit and sodium component scores were associated with lower CRP levels among women. In addition, women reported higher average consumption of fruit than men, similar to results of a population study of adults in 16 US states [36] and to a clinical study of people with type 2 diabetes in Canada [37]. One possible reason for the absence of an association between the total score of HEI-2005 with CRP among women may be related to the finding that a higher score for the oils component was associated with higher CRP levels among women, as opposed to lower levels among men. The contradictory findings for the oils component may point to a limitation of this HEI-2005 component. The oils component, “non-hydrogenated vegetable oils, and oils in fish, nuts and seeds”, does not capture extra intake of oils [38] and includes a variety of food items, which precludes our ability to discern the sources of ingested oil. While oils rich in long-chain n-3 fatty acids (e.g., from fish oil) have advantageous effect on CRP [39], oils rich in n-6 fatty acids (e.g., from sunflower oil) may have pro-inflammatory effects [40]. These findings suggest that the impact of specific components of the HEI-2005, in addition to the total dietary score, should be taken into account when anti-inflammatory properties of diet are evaluated.
Another possible explanation for the discrepancy observed between men and women may be related to sex differences in the pathogenesis of inflammation-induced cardiovascular disease. Women have higher CRP than men at any given BMI [41]. In addition, women with diabetes have twice the risk of developing cardiovascular disease compared with men with diabetes [16]. Sex differences in cardiovascular disease risk may be related to the higher truncal fat content in women with diabetes than in men with diabetes [42]. Excess body fat may have confounded the effect of physical activity among women in our study and in other studies that failed to find an association between physical activity and CRP among women [43-44]. Whether different types or intensities of interventions are needed to reduce CRP in women than in men is not clear. Nevertheless, intensive lifestyle intervention for weight loss resulted in a similar reduction in CRP in gender-specific analyses among people with impaired glucose tolerance [45] and with type 2 diabetes [46]. It is also worth noting that although many studies have found an association between smoking and CRP levels [8, 47], other studies have shown no significant association between smoking intensity and CRP levels [48]. Also, smoking cessation may result in a delayed (rather than temporally convergent) decrease in CRP [49]. Accordingly, further research is needed to examine both the range of environmental influences and also the apparent differences between men and women with respect to the associations between inflammation and a variety of lifestyle factors.
The mechanisms by which physical activity and a healthy diet may reduce CRP are not yet clear. One possible mechanism is that physical activity and a healthy diet may reduce inflammation by decreasing body weight and fatness [50]. However, the effect of physical activity and diet on inflammation is at least partly independent of weight loss [51, 52]. Physical activity can result in production of anti-inflammatory cytokines such as interleukin-6 (IL-6) from muscle [53]. Although, chronic elevation of IL-6 has pro-inflammatory effects, transient increase in IL-6 during exercise triggers a cascade of anti-inflammatory effects by increasing the production of anti-inflammatory cytokines IL-10 and IL-1 receptor antagonist [54]. Thus, pro-inflammatory marker IL-6 may have paradoxical anti-inflammatory effects during exercise. In addition, a healthy diet may decrease oxidative stress and associated inflammation by improving hyperglycemia, insulin sensitivity and antioxidant properties [55, 56].
This study has several limitations. First, the data are cross-sectional, and thus temporal associations could not be determined. Although it is biologically plausible that diet and physical activity behaviors and obesity preceded any elevations in CRP, we cannot dismiss the possibility of an indirect effect between lifestyle factors and CRP through as-yet-unknown mechanisms. Second, use of self-reported measures may lead to some misclassification bias. Obese subjects, particularly women, underreport their energy intake [57]. Since obesity is associated with higher levels of CRP, this underreporting could have attenuated the association between CRP and diet. Third, women with diabetes have higher CRP levels than men with diabetes [16], as was observed in this study. Thus, using a single cut-off point for both genders possibly decreased specificity in women and sensitivity in men and influenced the results. This possibility may be supported by the discrepancy in the findings for physical activity between logistic regression and linear regression, in which CRP was entered as a continuous instead of categorical variable. Fourth, the small sample size in some categories limited the precision of estimates, as reflected by wide confidence intervals. Fifth, our results may not apply to respondents younger than 30 years old, because they were excluded in this study, and because the majority of women in this analysis was postmenopausal, caution should be used in generalizing the results to premenopausal women. Finally, it is possible that other confounders could have affected the associations we observed. Potential confounders might include measures of co-morbidity, use of vitamin supplements, and biomarkers known to be associated with CRP, such as for cholesterol and triglycerides. Cholesterol and triglyceride measures were available for less than half of NHANES respondents, thus we did not include these biomarkers in our analysis. Nevertheless, important strengths of this study include the use of NHANES data with a large sample of U.S. men and women with diabetes and the diversity of the sample in terms of other demographic characteristics.
In conclusion, our results extend previous reports on the association between CRP and lifestyle factors to people with type 2 diabetes. In both men and women, obesity was a strong predictor of elevated CRP. In addition, healthier diet quality and higher levels of physical activity were associated with lower CRP levels among men but not among women. Given that systemic inflammation in diabetes may be at least partly related to lifestyle factors preventive strategies should address lifestyle modifications in these high-risk individuals. Our results provide further evidence to support current diabetes education programs’ emphases on weight management as well as improving diet quality and physical activity.
Acknowledgements
SJ was supported by the Washington University School of Medicine. DBJ was supported by the Health Behavior, Communication, and Outreach Core of the Siteman Cancer Center, which is supported in part by the National Cancer Institute Cancer Center Support Grant (P30 CA91842) to the Siteman Cancer Center and the Clinical and Translational Sciences Award (UL1 RR024992) to Washington University School of Medicine. NOD was supported in part by grants from NIH (DK 56260, DK 52574, HL 38180).
Footnotes
Conflict of Interest Statement: The authors have no conflict of interest to disclose.
References
- 1.National Institute of Diabetes and Digestive and Kidney Diseases [July 10, 2011];National diabetes statistics fact sheet, [article online] 2008 Available at http://diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm.
- 2.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 5.Forman D, Bulwer BE. Cardiovascular disease: Optimal approaches to risk factor modification of diet and lifestyle. Curr Treat Options Cardiovasc Med. 2006;8:47–57. doi: 10.1007/s11936-006-0025-7. [DOI] [PubMed] [Google Scholar]
- 6.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–5. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 7.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45:1563–9. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 8.Bazzano LA, He J, Muntner P, Vupputuri S, Whelton PK. Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Ann Intern Med. 2003;138:891–897. doi: 10.7326/0003-4819-138-11-200306030-00010. [DOI] [PubMed] [Google Scholar]
- 9.King DE, Egan BM, Geesey ME. Relation of dietary fat and fiber to elevation of C-reactive protein. Am J Cardiol. 2003;92:1335–9. doi: 10.1016/j.amjcard.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Gao X, Bermudez OI, Tucker KL. Plasma C-reactive protein and homocysteine concentrations are related to frequent fruit and vegetable intake in Hispanic and non-Hispanic White elders. J Nutr. 2004;134:913–8. doi: 10.1093/jn/134.4.913. [DOI] [PubMed] [Google Scholar]
- 11.Jensen MK, Koh-Banerjee P, Franz M, Sampson L, Grønbæk M, Rimm EB. Whole grains, bran, and germ in relation to homocysteine and markers of glycemic control, lipids, and inflammation. Am J Clin Nutr. 2006;83:275–283. doi: 10.1093/ajcn/83.2.275. [DOI] [PubMed] [Google Scholar]
- 12.Ford ES. Body mass index, diabetes, and C-reactive protein among U.S. adults. Diabetes Care. 1999;22:1971–1977. doi: 10.2337/diacare.22.12.1971. [DOI] [PubMed] [Google Scholar]
- 13.Qi L, van Dam RM, Liu S, Franz M, Mantzoros C, Hu FB. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care. 2006;29:207–11. doi: 10.2337/diacare.29.02.06.dc05-1903. [DOI] [PubMed] [Google Scholar]
- 14.Balducci S, Zanuso S, Nicolucci A, et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis. 2010;20:608–617. doi: 10.1016/j.numecd.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Zoppini G, Targher G, Zamboni C, et al. Effects of moderate-intensity exercise training on plasma biomarkers of inflammation and endothelial dysfunction in older patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2006;16:543–549. doi: 10.1016/j.numecd.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106:679–684. doi: 10.1161/01.cir.0000025403.20953.23. [DOI] [PubMed] [Google Scholar]
- 17.Wingard DL, Barrett-Connor E. Heart disease and diabetes. In: Harris MI, Cowie CC, Stern MP, Boyko EJ, Rieber GE, Bennett PH, editors. Diabetes in America. 2nd ed. National Institute of Health, National Diabetes Data Group; Bethesda, Md: 1995. pp. 429–48. publ. no. NIH 95-1468. [Google Scholar]
- 18.National Center for Health Statistics . NHANES 1999-2000, 2001-2002, 2003-2004. U.S. Department of Health and Human Service, Centers for Disease Control and Prevention, National Center for Health Statistics; Hyattsville, MD: [May 9, 2011]. Available at http://www.cdc.gov/nchs/nhanes.htm. [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS) National Health and Nutrition Examination Laboratory Protocol. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, MD: [May 9, 2011]. NHANES 1999-2000, 2001-2002, 2003-2004 Available at http://www.cdc.gov/nchs/nhanes.htm. [Google Scholar]
- 20.Pearson TA, Mensah GA, Alexander RW, et al. Centers for Disease Control and Prevention. American Heart Association Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 21.Library NA. [May 9, 2011];National Nutrient Database for Standard Reference: U.S. Department of Agriculture Web site. http://fnic.nal.usda.gov/nal_display/index.php?info_center=4&tax_level=1.
- 22.Guenther PM, Krebs-Smith SM, Reedy J, Britten P, Juan Wen Y. Center for Nutrition Policy and Promotion factsheet No. 1. Revised 2008. US Department of Agriculture, Center for Nutrition Policy and Promotion; Alexandria, VA: [May 16, 2011]. Healthy Eating Index 2005. Available at http://www.cnpp.usda.gov/Publications/HEI/healthyeatingindex2005factsheet.pdf. [Google Scholar]
- 23.U.S. Department of Health and Human Services. U.S. Department of Agriculture . Dietary guidelines for Americans, 2005. U.S. Government Printing Office; Washington (DC): 2005. [Google Scholar]
- 24.Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc. 1995;95:1103–8. doi: 10.1016/S0002-8223(95)00300-2. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Department of Agriculture. Center for Nutrition Policy and Promotion [May 16, 2011];HEI2005_NHANES0102.txt. Available at http://www.cnpp.usda.gov/HealthyEatingIndex-2005report.htm.
- 26.George SM, Neuhouser ML, Mayne ST, et al. Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2010;19:2220–2228. doi: 10.1158/1055-9965.EPI-10-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Department of Health and Human Services . Physical activity guidelines for Americans: be active, healthy, and happy. Washington (DC): 2011. [Google Scholar]
- 28.World Health Organization . Report of a WHO consultation. World Health Organization; Geneva, Switzerland: 2000. Obesity: preventing and managing the global epidemic. [PubMed] [Google Scholar]
- 29.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 30.Ridker P, Danielson E, Fonseca F, et al. JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 31.King DE, Mainous AG, 3rd, Buchanan TA, Pearson WS. C-reactive protein and glycemic control in adults with diabetes. Diabetes Care. 2003;26:1535–9. doi: 10.2337/diacare.26.5.1535. [DOI] [PubMed] [Google Scholar]
- 32.American Diabetes Association Standards of medical care in diabetes--2011. Diabetes Care. 2011;34(Suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Festa A, D'Agostino R, Jr, Tracy RP, Haffner SM, Insulin Resistance Atherosclerosis Study Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131–7. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 34.Ford ES, Mokdad AH, Liu S. Healthy Eating Index and C-reactive protein concentration: findings from the National Health and Nutrition Examination Survey III, 1988-1994. Eur J Clin Nutr. 2005;59:278–83. doi: 10.1038/sj.ejcn.1602070. [DOI] [PubMed] [Google Scholar]
- 35.Boynton A, Neuhouser ML, Wener MH, et al. Associations between healthy eating patterns and immune function or inflammation in overweight or obese postmenopausal women. Am J Clin Nutr. 2007;86:1445–1455. doi: 10.1093/ajcn/86.5.1445. [DOI] [PubMed] [Google Scholar]
- 36.Serdula MK, Coates RJ, Byers T, Simoes E, Mokdad AH, Subar AF. Fruit and vegetable intake among adults in 16 states: results of a brief telephone survey. Am J Public Health. 1995;85:236–9. doi: 10.2105/ajph.85.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarvandi S, Gougeon R, Bader A, Dasgupta K. Differences in food intake among obese and non-obese women and men with type 2 diabetes. Am Coll of Nutr. 2011;30:225–232. doi: 10.1080/07315724.2011.10719964. [DOI] [PubMed] [Google Scholar]
- 38.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108:1896–1901. doi: 10.1016/j.jada.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Ciubotaru I, Lee Y-S, Wander RC. Dietary fish oil decreases C-reactive protein, interleukin-6, and triacylglycerol to HDL-cholesterol ratio in postmenopausal women on HRT. J Nutr Biochem. 2003;14:513–521. doi: 10.1016/s0955-2863(03)00101-3. [DOI] [PubMed] [Google Scholar]
- 40.Hennig B, Lei W, Arzuaga X, Ghosh DD, Saraswathi V, Toborek M. Linoleic acid induces proinflammatory events in vascular endothelial cells via activation of PI3K/Akt and ERK1/2 signaling. J Nutr Biochem. 2006;17:766–772. doi: 10.1016/j.jnutbio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–39. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen M, Bruunsgaard H, Weis N, et al. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech Ageing Dev. 2003;124:495–502. doi: 10.1016/s0047-6374(03)00027-7. [DOI] [PubMed] [Google Scholar]
- 43.Valentine RJ, Vieira VJ, Woods JA, Evans EM. Stronger relationship between central adiposity and C-reactive protein in older women than men. Menopause. 2009;16:84–89. doi: 10.1097/gme.0b013e31817fcb8f. [DOI] [PubMed] [Google Scholar]
- 44.Albert MA, Glynn RJ, Ridker PM. Effect of physical activity on serum C-reactive protein. Am J Cardiol. 2004;93:221–225. doi: 10.1016/j.amjcard.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 45.Haffner S, Temprosa M, Crandall J, et al. Diabetes Prevention Program Research Group Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54:1566–72. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belalcazar LM, Reboussin DM, Haffner SM, et al. A 1-year lifestyle intervention for weight loss in individuals with type 2 diabetes reduces high C-reactive protein levels and identifies metabolic predictors of change. Diabetes Care. 2010;33:2297–2303. doi: 10.2337/dc10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wannamethee SG, Lowe GDO, Shaper AG, Rumley A, Lennon L, Whincup PH. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur Heart J. 2005;26:1765–1773. doi: 10.1093/eurheartj/ehi183. [DOI] [PubMed] [Google Scholar]
- 48.Asthana A, Johnson HM, Piper ME, Fiore MC, Baker TB, Stein JH. Effects of smoking intensity and cessation on inflammatory markers in a large cohort of active smokers. Am Heart J. 2010;160:458–63. doi: 10.1016/j.ahj.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hastie CE, Haw S, Pell JP. Impact of smoking cessation and lifetime exposure on C-reactive protein. Nicotine Tob Res. 2008;10:637–642. doi: 10.1080/14622200801978722. [DOI] [PubMed] [Google Scholar]
- 50.Nicklas BJ, Ambrosius W, Messier SP, et al. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79:544–551. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 51.You T, Berman DM, Ryan AS, Nicklas BJ. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab. 2004;89:1739–1746. doi: 10.1210/jc.2003-031310. [DOI] [PubMed] [Google Scholar]
- 52.Katcher HI, Legro RS, Kunselman AR, et al. The effects of a whole grain–enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am J Clin Nutr. 2008;87:79–90. doi: 10.1093/ajcn/87.1.79. [DOI] [PubMed] [Google Scholar]
- 53.Pedersen BK. The anti-inflammatory effect of exercise: Its role in diabetes and cardiovascular disease control. Essays Biochem. 2006;42:105–117. doi: 10.1042/bse0420105. [DOI] [PubMed] [Google Scholar]
- 54.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 55.Qi L, Hu FB. Dietary glycemic load, whole grains, and systemic inflammation in diabetes: the epidemiological evidence. Curr Opin Lipidol. 2007;18:3–8. doi: 10.1097/MOL.0b013e328011c6e0. [DOI] [PubMed] [Google Scholar]
- 56.Chun OK, Chung SJ, Claycombe KJ, Song WO. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in U.S. adults. J Nutr. 2008;138:753–760. doi: 10.1093/jn/138.4.753. [DOI] [PubMed] [Google Scholar]
- 57.Johnson RK, Goran MI, Poehlman ET. Correlates of over- and underreporting of energy intake in healthy older men and women. Am J Clin Nutr. 1994;59:1286–90. doi: 10.1093/ajcn/59.6.1286. [DOI] [PubMed] [Google Scholar]