Abstract
Metabolic syndrome is a major health issue in the western world. An elevated pro-inflammatory state is often found in patients with metabolic diseases such as type 2 diabetes and obesity. Atherosclerosis is one such clinical manifestation of pro-inflammatory state associated with the vasculature. The exact mechanism by which metabolic stress induces this pro-inflammatory status and promotes atherogenesis remained elusive until the discovery of the inflammasome protein complex. This complex is composed of pro-caspase-1 and pathogen sensors. Activation of inflammasome requires the transcriptional upregulation of inflammasome components and the post-translational assembly. Three models of inflammasome assembly have been proposed: 1) the ion channel model; 2) the reactive oxygen species (ROS) model; and 3) the lysosome model. In either case, inflammasome activation triggers the auto-activation of pro-caspase-1 into its mature form. Caspase-1, which was first discovered as the IL-1β converting enzyme, is known to be a major player in inflammatory and cell death pathways. Many endogenous metabolic ligands have been experimentally shown to activate inflammasome, and thus initiate the subsequent inflammation process. Further understanding of the distinct molecular mechanism by which metabolic ligands activates inflammasome could lead to developing novel therapeutic interventions for atherosclerosis and other clinical problems related to metabolic diseases.
Keywords: inflammasomes, Caspase-1, ROS, Vascular Inflammation, Interleukin-1 beta, Atherosclerosis, Review
2. INSTRODUCTION
Metabolic syndrome is a combination of medical disorders such as hypertension, insulin resistence, hyperlipidemia and central obesity that, when occurring together, significantly increase the risk for coronary artery disease (CAD), stroke, and type 2 diabetes (T2D) (1). For instance, it is the metabolic stresses on the vasculature that lead to endothelial cell activation, endothelial dysfunction, and local vascular inflammation resulting in a pathogenic process called atherosclerosis, a leading cause of CAD and stroke. In turn, these cardiovascular diseases are the leading causes of morbidity and mortality in metabolic syndrome patients.
Atherosclerosis (also known as arteriosclerotic vascular disease or ASVD) is a condition commonly referred to as a hardening or furring of the arteries. Hyperlipidemia is a major risk factor for atherosclerosis, and correction of dyslipidemia is the mainstay treatment for symptomatic cardiovascular disease. Other endogenous signals including glucose, oxidized LDL (oxidized-LDL) ,and free fatty acids (FFAs), which are elevated in patients with obesity and T2D (2) have also been known to account for atherogenesis. Despite the growing evidence that shows the causal effect of metabolic syndrome to atherosclerosis, the exact biological mechanism is poorly understood.
Recently studies have demonstrated that several endogenous metabolic stress molecules, such as oligomers of islet amyloid polypeptide (3), glucose (4), ceramid (5), oxidized low density lipoprotein(6) and cholesterol crystals, can be sensed by NLRP3 (nucleotide-binding domain and leucine-rich repeat containing (NLR) family, pyrin domain containing 3), a cytosolic pathogen associated molecular pattern (PAMP) recognition receptor similar to Toll-like receptors (TLRs) (4–7) and stimulate NLRP3 inflammasome complex assembly (Table 1). The inflammasome is a large, multiprotein complex, in which pro-caspase-1 is cleavaged into its mature p20-p10 heterodimeric/tetrameric activated proteinase. Activated caspase-1 then is capable to cleave off the N-terminal 117 amino acids from pro-interleukin (IL)-1β(269 amino acids (aa) in length, 30kD in size) and the N-terminal 35 amino acids from pro-IL-18 (192 aa in length, 21 kD in size) into their bioactive forms of 17kD, respectively. Thus, caspase-1 functions as the key bridge linking the metabolic stresses and innate immune sensors to pro-inflammatory cytokine production and initiation of vascular inflammation.
Table 1.
Endogenous activators of Nalp3 inflammasome
| Activator | Signal 1 | Signal 2 | Mechanism | Clinic Relevance | Reference (PMID) |
|---|---|---|---|---|---|
| Palmitate | √ | ROS | Metabolic Syndrome | 21478880 | |
| Ceramide | √ | √ | ROS | Metabolic Syndrome | 21217695 |
| Glucose | √ | √ | ROS | Metabolic Syndrome | 20023662 |
| oxLDL | √ | √ | ROS | Metabolic Syndrome | 20428172 |
| Cholesterol Crystals | √ | Lysosomal Damage | Metabolic Syndrome | 20428172 | |
| Islet Amyloid Polypeptide | √ | Lysosomal Damage | Metabolic Syndrome | 20835230 | |
| Monosodium Urate (Uric Acid) | √ | Particle Endocytosis | Gout | 16407889 | |
| Calcium Pyrophosphate Dihydrate (CPPD) | √ | Particle Endocytosis | Pseudogout | 16407889 | |
| Amyloid β | √ | Lysosomal Damage | Alzheimer's Disease | 18604209 | |
| Hyaluronan | √ | Particle Endocytosis | Injure | 19258328 | |
| ATP | √ | K+ Efflux | Injure | 16407890 |
In addition, recent clinical studies reported the following findings: 1) activated caspase-1 is found in patients’ vulnerable atherosclerotic plaques (8); 2) elevated serum concentrations of caspase-1 are found in patients with acute angina(9); and 3) Familiar Mediterranean fever (FMF) is a common hereditary auto-inflammatory disorder characterized by recurrent febrile attacks and polyserositis, resulting from missense mutations of Mediterranean FeVer gene that alters the structure and function of pyrin protein (10). Major role of pyrin is the regulation of caspase-1 activation. The mutation M694V of pyrin is an independent risk factor for developing acute myocardial infarction in the Sicilian population (11). Lastly, endothelium-dependent flow-mediated dilation is reduced and intima-media thickness of the carotid arteries is increased in patients with FMF (12, 13). These clinical reports clearly demonstrate the pathophysiology of atherogenesis and caspase-1 activation. In this review, we will focus on the recent progress in the understanding of how a prototype pro-inflammatory caspase, caspase-1, gets activated by metabolic stresses and its subsequent role in vascular inflammation.
3. INFLAMMATORY CASPASE FAMILY
Apoptosis, as a format of programmed cell death, serves as an evolutionary conserved cellular protective mechanism. Similarly, inflammation also serves as a tissue protective mechanism in mammalian species. Most of the five cardinal features of inflammation including redness/rubor, swelling/tumor, pain/dolor, heat/calor, and dysfunction results from the vascular responses to causative stimuli including vasodilation, increased blood flow, extravasation of fluid (permeability) and cellular influx (chemotaxis). However, since apoptosis results in no inflammation in response to inflammatory stimuli, vascular cells in the affected tissue would either inflame or perish via apoptosis or something in between, i.e. newly termed cell death form “pyroptosis” (14). Pyroptosis, or caspase 1-dependent cell death, which is inherently inflammatory, can be triggered by various pathological stimuli, such as stroke, heart attack or cancer, and is crucial to control microbial infections. Therefore, activation of either apical/initiator apoptotic caspases or inflammatory caspases will determine the fate of the protective responses of the cells.
Caspases (cysteinyl aspartate proteases) are an evolutionarily conserved class of intracellular proteases. The history of caspases’ characterization began with the identification of caspase-1 as the IL-1β-converting enzyme (15). The discovery of the homology of caspase-1 to ced-3, which is involved in programmed cell death in Caenorhabditis elegans, suggested the key role of caspases in apoptosis.(16) The role of apoptotic caspases (caspase-2, 3, 6, 7, 8, 9, and 10) has been well studied (17). However, several members of caspase family have gained prominence as critical mediators in inflammation and innate immune responses. These caspases are called inflammatory caspases (also known as the group 1 caspases). Three human caspases (caspase-1, caspase-4, and caspase-5) and three mouse caspases (caspase-1, caspase-11, and caspase-12) have been categorized into the inflammatory caspase subfamily (18). All inflammatory caspases share a conserved caspase activation and recruitment domain (CARD) at the N-terminus. Sequence analysis suggests that human caspase-1 and mouse caspase-1 are likely orthologues, while human caspase-4 and caspase-5 have originated from a duplication of mouse caspase-11 (19). Human caspase-12 homolog lost its C-terminus and became enzymatic inactivated through evolution (20).
Activated caspase-1, the prototype of inflammatory caspases, can process pro-IL-1β and pro-IL-18, two critical pro-inflammatory cytokines, into their mature forms. A more sophisticated analysis from our laboratory (21) demonstrated the existence of up to 70 protein substrates for caspase-1, most of which play a role in vascular inflammation, vascular function and atherogenesis. Of note, no specific substrates have been characterized for human caspase-4 and caspase-5 and mouse caspase-11 and caspase-12. Therefore, the pathophysiological functions of these caspases are poorly understood.
One report showed that caspase-11-deficient macrophages have insufficiency of caspase-1 activation and IL-1β production when infected with Escherichia coli, Citrobacter rodentium or Vibrio cholera. Similar data was not observed when these macrophages were treated with endogenous non-infectious stimuli, such as adenosine-5'-triphosphate (ATP) and monosodium urate (MSU) crystals. The authors of the studies thus proposed that caspase-11 was required for non-canonical activation of caspase-1 (22). Murine caspase-12 may be functional as the dominant negative regulator of caspase-1 and block caspase-1 activation (18). This argument was supported by the findings that caspase-12-deficient mice have higher IL-1β and Il-18 levels but not tumor necrosis factor-α (TNF-α) and IL-6 as compared to wild-type littermates after bacterial infection. These results suggest that it is likely that other members of inflammatory caspases are involved inflammatory process via facilitating or suppressing the caspase-1 activation.
3.1 INFLAMMASOMES AND CASPASE-1 ACTIVATION
Pro-caspase-1 is present in the cell cytosol as an inactive zymogen and requires a protein complex assembly for auto-activation. This protein platform, which is composed of NLR family member and pro-caspase-1, has been called the “inflammasome” as an analogy to the “apoptosome” that controls caspase-9 activation during mitochondrion-dependent apoptosis. Similarly,caspase-8 and caspase-2 activation are controlled by a protein complex called the death inducing signaling complexes (DISCs) and the p53-induced protein with a death domain complex (PIDDosome) respectively (23). These protein complex assemblies add an extra layer of regulation in addition to the existing regulatory mechanisms including transcription, RNA splicing, mRNA stability, microRNA-mediated mRNA regulation, translation, and post-translational modifications. These models of caspase activation provide several functional advantages, such as capacity for sensing stimuli and rapid action using pre-existing protein components without new gene expression.
The NLR family comprises of 22 cytoplasmic proteins that include 5 members of the NOD (Nucleotide-binding oligomerization domain) subfamily, 14 NLRP members [domain present in NAIP, CIITA, HET-E (Podospora anserine incompatibility locus protein) and telomerase associated protein (NACHT)-, leucine-rich repeat domain (LRR)- and pyrin-domain-containing proteins], interleukin 1β-converting enzyme protease activating factor (IPAF), neuronal apoptosis inhibitor protein (NAIP), and major histocompatibility complex (MHC) class II transactivator (CIITA). Similar to apoptotic protease activating factor 1 (APAF-1) in the caspase-9 activating protein complex, NLRs have a tripartite structure, consisting of a C-terminal leucine-rich repeat domain, a central nucleotide-binding oligomerization (NOD or NACHT) domain, and an N-terminal effector domain. The effector domain facilitate downstream signaling via protein-protein interaction, which can be either a CARD, a pyrin domain (PYD), or a baculovirus inhibitor of apoptosis repeat domain. Thus, NLRs can either directly bind to caspase-1 through a CARD-CARD domain interaction, such as IPAF or associated with caspase-1 through an adaptor protein called apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), such as NLRP1 and NLRP3. Up to now, several members of NLRs have been documented to assemble into inflammasomes, including NLRP1, NLRP3, NLRC4 (IPAF), NFLRP6 and NLRP12 (24). These NLRs sense different endogenous and exogenous stimuli and form the inflammasome complex for caspase-1 activation. For example, NLRP1 inflammasome was the first identified inflammasome, and was found to be the primary mediator of susceptibility to anthrax lethal toxin (25). On the other hand, IPAF inflammasome (26) can sense flagellin derived from Legionella P., Salmonella T., pseudomonas A. and shigella F. Of note, two members of the pyrin domain (PYD) and HIN domain-containing (PYHIN), AIM2 and IFI16, were reported to assemble the NLR-independent inflammasomes. These receptors can directly engage with double-stranded DNA (dsDNA) ligands via the DNA-binding HIN domains (27, 28),(29).
3.2 Models of NLRP3 activation
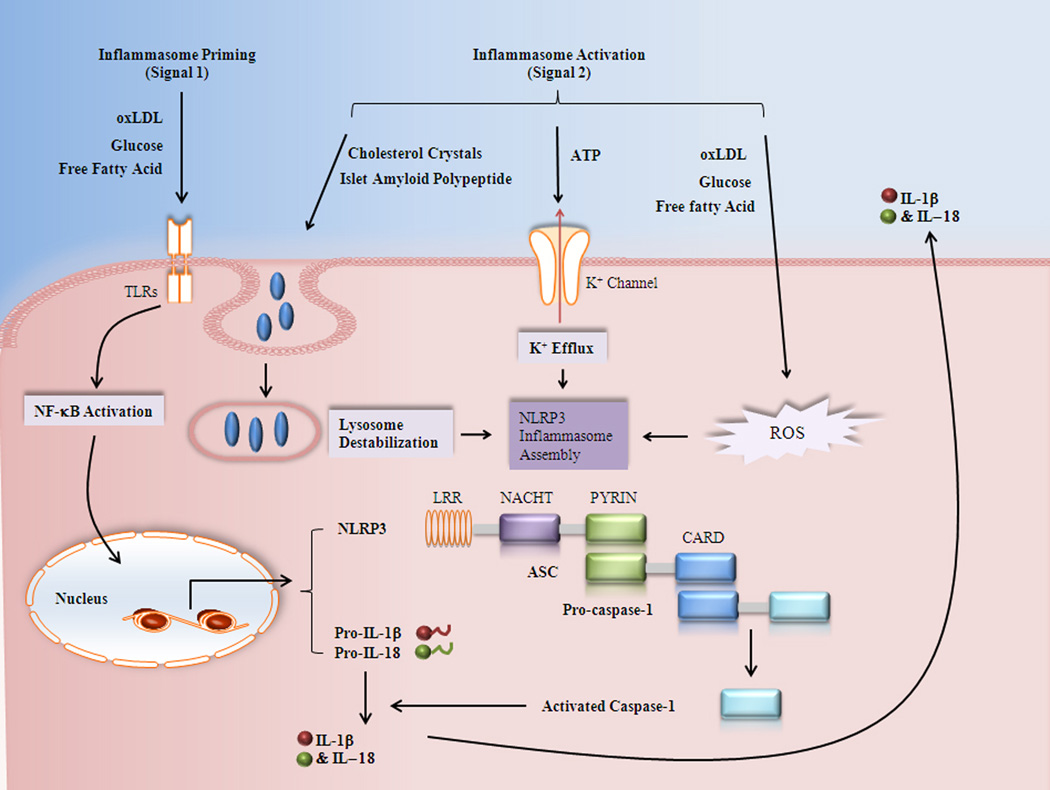
NLRP3 inflammasome is the most widely studied inflammasome, which is found to be capable of sensing a number of stimuli. It is commonly accepted that activation of NLRP3 inflammasome involves both transcriptional (signal 1) and post-translational (signal 2) regulation (Figure 1).
Figure 1.
proposed models of metabolic stress induced nlrp 3 inflammasome activation. The signal 1 of NLRP3 inflammasome activation proceeds via TLR/NF-κB to upregulate the mRNA levels of inflammasome components. Three models have been proposed to activate NLRP3 inflammasome assembly (signal 2) by metabolic stress, including ion channel model, ROS model, and lysosome model. Pro-caspase-1 is processed into activated caspase-1 via inflammasome, which is then capable to cleavage pro-IL-1β and pro-IL-18 into their mature forms. The bioactive forms of IL-1β and IL-18 can be secreted out and further induce inflammatory response. LRR : leucine-rich repeat domain; NACHT: nucleotide-binding oligomerization (NOD or NACHT) domain; CARD : Caspase activation and recruitment domains.
Signal 1 of inflammasome activation generally involves the upregulation of inflammasome component via TLR/NF-κB pathway. The Myeloid cells are maintaining a low level of pro-IL-1β expression when resting. As the major read-out of inflammasome activation is the secretion of matured IL-1β, researchers always perform a priming step to raise the pro-IL-1β level before treating the cells with the actual stimulation of the inflammasome activators. LPS is a widely used inducer for pro-IL-1β, which is accepted as the signal 1 of inflammasome activation (30). However, a large range of NF-κB activators such as TNF, CpG, and IL-1 itself can be used instead of LPS (31). The expression of NLRP3 is relatively low in many cell types and thus requires a priming signal to be induced (32, 33).
Signal 2 induces the assembly of NLRP3 oligomer. The list of NLRP3 inflammasome activators includes a wide variety of chemically and biologically unrelated PAMPs and DAMPs (danger-associated molecular patterns) (34). Such diversity of stimuli leads to the question by what mechanisms these structurally distinct molecules activate NLRP3 inflammasome activation. Although this question has not been fully answered, a few hypothetical mechanisms have been proposed (35).
Extracellular ATP, as a NLRP3 agonist, is a major danger signal released at the sites of cellular injury and necrosis. The first model suggests that ATP-mediated inflammasome activation depends on the activation of the ATP-gated P2X7 purinergic receptor, which is an ATP-gated ion channel responsible for potassium (K+) efflux through the cell membrane. In agreement with this model, an in vitro experiment has already demonstrated that NLRP inflammasome assembly and procaspase-1 recruitment occur spontaneously at K+ concentrations below 90 mM, but that this is prevented at higher concentrations. Thus, low intracellular K+ may be at least a common trigger of NLRP-inflammasome activation (36). In addition, blockade of K+ efflux induced by NLRP3 activators suppresses inflammasome activation as measured by caspase-1 activation and IL-1β maturation. ATP-dependent activation of NLRP3 has also been linked to another type of P2X7 receptor interaction channel, pannexin-1. Muramyl dipeptide (MDP), the microbial activator for NLRP1/3 inflammasome, can be phagocytosed and translocated into primary macrophages and accumulates in the acidified endosomal compartments. Upon ATP stimulation, MDP is then rapidly released from acidified vesicles into the cytosol through a pathway dependent on pannexin-1 function. One explanation is that pannexin-1 will form a hemi-channel that will allow extracellular NLRP3 activator, especially bacterial products, to access the cytoplasm and interact with and activate NLRP3 directly (37). Moreover, any pore formation in the plasma membrane would lead to K+ efflux from the cells, which can cooperate with the K+ channels in controlling the intracellular K+ concentration after ATP stimulation.
The second model of NLRP3 inflammasome activation claims that considering the size of many NLRP3 activators such as MSU crystals or the particulate asbestos, they are too large for cytoplasmic translation, but may lead to phagosomal destabilization and lysosome rupture during phagocytosis. Supporting evidence for this model shows that crystal-induced NLRP3 activation can be impaired by the inhibition of the lysomal protein, cathepsin B and phagocytosis impairs (3, 30). Consistence with this hypothesis, sterile lysomomal rapture itself (in the absence of any microbial stimuli) is sufficient to induce NLRP3 activation in human cells. However, the detailed molecular mechanism of this model is still unclear.
The third model proposes that NLRP3 is a general sensor for ROS generation to the inflammasome. Accompanied with inflammasome activation, ROS production is observed in all the NLRP3 activator-treated cells, including ATP and the particle activators. ROS blockade via knockdown of nicotinamide adenine dinucleotide phosphate (NAPDH) oxidase subunit expressions or the use of antioxidants impairs inflammasome activation (38, 39). These findings indicate that ROS generation is essential for NLRP3 inflammasome activation. A recent study demonstrated that NLRP3 agonist can induce the dissociation of thioredoxin-interacting protein (TXNIP; also known as VDUP1) (4) from its inhibitory protein, oxidoreductase thioredoxin in a ROS-sensitive manner. After that, free TXNIC binds with NLRP3 through LLRs and leads to NLRP3 inflamamsome activation. This report provides some insight into the mechanism of ROS-dependent inflammasome activation in molecular levels. However, TXNIP deficiency only partially impairs the activation of the NLRP3 inflammasome and subsequent secretion of IL-1β, which indicates that other pathways may also be involved.
Although evidence can be found to support each of these three models, much is still unknown regarding the specific details. In addition, these three models are not functionally independent but rather they might be interactive. For example, ROS generation is frequently accompanied by K+ efflux (40). It is possible that low K+ concentration can induce ROS production or vice versa. Incomplete or “frustrated phagocytosis” can also induce ROS production (39). Future studies are needed to clarify these issues.
3.3 Downstream functions of caspase-1 activation
The consequence of inflammasome activation is the maturation of caspase-1. Caspase-1 was firstly recognized as the IL-1β converting enzyme and is known to be required to process pro-IL-1β and pro-IL-18 into their mature form (41). Both cytokines attain a potent pro-inflammatory activity and are capable to induce a wide variety of biological effects. During infection, local injury or immunological challenge, IL-1β can be induced and then can regulate systemic and local inflammation by upregulating the expression of many effector proteins through IL-1RI and Nuclear factor (NF)-κB pathway (42). These effector proteins including cytokines/chemokines (43) (e.g., monocyte chemotactic protein 1), adhesion molecules (44) (e.g., vascular cell adhesion molecule 1 and intercellular adhesion molecule 1), nitric-oxide synthase (45), and matrix metalloproteinases (46), will then mediate several immune responses such as generating fever, activating lymphocytes and promoting leukocyte transmigration to the injury site. IL-18, although less effective than IL-1β, also plays key roles in a various disease models, including atherosclerosis (47), lupus erythematosus (48), acute graft-versus-host disease (49), hepatitis (50), and obesity (51). Therefore, it is a rational conclusion that IL-1β and IL-18 are the most important effector proteins for inflammasome activation. However, not all inflammasome functions can be explained by the effects of IL-1β and IL-18. For example, caspase-1-deficient mice are resistant to lipopolysaccharide (LPS)-induced shock, whereas this phenotype was not observed in both IL1β and IL-18-deficient mice (52). Additional evidence shows that aside from IL-1β and IL-18, caspase-1 can also mediate the cleavage and/or secretion of numerous other cytoplasmic targets, which indicates that caspase-1 can regulate different cellular functions beyond IL-1β and IL-18 activation.
Unlike other members of the caspase family, caspase-1 is not involved in the apoptosis pathway. Caspase-1 activation drives a specialized form of cell death known as pyroptosis, which is caspase-1-dependent, pro-inflammatory cell death. It was firstly discovered in macrophages when infected with shigella flexner, (53), and subsequently observed in macrophages and dendritic cells when infected with other bacteria (54). In contrast to apoptosis, which is an immunologically “silent”, pyroptosis features the release of pro-inflammatory intracellular contents. A rapid plasma-membrane rupture and plasma-membrane pore formation are also observed during pyroptosis via a caspase-1-dependent manner (55). The pores formed on the plasma membrane produce a net increase in osmotic pressure and induce water influx and cell swelling resulting in osmotic lysis and release of inflammatory-cellular contents (56). Thus, the pyroptotic cell death allows the eliminating infected cells, as well as alerting the immune system for further responses. Although the mechanism of caspase-1-dependent cell death is poorly understoody, it seems to be independent of IL-1β and IL-18 (54), which indicates that pyroptosis involves unknown caspase-1 pathways requiring further investigation.
Besides IL-1β and IL-18, caspase-1 can also regulate production and/or secretion of several other cytokines. The evidence is that the plasma level of IL-1α, TNF-α and IL-6 were also down-regulated in the caspase-1-deficient mice after LPS shock compared to wide-type controls (57). More recent proteomic approaches have confirmed that caspase-1 is a regulator for unconventional protein secretion (58). Most secreted proteins contain signal peptides that mediate its secretion via the classical endoplasmic reticulum (ER) and Golgi pathway. However, a small amount of proteins including the major caspase-1 substrates, IL-1β and IL-18 are known to be released by unconventional protein secretion pathways which are independent of the ER and Golgi (59). Caspase-1 was demonstrated to be essential for the secretion of some other proteins which are secreted non-classically, including IL-1α, high mobility group box-1 (HMGB1) and fibroblast growth factor-2 (FGF2). It is likely that caspase-1 regulates the secretion of these proteins via protein-protein binding. However, the distinct pathway of the unconventional protein secretion needs further studies.
In addition, caspase-1 can regulate NF-κB activation (60). One group reported that caspase-1 can regulate NF-κB and P38 MAPK activity which is independent of caspase-1 catalytic activity (61). The authors also proposed that caspase-1 may interact with the receptor interacting protein-2 (RIP2) through CARD domain. Furthermore, overexpression of the caspase-1-like protein COP, which in contrast to caspase-1 only consists of a CARD domain, is capable of activating NF-κB via RIP2 (62). It was proposed that caspase-1 was able to cleavage MyD88 adapter-like (MAL) at the its TIR domain which mediates its binding to TLR and thus regulates TLR/MyD88/NF-κB pathway. This is supported by the evidence that impaired NF-κB activation and TNF-α production were observed in caspase-1-deficient macrophage in response to TLR2 or TLR4 stimulation (60). Interestingly, other members of inflammatory caspases were found to regulate NF-κB activity. For instance, LPS treatment leads to the interaction of caspase-4 and TNF receptor associated factor 6 (TRAF6) via a TRAF6-binding motif which may boost the NF-κB signaling pathway while deficiency of caspase-4 in THP-1 leads to a diminished NF-κB activity upon LPS stimulation (63). Caspase-12, which is a decoy protein for caspase-1, on the other hand, can blunt NF-κB activation by binding to RIP2 and displacing TRAF6 from the RIP2 signaling complex (64).
In summary, activated caspase-1 is capable of regulating the secretion of unconventional proteins via either directly cleavage process (eg. IL-1β and IL-18) or a yet unknown process involved in protein-protein interaction. It could also regulate TLR/MyD88/NF-κB through interacting with RIP2 and MAL. Furthermore, it would lead to cell membrane pore formation and eventually a caspase-1-dependent cell death featured with cell lysis and pro-inflammatory cellular content release.
4. INFLAMMASOME AND METABOLIC STRESS
4.1 Inflammasome and wargurg effect
The Warburg effect generally refers to the aerobic glycolysis process adopted by cancer cells to generate enough energy for cellular process (65). Most differentiated cells primarily metabolize glucose to carbon dioxide by oxidation of glycolytic pyruvate in the mitochondrial Krebs cycle (the tricarboxylic acid cycle) to maximal the production of ATP under the presence of oxygen (known as “oxidative phosphorylation”, generating 36 mol ATP/ mol glucose). Only when there is insufficient oxygen these cells will produce large amount of lactate (known as “anaerobic glycolysis”, generating 2 mol ATP/ mol glucose). However, most cancer cells employ the “aerobic glycolysis” process for energy production. This process generates 4 mol ATP/mol glucose which is less efficiency than oxidative phosphorylation. However, the beneficial effect of it on cancer cells is still unclear.
Interestingly, LPS also promote a profound metabolic switch from oxidative phosphorylation to aerobic glycolysis in both macrophages and dendritic cells. (66) However, the mechanism of the Warburg effect in activated inflammatory cells remains largely unknown. One seminal study demonstrated that in LPS activated macrophages, the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK2) from the liver type-PFK2 (L-PFK2) to the more active ubiquitous PFK2 (uPFK2) isoform which potentiates the glycolytic flux (67). It is also supported by the phenomenon that interference of uPFK2 attenuated macrophage activation in responses to LPS (67). The mechanism of the Warburg effect in activated inflammatory cells may also involve a signal from mitochondria as LPS directly suppresses mitochondrial energy production. Significant decreases in mRNA level of the mitochondrial respiratory chain complex I-V genes in blood leukocyte occur after intravenous administration of LPS to healthy human subjects (68). Moreover, the rate of mitochondrial-dependent β-oxidation of free fatty acids and rate of mitochondrial-dependent oxygen consumption are significantly suppressed after LPS stimulation of dendritic cells (69).
LPS induced Warburg effect in inflammatory immune cells interacts with LPS induced inflammasome activation, especially for the priming step of the activation. Treatment of macrophages with the glycolytic inhibitor 2-deoxyglucose (2-DG) prevents the induction of IL-1β mRNA induced by LPS but does not affect transcription of the gene encoding TNF-α. In addition, 2-DG prevented islet amyloid polypeptide (IAPP) induced IL-1β release in a dose-dependent manner and this effect is only seen when 2-DG is added before but not after LPS priming suggesting that the Warburg effect is only required for priming (signal 1) but not activation (signal 2) of the inflammasome (7).
Furthermore, inflammasome activation can reciprocally regulate glycolysis process. It has been shown that a series of glycolytic enzymes including aldolase, triose-phosphate isomerase, glyceraldehyde-3-phosphate dehydrogenase, α-enolase, and pyruvate kinase are directly cleaved by caspase-1 (70). Cleavage forms of these enzymes were detected in macrophage and diaphragm muscle after Salmonella typhimurium infection and septic shock along with caspase-1 activation. However it was not observed in caspase-1-deficient cells (70). This report supports that inflammasome activation can inhibit glycolysis. Nonetheless, whether this phenomenon is responsible for reversing the Warburg effect in activated inflammatory cells as a feedback mechanism needs to be further investigated.
4.2 Inflammasome and atherosclerosis
Atherosclerosis, which is a pathogenic condition involved in a chronic vascular inflammation, accounts for the morbidity and mortality of metabolic diseases, including T2D and obesity. Elevated amounts of glucose, oxidized LDL and free fatty acids were also commonly found in patients with obesity, T2D or atherosclerosis. These endogenous molecules may induce pro-inflammatory cytokines production from macrophages and other cell types and accelerate the chronically pro-inflammatory state resulting atherosclerosis. Both IL-1β and IL-18 are considered to be the major contributors of atherogenesis (47, 71). IL-1β is one of the cytokines commonly expressed in human atherosclerotic plaques (72), and IL-18 has also been shown to be highly expressed in the atherosclerotic plaques as compared to normal arteries localized primarily in plaque macrophages (73). IL-1β deficiency decreases the severity of atherosclerosis in ApoE-deficient mice (43). In addition, the data from IL-18-deficient ApoE−/− mice showed reduced atherosclerosis despite the increased level of serum cholesterol supporting the proatherogenic role of IL-18 (74). In contrast, atherosclerotic lesion size in IL-1Ra+/−/ApoE−/− mice is significantly increased as compared to IL-1Ra+/+/ApoE−/− mice (75). However, the question of how endogenous metabolic stress could induce IL-1β and IL-18 production and thus induce atherogenesis remains unanswered.
An exciting development in this field is the identification of the roles of pathogen-recognition receptors (PRRs) in the pathogenesis of atherosclerosis. Toll-like receptors, as the major member of PRRs, recognize a variety of conserved PAMPs derived from bacteria, viruses, protozoa, and fungi, and then signal an upregulation of a range of immune and inflammatory genes. Genetic deficiency of TLRs (eg: TLR2, TLR4) in atherogenic mice significantly decrease the size of atherosclerotic lesions (76), suggesting the significant role of TLRs in atherogenesis. A recent study demonstrated that synergistic activation of TLRs and other cytosolic sensing receptor families including NLRs led to the assembly of a multiprotein complex, termed inflammasome, which then activate caspase-1 and its subsequent substrates (reference). As described previously, activated caspase-1 can induce inflammation by cleaving pro-inflammatory cytokines (IL-1β and IL-18) into their mature forms or control inflammatory pathway by regulating NF-κB activity.
Recent reports demonstrate that several endogenous molecules (oligomers of islet amyloid polypeptide (3), glucose (4), ceramid (3), oxidized LDL (6), and cholesterol crystals (6)) that are abundant in T2D and atherosclerosis patients can induce NLRP3 inflammasome activation resulting in IL-1βand IL–18 production. Both human and animal data support the role of caspase-1 activation in the development of cardiovascular disease. Activated caspase-1 was observed in ruptured plaque but not stable plaque in patients with sudden coronary death (8), and patients with high level of plasma caspase-1 were found to have significantly lower survival rate after myocardial infarction (77). Moreover, a recent study showed that LDL receptor-deficient mice, a mouse model of atherosclerosis, have significantly reduced aortic lesion size and serum IL-18 level when reconstituted with NLRP3-, ASC-, and IL-1β-deficient bone marrow (6) (Table 2). However, this finding is not consistent with the data generated from Dr.’s J Tschopp’s lab (78). His data showed that there was no significant difference in atherosclerosis progression, infiltration of plaques by macrophages, plaque stability, and phenotype across the genotypes when comparing ApoE−/−/NLRP3−/−, ApoE−/−/ASC−/− and ApoE−/−/caspase-1−/− double-knockout mice to ApoE−/− mice after of 11-week feeding of high fat diet. One explanation for this discrepancy is that ApoE−/− mice usually form a more severe atherosclerotic lesion as compared to LDLr−/− mice, especially after 11-week high fat diet. It is possible that NLRP3 inflammasome regulate early atherogenesis while in later stage, this affect is negated by other factors.
Table 2.
Inflammasome components and atherosclerosis
| Inflammasome Components |
Animal Genotype |
Genomic Background |
Age (weeks) |
High Fat Fed (weeks) |
Gender | Atherosclerosis Lesion |
References (PMID) |
|---|---|---|---|---|---|---|---|
| NLRP3 | NLRP3−/− /ApoE −/− LDLr−/−x BMT NLRP3−/− * |
C57BL/6 C57BL/6 |
15 16 |
11 8 |
Not Specified Female |
No Difference Decrease |
21451572 20428172 |
| Caspase-1 | Caspase-1−/− /ApoE −/− Caspase-1−/− /ApoE −/− |
C57BL/6 C57BL/6 |
16 15 |
8 11 |
Both Male and Female Not Specified |
Decrease No Difference |
22265992 21451572 |
| ASC | ASC−/−/ApoE − /− LDLr−/−x BMT ASC−/− |
C57BL/6 C57BL/6 |
15 16 |
11 8 |
Not Specified Female |
No Difference Decrease |
21451572 20428172 |
| IL-1β | IL-1β −/−/ApoE −/− LDLr−/−x BMT IL-1β−/− |
DBA/1 C57BL/6J C57BL/6 |
24 16 |
0 8 |
Male Female |
Decrease Decrease |
12615675 20428172 |
| IL-18 | IL-18−/−/ApoE −/− |
C57BL/6 | 24 | 0 | Male | Decrease | 12829194 |
BMT: Bone Marrow Transplantation
4.3 Inflammasome and vascular cells
Macrophage is considered to be the major source of IL-1β in vivo, and thus majority of inflammasome related work were done in macrophages. However, vascular cells including smooth muscle cells (SMCs) and endothelial cells (ECs) can also produce IL-1β and other cytokines but to a lesser amount (79). This indicates that cells may express inflammasome components and activate inflammasome to a different extent. Experimental data showed that granulocytes, monocytes (very weakly), dendritic cells, and B and T cells all express NLRP1 and NLRP3. The highest levels of NLRP1 are found in T cells and Langerhans cells. To further our understanding of the level of expression of TLRs, NLRs, inflammasome components, and proinflammatory caspases, we took different approaches and examined the mRNA transcript levels of these genes (80). Based on the expression data of three major inflammasomes (NLRP1, NLRP3, and IPAF inflammasomes), the examined tissues were grouped into three tiers: 1) the first tier tissues including brain, placenta, blood, and thymus express inflammasome(s) in a constitutive status; 2) the second tier tissues have inflammasome(s) in nearly-ready expression status (with the requirement of upregulation of one component); and 3) the third tier tissues, like heart and bone marrow, require upregulation of at least two components in order to assemble functional inflammasomes. This model leads to a new concept of third tier tissues inflammation privilege, which provides insight in the differences of tissues in initiating inflammatory response. Notably, vascular tissue is classified in the second/third tier of the inflammation privilege which indicates that vascular cells are protected from inflammasome activation and IL-1β production during acute inflammation and require an upregulation of inflammasome components for inflammasome activation.
5. REGULATION OF INFLAMMASOME AS A THERAPEUTIC TARGET
As previously reviewed, activation of inflammasome complexes leads to caspase-1 activation, which then regulate various facets of inflammatory response. Pharmacologically controlling of this process can regulate the pathogenesis of many inflammatory diseases, including atherosclerosis and T2D. In fact, glyburide, which is used in the treatment of T2D, was found to inhibit NLRP3 inflammasome activity (81). Orally active inhibitors of caspase-1, such as VX-740 (pralnacasan) and VX-765, have been used in clinical trials in patients with rheumatoid arthritis, and found to decrease disease progression (82).This supports the potential therapeutic role of caspases-1 inhibitors in other inflammatory diseases like atherosclerosis.
6. CONCLUSION
Discovery of the inflammasome has greatly enhanced our understanding on how metabolic stress activates our immune system. Activation of inflammasome leads to the maturation of caspase-1, which is the major enzyme that process pro-IL-1β and pro-IL-18 into their bioactive forms. In this review, we summarized the potential models of how endogenous metabolic ligands activate NLRP3 inflammasome, including the ion channel model, the ROS model, and the lysosome model. However, distinct molecular pathway of these models is still unclear. In addition, we also reviewed the downstream effects of caspase-1 activation beyond cytokines maturation and it is believed that caspase-1 is involved in inflammatory process in various aspects. Atherosclerosis is the major consequence of metabolic diseases on macro vessel. Several endogenous ligands, including oxLDL, cholesterol crystals, and FFA have been documented to activate inflammasome and promote atherogenesis. Clinical data also supports that during metabolic stress induced vascular inflammation, in particularly atherosclerosis, caspase-1 is a major player. Considering that abolishing IL-1β activity has already been targeted for potential therapy for many metabolic diseases, we proposed that caspase-1 may be the most potent therapeutic target to combat metabolic syndrome and related clinical complications.
ACKNOWLEDGEMENTS
This work was partially supported by the National Institutes of Health Grants HL094451 and HL108910 (XFY), HL67033, HL110764 and HL77288 (HW).
References
- 1.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 2.Masters SL, Latz E, O'Neill LA. The inflammasome in atherosclerosis and type 2 diabetes. Sci Transl Med. 2011;3:81ps17. doi: 10.1126/scitranslmed.3001902. [DOI] [PubMed] [Google Scholar]
- 3.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 5.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nunez G, Yodoi J, Kahn SE, Lavelle EC, O'Neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolodgie FD, Narula J, Burke AP, Haider N, Farb A, Hui-Liang Y, Smialek J, Virmani R. Localization of apoptotic macrophages at the site of plaque rupture in sudden coronary death. Am J Pathol. 2000;157:1259–1268. doi: 10.1016/S0002-9440(10)64641-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ankersmit HJ, Weber T, Auer J, Roth G, Brunner M, Kvas E, Moser B, Spreitzer S, Lassnig E, Maurer E, Hartl P, Wolner E, Boltz-Nitulescu G, Eber B. Increased serum concentrations of soluble CD95/Fas and caspase 1/ICE in patients with acute angina. Heart. 2004;90:151–154. doi: 10.1136/hrt.2003.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozcakar ZB, Yalcinkaya F. Vascular comorbidities in familial Mediterranean fever. Rheumatol Int. 2011;31:1275–1281. doi: 10.1007/s00296-011-1845-7. [DOI] [PubMed] [Google Scholar]
- 11.Grimaldi MP, Candore G, Vasto S, Caruso M, Caimi G, Hoffmann E, Colonna-Romano G, Lio D, Shinar Y, Franceschi C, Caruso C. Role of the pyrin M694V (A2080G) allele in acute myocardial infarction and longevity: a study in the Sicilian population. J Leukoc Biol. 2006;79:611–615. doi: 10.1189/jlb.0705416. [DOI] [PubMed] [Google Scholar]
- 12.Akdogan A, Calguneri M, Yavuz B, Arslan EB, Kalyoncu U, Sahiner L, Karadag O, Ertenli I, Kiraz S, Aytemir K, Akata D, Tokgozoglu L, Oto A. Are familial Mediterranean fever (FMF) patients at increased risk for atherosclerosis? Impaired endothelial function and increased intima media thickness are found in FMF. J Am Coll Cardiol. 2006;48:2351–2353. doi: 10.1016/j.jacc.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Ugurlu S, Seyahi E, Cetinkaya F, Ozbakir F, Balci H, Ozdogan H. Intima-media thickening in patients with familial Mediterranean fever. Rheumatology (Oxford) 2009;48:911–915. doi: 10.1093/rheumatology/kep131. [DOI] [PubMed] [Google Scholar]
- 14.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kostura MJ, Tocci MJ, Limjuco G, Chin J, Cameron P, Hillman AG, Chartrain NA, Schmidt JA. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A. 1989;86:5227–5231. doi: 10.1073/pnas.86.14.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 17.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 18.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 19.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to auto inflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Xue Y, Daly A, Yngvadottir B, Liu M, Coop G, Kim Y, Sabeti P, Chen Y, Stalker J, Huckle E, Burton J, Leonard S, Rogers J, Tyler-Smith C. Spread of an inactive form of caspase-12 in humans is due to recent positive selection. Am J Hum Genet. 2006;78:659–670. doi: 10.1086/503116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen J, Yin Y, Mai J, Xiong X, Pansuria M, Liu J, Maley E, Saqib NU, Wang H, Yang XF. Caspase-1 recognizes extended cleavage sites in its natural substrates. Atherosclerosis. 2010;210:422–429. doi: 10.1016/j.atherosclerosis.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 23.Mace PD, Riedl SJ. Molecular cell death platforms and assemblies. Curr Opin Cell Biol. 2010;22:828–836. doi: 10.1016/j.ceb.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13:333–332. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 27.Hornung V, Latz E. Intracellular DNA recognition. Nat Rev Immunol. 2010;10:123–130. doi: 10.1038/nri2690. [DOI] [PubMed] [Google Scholar]
- 28.Kerur N, Veettil MV, Sharma-Walia N, Bottero V, Sadagopan S, Otageri P, Chandran B. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011;9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guarda G, Zenger M, Yazdi AS, Schroder K, Ferrero I, Menu P, Tardivel A, Mattmann C, Tschopp J. Differential expression of NLRP3 among hematopoietic cells. J Immunol. 2011;186:2529–2534. doi: 10.4049/jimmunol.1002720. [DOI] [PubMed] [Google Scholar]
- 33.Hornung V, Latz E. Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol. 2010;40:620–623. doi: 10.1002/eji.200940185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffman HM, Brydges SD. Genetic and molecular basis of inflammasome-mediated disease. J Biol Chem. 2011;286:10889–10896. doi: 10.1074/jbc.R110.135491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 36.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 37.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Saha N, Moldovan F, Tardif G, Pelletier JP, Cloutier JM, Martel-Pelletier J. Interleukin-1beta-converting enzyme/caspase-1 in human osteoarthritic tissues: localization and role in the maturation of interleukin-1beta and interleukin-18. Arthritis Rheum. 1999;42:1577–1587. doi: 10.1002/1529-0131(199908)42:8<1577::AID-ANR3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 42.O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev. 2008;226:10–18. doi: 10.1111/j.1600-065X.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 43.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1 beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Feuerstein GZ, Gu JL, Lysko PG, Yue TL. Interleukin-1 beta induces expression of adhesion molecules in human vascular smooth muscle cells and enhances adhesion of leukocytes to smooth muscle cells. Atherosclerosis. 1995;115:89–98. doi: 10.1016/0021-9150(94)05503-b. [DOI] [PubMed] [Google Scholar]
- 45.Perrella MA, Patterson C, Tan L, Yet SF, Hsieh CM, Yoshizumi M, Lee ME. Suppression of interleukin-1beta-induced nitric-oxide synthase promoter/enhancer activity by transforming growth factor-beta1 in vascular smooth muscle cells. Evidence for mechanisms other than NF-kappaB. J Biol Chem. 1996;271:13776–13780. doi: 10.1074/jbc.271.23.13776. [DOI] [PubMed] [Google Scholar]
- 46.Ito A, Mukaiyama A, Itoh Y, Nagase H, Thogersen IB, Enghild JJ, Sasaguri Y, Mori Y. Degradation of interleukin 1beta by matrix metalloproteinases. J Biol Chem. 1996;271:14657–14660. doi: 10.1074/jbc.271.25.14657. [DOI] [PubMed] [Google Scholar]
- 47.Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E (−/−) mice through release of interferon-gamma. Circ Res. 2002;90:E34–E38. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- 48.Favilli F, Anzilotti C, Martinelli L, Quattroni P, De Martino S, Pratesi F, Neumann D, Beermann S, Novick D, Dinarello CA, Boraschi D, Migliorini P. IL-18 activity in systemic lupus erythematosus. Ann N Y Acad Sci. 2009;1173:301–309. doi: 10.1111/j.1749-6632.2009.04742.x. [DOI] [PubMed] [Google Scholar]
- 49.Reddy P, Ferrara JL. Role of interleukin-18 in acute graft-vs-host disease. J Lab Clin Med. 2003;141:365–371. doi: 10.1016/S0022-2143(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 50.Kimura K, Kakimi K, Wieland S, Guidotti LG, Chisari FV. Interleukin-18 inhibits hepatitis B virus replication in the livers of transgenic mice. J Virol. 2002;76:10702–10707. doi: 10.1128/JVI.76.21.10702-10707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, Tack CJ, van Krieken H, Kim SH, Stalenhoef AF, van de Loo FA, Verschueren I, Pulawa L, Akira S, Eckel RH, Dinarello CA, van den Berg W, van der Meer JW. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 52.Sarkar A, Hall MW, Exline M, Hart J, Knatz N, Gatson NT, Wewers MD. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1beta and interleukin-18. Am J Respir Crit Care Med. 2006;174:1003–1010. doi: 10.1164/rccm.200604-546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, Smith MR, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. Embo J. 1996;15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 54.Monack DM, Detweiler CS, Falkow S. Salmonella pathogenicity island 2-dependent macrophage death is mediated in part by the host cysteine protease caspase-1. Cell Microbiol. 2001;3:825–837. doi: 10.1046/j.1462-5822.2001.00162.x. [DOI] [PubMed] [Google Scholar]
- 55.Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105:4312–4317. doi: 10.1073/pnas.0707370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 57.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 58.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 59.Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. Embo J. 1990;9:1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Staal J, Bekaert T, Beyaert R. Regulation of NF-kappaB signaling by caspases and MALT1 paracaspase. Cell Res. 2011;21:40–54. doi: 10.1038/cr.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lamkanfi M, Kalai M, Saelens X, Declercq W, Vandenabeele P. Caspase-1 activates nuclear factor of the kappa-enhancer in B cells independently of its enzymatic activity. J Biol Chem. 2004;279:24785–24793. doi: 10.1074/jbc.M400985200. [DOI] [PubMed] [Google Scholar]
- 62.Lamkanfi M, Denecker G, Kalai M, D'Hondt K, Meeus A, Declercq W, Saelens X, Vandenabeele P. INCA, a novel human caspase recruitment domain protein that inhibits interleukin-1beta generation. J Biol Chem. 2004;279:51729–51738. doi: 10.1074/jbc.M407891200. [DOI] [PubMed] [Google Scholar]
- 63.Lakshmanan U, Porter AG. Caspase-4 interacts with TNF receptor-associated factor 6 and mediates lipopolysaccharide-induced NF-kappaB-dependent production of IL-8 and CC chemokine ligand 4 (macrophage-inflammatory protein-1) J Immunol. 2007;179:8480–8490. doi: 10.4049/jimmunol.179.12.8480. [DOI] [PubMed] [Google Scholar]
- 64.LeBlanc PM, Yeretssian G, Rutherford N, Doiron K, Nadiri A, Zhu L, Green DR, Gruenheid S, Saleh M. Caspase-12 modulates NOD signaling and regulates antimicrobial peptide production and mucosal immunity. Cell Host Microbe. 2008;3:146–157. doi: 10.1016/j.chom.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garedew A, Henderson SO, Moncada S. Activated macrophages utilize glycolytic ATP to maintain mitochondrial membrane potential and prevent apoptotic cell death. Cell Death Differ. 2010;17:1540–1550. doi: 10.1038/cdd.2010.27. [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, Cascante M, Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 68.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 69.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem. 2007;282:36321–36329. doi: 10.1074/jbc.M708182200. [DOI] [PubMed] [Google Scholar]
- 71.Merhi-Soussi F, Kwak BR, Magne D, Chadjichristos C, Berti M, Pelli G, James RW, Mach F, Gabay C. Interleukin-1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein E-knockout mice. Cardiovasc Res. 2005;66:583–593. doi: 10.1016/j.cardiores.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 72.Galea J, Armstrong J, Gadsdon P, Holden H, Francis SE, Holt CM. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol. 1996;16:1000–1006. doi: 10.1161/01.atv.16.8.1000. [DOI] [PubMed] [Google Scholar]
- 73.Mallat Z, Corbaz A, Scoazec A, Besnard S, Leseche G, Chvatchko Y, Tedgui A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001;104:1598–1603. doi: 10.1161/hc3901.096721. [DOI] [PubMed] [Google Scholar]
- 74.Elhage R, Jawien J, Rudling M, Ljunggren HG, Takeda K, Akira S, Bayard F, Hansson GK. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc Res. 2003;59:234–240. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 75.Isoda K, Sawada S, Ishigami N, Matsuki T, Miyazaki K, Kusuhara M, Iwakura Y, Ohsuzu F. Lack of interleukin-1 receptor antagonist modulates plaque composition in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1068–1073. doi: 10.1161/01.ATV.0000127025.48140.a3. [DOI] [PubMed] [Google Scholar]
- 76.Erridge C. The roles of pathogen-associated molecular patterns in atherosclerosis. Trends Cardiovasc Med. 2008;18:52–56. doi: 10.1016/j.tcm.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 77.Blankenberg S, Godefroy T, Poirier O, Rupprecht HJ, Barbaux S, Bickel C, Nicaud V, Schnabel R, Kee F, Morrison C, Evans A, Lackner KJ, Cambien F, Munzel T, Tiret L. Haplotypes of the caspase-1 gene, plasma caspase-1 levels, and cardiovascular risk. Circ Res. 2006;99:102–108. doi: 10.1161/01.RES.0000232324.87983.4b. [DOI] [PubMed] [Google Scholar]
- 78.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Menu P, Pellegrin M, Aubert JF, Bouzourene K, Tardivel A, Mazzolai L, Tschopp J. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moyer CF, Sajuthi D, Tulli H, Williams Jk. Synthesis of IL-1 alpha and IL-1 beta by arterial cells in atherosclerosis. Am J Pathol. 1991;138:951–960. [PMC free article] [PubMed] [Google Scholar]
- 81.Yin Y, Yan Y, Jiang X, Mai J, Chen NC, Wang H, Yang XF. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int J Immunopathol Pharmacol. 2009;22:311–322. doi: 10.1177/039463200902200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Randle JC, Harding MW, Ku G, Schonharting M, Kurrle R. ICE/Caspase-1 inhibitors as novel anti-inflammatory drugs. Expert Opin Investig Drugs. 2001;10:1207–1209. doi: 10.1517/13543784.10.7.1207. [DOI] [PubMed] [Google Scholar]