Abstract
Elevated homocysteine levels and low vitamin B12 and folate levels have been associated with deteriorated bone health. This systematic literature review with dose-response meta-analyses summarizes the available scientific evidence on associations of vitamin B12, folate, and homocysteine status with fractures and bone mineral density (BMD). Twenty-seven eligible cross-sectional (n = 14) and prospective (n = 13) observational studies and one RCT were identified. Meta-analysis on four prospective studies including 7475 people showed a modest decrease in fracture risk of 4% per 50 pmol/L increase in vitamin B12 levels, which was borderline significant (RR = 0.96, 95% CI = 0.92 to 1.00). Meta-analysis of eight studies including 11511 people showed an increased fracture risk of 4% per μmol/L increase in homocysteine concentration (RR = 1.04, 95% CI = 1.02 to 1.07). We could not draw a conclusion regarding folate levels and fracture risk, as too few studies investigated this association. Meta-analyses regarding vitamin B12, folate and homocysteine levels, and BMD were possible in female populations only and showed no associations. Results from studies regarding BMD that could not be included in the meta-analyses were not univocal.
1. Introduction
Osteoporosis is a chronic, multifactorial disorder which is characterized by low bone mass and microarchitectural deterioration of bone tissue [1]. Its major consequence is fractures. Especially hip fractures are frequently associated with institutionalization and increased mortality, and thus with an increased social and economic burden. This burden is expected to increase substantially in Europe in the coming decades due to a rise in life expectancy [2].
Elevated homocysteine concentrations and low vitamin B12 and folate status have been associated in several studies with lower bone mineral density (BMD) and higher fracture risk in elderly [3–11].
An elevated plasma homocysteine level (>15 μmol/L) is prevalent in 30–50% of people older than 60 years [12–14]. The cause is multifactorial; a combination of environmental and genetic factors, nutrition, lifestyle, and hormonal factors [15]. Vitamin B12 and folate are major determinants of homocysteine metabolism [16, 17] and supplementation with vitamin B12 and folic acid has been shown to be effective in normalizing homocysteine levels [18, 19]. Reversing elevated homocysteine levels through folic acid and vitamin B12 supplementation could theoretically prevent the problem of impaired bone health and osteoporosis. However, at present, no consensus is reached on the magnitude of the association between vitamin B12, folate, homocysteine, and bone health nor on the possible effect of vitamin B12 and folate supplementation on bone health.
Up until now one systematic review including a meta-analysis summarized the evidence on homocysteine and fracture risk, showing that higher homocysteine levels significantly increase the risk of fracture [20]. No meta-analyses are known on the topic of folate and vitamin B12 in relation to bone health. The purpose of this review is to provide a systematic overview, where possible including pooled estimates of the dose-response association, of the scientific evidence available from randomized controlled trials (RCTs), prospective cohort, and cross-sectional studies addressing vitamin B12, folate, and homocysteine levels in association with bone health, that is, fracture risk and BMD, in adults and elderly people.
2. Methods
This systematic review with dose-response meta-analyses was conducted within the scope of the EURRECA (European Micronutrient Recommendations Aligned) Network of Excellence (http://www.eurreca.org/) [21]. We followed a standardized methodology which is described in short below.
2.1. Search Strategy and Selection of Articles
We conducted systematic literature searches for (1) vitamin B12, (2) folate, and (3) homocysteine. The electronic databases MEDLINE, EMBASE, and Cochrane Library Central were searched, using search terms in “MeSH” terms and “title” and “abstract” on study designs in humans, vitamin B12, folate, homocysteine, and intake or status. The full Medline search strategy is available online, (see Appendix 1 in Supplementary Material at http://dx.doi.org/10.1155/2013/486186).
To be able to use the same search to identify publications on other health related outcomes both in adults and elderly and in younger population groups, no terms were added to limit the search to health outcome or study population. Moreover, by using a broad search we expected a more complete retrieval of relevant publications. In this review only the results on vitamin B12, folate, and homocysteine status (i.e., biomarkers measured in serum or plasma) in relation to bone health indicators (fracture risk and BMD) are presented. In addition to the search, reference lists of 10 review articles were checked to identify potentially relevant references that were not identified with the multidatabase search. The search was not limited by language. This review contains studies up to July 2012.
We selected articles in two steps. The first selection step included screening for title and abstract by three independent investigators (J. P. van Wijngaarden, E. L. Doets, SB). In the second selection step, full texts of the selected abstracts were evaluated on basis of predefined inclusion criteria by four investigators (J. P. van Wijngaarden, E. L. Doets, A. Szczecińska, MP).
For the purpose of alignment and quality control 10% of the references in each selection step was screened and selected in duplicate by two investigators independently. Results were compared and discrepancies were resolved by unanimous consensus among all investigators.
Studies were eligible for inclusion if they were conducted in apparently healthy human subjects aged ≥18 y. Furthermore, studies had to report fracture incidence, fracture risk, or bone mineral density (BMD) as a health outcome and had to report baseline data on the outcome measure.
Observational studies were included if they (1) had a prospective cohort, nested case-control, or cross-sectional design, and (2) addressed serum/plasma concentration of markers indicating vitamin B12 status (serum/plasma vitamin B12, methylmalonic acid (MMA), holotranscobalamin (holoTC)), folate status (serum/plasma folate or erythrocyte folate), or homocysteine status (serum/plasma homocysteine). Intervention studies were included if they (1) had a randomized controlled trial design, (2) studied the effects of vitamin B12 or folic acid supplements, fortified foods or micronutrient intake from natural food sources and included a placebo or untreated comparison group, and (3) had a minimum intervention duration of six months.
2.2. Data Extraction and Statistical Analysis
We extracted data for each of the identified studies on population characteristics, study design, assessment of vitamin B12, folate and homocysteine status, and fracture risk or bone mineral density.
Opportunities for meta-analysis were evaluated based on comparability of health outcome and status marker. If less than three comparable studies were available, results were qualitatively described. If three or more comparable studies were available, the results of these individual studies were expressed in a standardized format to allow comparison in the form of a continuous dose-response meta-analysis that pools the regression coefficient (β) (SE) from multiple adjusted models. We chose to express association measures for serum/plasma vitamin B12 per 50 pmol/L. When βs were not reported in the original article, we transformed Relative Risk (RR), Hazard Ratio (HR), or Odds Ratio (OR) to βs, using a standardized method [22]. The transformations to obtain βs and SEs and statistical analyses were performed using R statistics version 2.9.2 (http://www.R-project.org/), with statistical significance defined as P < 0.05. HR and OR were considered as RR because the outcome was relatively rare. If articles reported insufficient data (missing data, inconsistencies, or any other uncertainties), we contacted corresponding authors for additional information.
We calculated summary estimates of comparable studies using random effects meta-analysis. Applying the methods of DerSimonian and Laird, the between study variance was estimated which was used to modify the weights for calculating the summary estimate [23]. Residual heterogeneity between studies was evaluated using Q-statistic and I 2-statistic.
In total, from 3 searches we identified 11837 potentially relevant articles, of which 9835 articles were excluded based on title and abstract. Of the remaining 2002 articles, 1961 articles were excluded based on full texts, leaving 41 articles. As the searches were partly overlapping and some articles addressed more than one association this resulted in 20 unique articles, 19 observational and 1 intervention. A search update on July 2nd, 2012 resulted in an additional 8 observational studies, which makes a total of 28 included articles. All addressed the association between vitamin B12, folate or homocysteine status, and fracture risk or BMD. The flow diagram of the process of screening and selection is shown in Figure 1.
Figure 1.
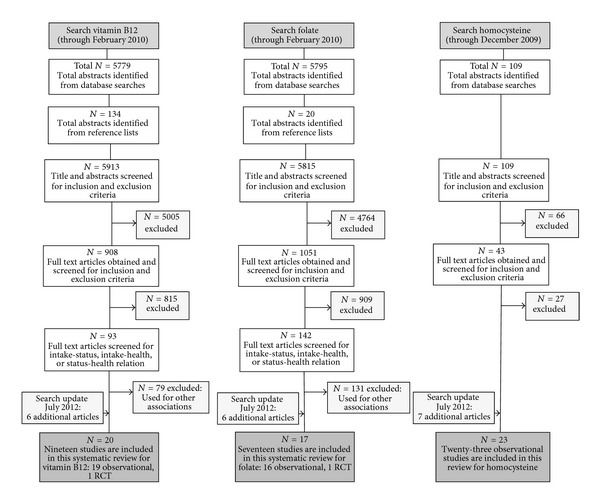
Flow diagram of screening and selection.
3. Results
3.1. Fractures
3.1.1. Vitamin B12
Four longitudinal observational studies [3, 24–26], including 7475 elderly people with 3 to 16 years of follow-up and a total of 458 cases addressed the association between serum/plasma vitamin B12 and fracture (Table 1). Pooled analysis of the association between 50 pmol/L increase in plasma/serum B12 and change in fracture risk showed an inverse association (RR = 0.96, 95% CI = 0.92 to 1.00) with no heterogeneity between studies (I 2 = 0%, P = 0.76) (Figure 2). This indicates that a vitamin B12 increase of 50 pmol/L tends to decrease the risk of fracture with 4%.
Table 1.
Studies regarding the association between vitamin B12 and bone health.
| Author Year |
Study characteristics Duration of follow-up (when applicable) Country Risk of bias |
Population characteristics: N (% men) Age (y) ± SD |
Vitamin B12 status pmol/L* Mean ± SD |
Outcome | Association type | Results* | ||
|---|---|---|---|---|---|---|---|---|
| Dhonukshe-Rutten et al. 2005 [3] |
Cohort (3 y) The Netherlands High risk |
1253 (48%) 75.5 ± 6.6 |
♀: 289 ± 99 ♂: 268 ± 89 |
Fracture (verified by physician or radiograph) |
β (SE) for association vitB12-fracture (per 50 pmol/L) |
♀: −0.09 (0.06)a, 1
♂: 0.02 (0.08)a, 1 |
||
|
| ||||||||
| Gjesdal et al. 2007 [24] | Cohort (12.6 y) Norway Low risk |
4761 (45%) 65–67 at baseline |
♀: 386.4 ± 372.0 ♂: 359.3 ± 276.2 |
Hip fracture (verified by hospital discharge diagnoses) |
β (SE) for association vitB12-hip fracture (per 50 pmol/L) |
♀: −0.03 (0.03)b, 2
♂: −0.06 (0.05)b, 2 |
||
|
| ||||||||
| McLean et al. 2008 [25] | Cohort (16 y) USA Low risk |
823 (41%) 75.3 ± 4.9 |
Deficient (<148): ♀ 9%/♂14.0% Low (148–257.9): ♀ 24.3%/♂32.5% Normal (≥258): ♀ 66.7%/♂53.5% |
Hip fracture (verified by review medical records) |
β (SE) for association vitB12-hip fracture (per 50 pmol/L) |
♀: −0.09 (0.06)c, 1
♂: −0.09 (0.11)c, 1 |
||
|
| ||||||||
| Ravaglia et al. 2005 [26] | Cohort (4 y) Italy Moderate risk |
702 (47%) 73.0 ± 6.0 |
Geometric mean (95% CI) 249.1 (203–272) |
Fracture (verified by review medical records) |
β (SE) for association vitB12-fracture (per 50 pmol/L) | 0.04 (0.08)d, 2 | ||
|
| ||||||||
| Bozkurt et al. 2009 [32] | Cross-sectional Turkey High risk |
178 (0%) 53.5 ± 8.0 |
247.7 ± 85.4 | BMD: LS, FN [DXA] |
Logistic regression for FN, LS and FN + LS combined for vitB12 status under the quintile value. β (SE) + P value |
LS: −2.3 (0.9) P = 0.017 FN: −0.4 (0.9) P = 0.669 LS + FN: 1.8 (0.8) P = 0.045e |
||
|
| ||||||||
| Bucciarelli et al. 2010 [33] | Cross-sectional Italy Moderate risk |
446 (0%) 65.1 ± 9.4 |
(geometric mean ± SD) 399.1 ± 1.6 |
BMD: FN, LS, TH [DXA, Prodigy, GE, Lunar] | β for association vitB12-TH BMD β (SE) (per 50 pmol/L) | −0.00105 (0.939)f, 2 | ||
|
| ||||||||
| Cagnacci et al. 2008 [34] | Cohort (5 y) Italy Moderate risk |
117 (0%) 54.4 ± 0.5 |
(Mean ± SE) 548.5 ± 40.5 |
BMD: LS [DXA: Lunar DPX] |
Regression for vitB12-BMD change β (SE) P value | −0.003 (0.012) P = 0.784g | ||
|
| ||||||||
| Dhonukshe-Rutten et al. 2003 [35] |
Cross-sectional The Netherlands Moderate risk |
194 (26%) 78.3 ± 5.5 |
♀ 288 ± 131 ♂ 238 ± 95 |
BMD: whole body [DXA, Lunar DPX-L] |
Multivariate regression, β for association vitB12-BMD β (95% CI) in women | ♀: 12.3·10−5 (0.2·10−5–2.4·10−4)h | ||
|
| ||||||||
| Gjesdal et al. 2006 [10] | Cross-sectional Norway Moderate risk |
5329 (43%) middle aged: 47–50 Older: 71–75 |
♀ 393.4 ± 235.8 ♂ 374.6 ± 230.7 |
BMD: TH [DXA, Lunar EXPERT-XL] |
OR (95% CI) for low BMD per category vitB12 status 1: <230 pmol/L 2: 230.0–279.9 pmol/L 3: 280.0–414.9 pmol/L 4: ≥415.0 pmol/L + P for trend |
♀: 1: 0.97 (0.68–1.37) 2: 0.87 (0.63–1.21) 3: 1.02 (0.82–1.27) 4: 1.00 (reference) P for trend = 0.61 |
♂: 1.22 (0.82–1.81) 1.14 (0.80–1.62) 0.97 (0.74–1.28) 1.00 (reference) P for trend = 0.25i |
|
|
| ||||||||
| Golbahar et al. 2004 [9] | Cross-sectional Iran Moderate risk |
271 (0%) 60.8 ± 6.8 |
(geometric mean ± SD) 339.5 ± 247.6 |
BMD: FN, LS [DXA, Lunar DPX-L] |
β (SE) for association vitB12-BMD (per 50 pmol/L) |
FN: 0.0002 (0.07)2
LS: 0.0114 (0.14)2 |
||
|
| ||||||||
| Haliloglu et al. 2010 [36] | Cross-sectional Turkey Moderate risk |
120 (0%) 54.4 ± 1.1 |
Osteoporotic: 216.0 ± 135.1 Osteopenic: 190.8 ± 97.4 Normal BMD: 251.0 ± 205.8 |
BMD: LS [DXA, Lunar DPX-L] |
ANOVA for difference in vitB12 status per BMD group compared to normal BMD group | No sign differences in vitB12 status between BMD groups | ||
|
| ||||||||
| Krivosikova et al. 2010 [37] | Cross-sectional Slovakia High risk |
272 (0%) 41.3 ± 19.8 |
273.2 ± 152.7 | BMD: FN, LS, trochanter, TH [DXA, Lunar DPX-L] |
Stepwise multivariate linear regression, β for association vitB12-BMD. β (SE) P value (per 50 pmol/L) |
FN: −2.0 (2.73) j, 2
LS: −1.15 (1.42) j, 2 TH: −0.5 (3.03) j, 2 |
||
|
| ||||||||
| Morris et al. 2005 [7] |
Cross-sectional USA Low risk |
1550 (48%) 68 |
Geometric mean (95% CI) Osteoporosis: 271 (243–302) Osteopenia: 309 (293–325) Normal: 310 (297–323) Serum MMA (nmol/L) Osteoporosis: 305 (276–337) Osteopenia: 251 (234–269) Normal: 241 (212–274) |
BMD: Trochanter, intertrochanter, FN, Ward's triangle, TH [DXA, Hologic QDR-1000] | OR (95% CI) for mean BMD in relation to quartile categories of vitB12 and MMA status + P for trend. Category medians: | Vit B12: Q1: 2.0 (1.0–3.9) Q2: 1.3 (0.6–2.7) Q3: 1.7 (0.8–3.3) Q4: 1.0 (reference) P for trend = 0.09 |
MMA: 1.0 (reference) 3.5 (1.4–8.5) 5.2 (2.0–13.1) 7.2 (3.4–15.2) P for trend <0.001k |
|
| B12 (pmol/L): Q1: 182 Q2: 268 Q3: 349 Q4: 495 |
MMA (nmol/L): 157 206 272 415 |
Among subjects with vitB12 <220 pmol/L mean BMD increased sign with increasing vitB12 (P = 0.01) | ||||||
|
| ||||||||
| Naharci et al. 2012 [38] | Cross-sectional Turkey Moderate risk |
264 (100%) 77.0 ± 6.0 |
26.7% low (<148, group I) 39.1% borderline (148–221, group II) 34.2% normal (>221, group III) |
BMD: FN, TH, trochanter, inter-trochanter [DXA, hologic QDR-4500] | Anova for differences in FN BMD between groups of serum vitB12 |
Sign differences FN BMD group I and II (P = 0.013) group I and III (P < 0.001) group II and III (P = 0.003) FN BMD was positively correlated with serum vitB12 (r = 0.362, P < 0.001) |
||
|
| ||||||||
| Ouzzif et al. 2012 [39] | Cross-sectional Morocco Moderate risk |
188 (0%) 57.8 ± 8.5 |
360.4 ± 149.2 |
BMD: FN, LS, TH, trochanter [DXA, Lunar prodigy] | Multivariate regression, β for association vitB12-BMD β (SE) (per 50 pmol/L) P value | LS: −7.85 (0.25) TH: −11.65 (0.02) |
P = 0.160L, 2
P = 0.007L, 2 |
|
|
| ||||||||
| Rumbak et al. 2012 [40] | Cross-sectional Croatia Low risk |
131 (0%) 54.0 ± 4.9 |
239.6 ± 97.0 | BMD: FN, LS, TH, radius [DXA, Lunar-prodigy] |
Stepwise multivariate regression, β for association vitB12-BMD for pre- and postmenopausal women β (SE) P value (per 50 pmol/L) |
Premenopausal: LS: −3.39 (8.91) P = 0.709m, 2 FN: 7.45 (10.07) P = 0.467m, 2 TH: −1.36 (7.53) P = 0.862m, 2 Postmenopausal: LS: 7.45 (8.98) P = 0.411m, 2 FN: 12.20 (8.97) P = 0.180m, 2 TH: 8.81 (8.63) P = 0.314m, 2 |
||
|
| ||||||||
| Stone et al. 2004 [11] | Cohort (5.9 y) USA Low risk |
83 (0%) 71.1 ± 4.4 |
352 ± 174 |
BMD: TH, FN (change) [DXA, Hologic QDR-1000] | t-test for difference in BMD change between low and normal vitB12 status | Participants with low vitB12 (≤207 pmol/L) had a more rapid decline in BMD (−1.91%/year) than part. with normal vitB12 (−0.10%/year), P < 0.05 | ||
|
| ||||||||
| Tucker et al. 2005 [6] | Cross-sectional USA Low risk |
2576 (44%) 58.8 ± 9.5 |
Distribution per category of plasma vitB12 status 1: ♀ 4.4%/♂4.7% ≤148 2: ♀ 6.9%/♂7.8% >148–185 3: ♀ 25.4%/♂28.2% >185–259 4: ♀ 63.3%/♂ 59.3% >259 |
BMD: FN, LS, TH, Trochanter, Ward [DXA, Lunar DPX-L] |
Differences in BMD per category of plasma vitB12 level, relative to category 1 |
♀: FN: no differences ♀: LS: vitB12 in cat 2 (P < 0.10), 3, 4 (P < 0.05) was assoc. with better BMD ♀: TH: vitB12 in cat 3, 4 (P < 0.10) was assoc. with better BMD ♂: FN: vitB12 in cat 2, 3, 4 was assoc. with better BMD (P < 0.05) ♂ LS: no differences ♂ TH: vitB12 in cat 2 (P < 0.10), 3, 4 (P < 0.05) was assoc. with better BMDn |
||
*Serum/plasma vitamin B12 concentrations were converted to pmol/L if applicable, using the following equation: 1 pg/mL = 1 ng/L = 0.738 pmol/L. subsequent outcomes were also converted. Where possible, subgroups were combined. BMD sites: LS: Lumbar Spine, FN: Femoral Neck, TH: Total Hip.
1 β(SE) as calculated from data provided by author; 2 β (SE) as calculated from presented data.
aadjusted for age, BMI, smoking, recurrent falling; badjusted for age, BMI, smoking, coffee intake, physical activity, vit D use, educational level, estrogen use in women; cadjusted for sex, age, height, weight, estrogen use in women; dadjusted for age, sex, education, osteoporosis drugs, creatinine, tHcy; eadjusted for duration of menopause, smoking, BMI, folic acid levels, tHcy levels; fadjusted for age, BMI, logtHcy, logFolate, creatinine clearance, smoking, alcohol intake; gAdjusted for age, weight, weight change; hadjusted for weight, height, energy intake; iadjusted for smoking, BMI, creatinin, coffee intake, physical activity, use of estrogen therapy; jadjusted for age, folate, tHcy, PTH, CTx, Ca, Cr; kAdjusted for age, sex, ethnicity, BMI, smoking, physical activity, creatinin, alcohol, coffee, energy, calcium, vitamin D zinc intake; Ladjusted for age, BMI, tHcy and folate; madjusted for Age, BMI, smoking, alcohol, physical activity, tHcy, Folate; nadjusted for energy, calcium, vitamin D intake, BMI, height, smoking, age, physical activity, calcium supplement, vitamin D supplement, alcohol, osteoporosis medication, season of measurement.
Figure 2.
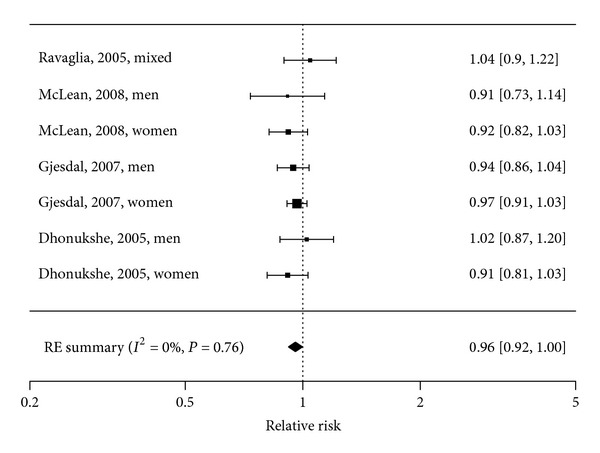
Forest plot of the association between vitamin B12 (50 pmol/L) and risk of fracture: Meta-Analysis of 4 observational studies.
3.1.2. Folate
Three longitudinal observational studies examined the association between plasma folate and fractures [24–26] (Table 2). One study showed that women, but not men, with plasma folate in the lowest quartile had a higher fracture risk (HR 2.40, 95% CI 1.50 to 3.84) compared to the highest (reference) quartile (P for trend <0.001) [24]. Ravaglia et al. (2005) [26] showed a significant association between low folate status and fracture risk when folate was analyzed as a dichotomous variable (lowest quartile of folate status versus other 3 quartiles), but when analyzed as a continuous variable, no significant association was observed [26]. One study did not observe an association [25].
Table 2.
Studies regarding the association between folate and bone health.
| Author Year |
Study characteristics Duration of follow-up (when applicable) Country Risk of bias |
Population characteristics: N (%men) Age (y) ± SD |
Folate status (nmol/L)* Mean ± SD |
Outcome | Association type | Results* | ||
|---|---|---|---|---|---|---|---|---|
| Gjesdal et al. 2007 [24] | Cohort (12.6 y) Norway Low risk |
4761 (45%) 65–67 at baseline |
♀ 6.0 ± 3.5 ♂ 5.2 ± 2.7 |
Hip fracture (verified by hospital discharge diagnoses) |
HR for hip fracture according to folate status 1: <2.9 2: 2.9–3.8 3: 3.9–6.5 4: ≥6.6 |
♀: 1: 2.40 (1.50–3.84) 2: 1.15 (0.68–1.94) 3: 1.02 (0.68–1.54) 4: 1.00 (reference)a |
♂: 1.00 (0.48–2.12) 0.80 (0.39–1.62) 0.81 (0.45–1.46) 1.00 (reference)a |
|
|
| ||||||||
| McLean et al. 2008 [25] | Cohort (16 y) USA Low risk |
960 (41%) 75.3 ± 4.9 |
Not shown | Hip fracture (verified by review medical records) |
HR for hip fracture according to folate status Normal: ≥11 Low: 7–10.9 Deficient: <7 |
Normal: 1.00 (reference) Low: 0.76 (0.43, 1.32) Deficient: 1.38 (0.91, 2.09)b |
||
|
| ||||||||
| Ravaglia et al. 2005 [26] | Cohort (4 y) Italy Moderate risk |
702 (47%) 73.0 ± 6.0 |
11.7 (9.0–12.2) mean (95% CI) |
Fracture (verified by review medical records) |
OR (95% CI) for risk of fracture at follow-up for each increment of 1 sd in the log-transformed serum folate value | 0.83 (0.59–1.19)c | ||
|
| ||||||||
| Baines et al. 2007 [41] | Cross-sectional Great Britain High risk |
328 (0%) 67.5 (40–85) mean (range) |
Osteoporosis: 8.1 ± 8.7# Osteopenia: 10.2 ± 4.6 Normal: 9.4 ± 6.3 |
BMD: os calcis/ heel bone [PIXI, GE Lunar] | ANOVA for difference between the normal, osteopenia and osteoporosis group |
FA status was significantly different between osteroporotic and osteopenic group (P = 0.049) |
||
|
| ||||||||
| Bozkurt et al. 2009 [32] | Cross-sectional Turkey High risk |
178 (0%) 53.5 ± 8.0 |
24.9 ± 7.9 |
BMD: FN, LS [DXA] |
Logistic regression for FN, LS and FN + LS combined. β (SE) + P value for assoc. BMD-folate status under the median value |
LS: −0.2 (0.2) P = 0.417 FN: −0.04 (0.2) P = 0.835 LS + FN: −0.03 (0.2) P = 0.896d |
||
|
| ||||||||
| Bucciarelli et al. 2010 [33] | Cross-sectional Italy Moderate risk |
446 (0%) 65.1 ± 9.4 |
(geometric mean ± SD) 3.8 ± 1.6 |
BMD: FN, LS, TH [DXA, Prodigy, GE, Lunar] | β for association folate-TH BMD β (SE) | 0.004 (0.018)e, 2 | ||
|
| ||||||||
| Cagnacci et al. 2008 [34] | Cohort (5 y) Italy Moderate risk |
117 (0%) 54.4 ± 0.5 |
(Mean ± SE) 20.6 ± 1.4 |
BMD: LS [DXA: Lunar DPX] |
Regression analysis for folate-BMD change β (SE) + P value | 1.602 (0.803) P = 0.048f | ||
|
| ||||||||
| Cagnacci et al. 2003 [8] | Cross-sectional Italy Moderate risk |
161 (0%) 53.3 ± 1.04 |
(Mean ± SE) 21.5 ± 4.3 |
BMD: LS [DXA: Lunar DPX] |
Regression analysis, r (P value) for association folate-BMD | r = 0.254 | (P < 0.002) | |
|
| ||||||||
| Gjesdal et al. 2006 [10] |
Cross-sectional Norway Moderate risk |
5329 (43%) middle aged: 47–50 Older: 71–75 |
♀ 8.9 ± 7.1 ♂ 7.3 ± 4.6 |
BMD: TH [DXA, Lunar EXPERT-XL] |
OR (95% CI) for low BMD per category folate status: 1: FA < 3.8 nmol/L 2: FA 3.8–4.9 nmol/L 3: FA 5.0–8.4 nmol/L 4: FA ≥ 8.5 nmol/L + P for trend Multivariate regression for folate-BMD β (SE) (per 50 nmol/L) |
♀: 1: 1.55 (1.07–2.23) 2: 1.18 (0.86–1.63) 3: 1.24 (0.99–1.56) 4: 1.00 (reference) P for trend = 0.02 |
♂: 0.81 (0.53–1.24) 0.96 (0.67–1.38) 1.15 (0.87–1.53) 1.00 (reference) P for trend = 0.26g |
|
| Elderly women: β = 0.05 (0.02)g, 2 | ||||||||
|
| ||||||||
| Golbahar et al. 2004 [9] | Cross-sectional Iran Moderate risk |
271 (0%) 60.8 ± 6.8 |
(geometric mean ± SD) 11.6 ± 6.5 |
BMD: FN, LS [DXA, Lunar DPX-L] | β for association folate-BMD β (SE) |
FN: 0.008 (0.019)h, 2
LS: 0.010 (0.018)i, 2 |
||
|
| ||||||||
| Haliloglu et al. 2010 [36] | Cross-sectional Turkey Moderate risk |
120 (0%) 54.4 ± 1.1 |
Osteoporotic: 12.2 ± 6.3 Osteopenic: 15.4 ± 7.4 Normal: 15.8 ± 8.3 |
BMD: LS [DXA, Lunar DPX-L] |
ANOVA for difference in folate status per BMD group (osteoporotic, osteopenic, compared to normal BMD group) | No significant differences in folate status between BMD groups | ||
|
| ||||||||
| Krivosikova et al. 2010 [37] | Cross-sectional Slovakia High risk |
272 (0%) 41.3 ± 19.8 |
23.8 ± 9.6 | BMD: FN, LS, trochanter, TH [DXA, Lunar DPX-L] |
Stepwise multivariate linear regression, β for association folate-BMD. β (SE) P value |
FN: −0.028 (0.054) P = 0.606 j, 2
LS: −0.001 (0.067) P = 0.988 j, 2 TH: −0.032 (0.060) P = 0.595 j, 2 |
||
|
| ||||||||
| Morris et al. 2005 [7] | Cross-sectional USA Low risk |
1550 (47%) 68 |
Osteoporosis: 17.2 (15.4–19.2) Osteopenia: 17.2 (16.0–18.5) Normal: 16.7 (15.3–18.3) Geometric mean (95% CI) |
BMD: Trochanter, intertrochanter, FN, Ward's triangle, TH [DXA, Hologic QDR-1000] | OR (95% CI) for mean BMD in relation to quartile categories of folate status + P for trend Category median (nmol/L): Q1: 8.0 Q2: 12.4 Q3: 20.3 Q4: 38.9 |
Q1: 1.1 Q2: 1.1 Q3: 1.5 Q4: 1.0 P for trend |
(0.5–2.3) (0.0.5–2.9) (0.7–3.4) (reference) = 0.83k |
|
|
| ||||||||
| Naharci et al. 2012 [38] | Cross-sectional Turkey Moderate risk |
264 (100%) 77.0 ± 6.0 |
low (<7.0, group I): 0.0% borderline (7.0–10.9, group II): 9.2% normal (>10.9, group III): 90.8% |
BMD: FN, TH, trochanter, intertrochanter [DXA, hologic QDR-4500] |
Independent sample t-test for differences in FN BMD between group II and III of serum folate | No significant differences in BMD (all sites) between group II and III of folate status | ||
|
| ||||||||
| Ouzzif et al. 2012 [39] | Cross-sectional Morocco Moderate risk |
188 (0%) 57.8 ± 8.5 |
15.6 ± 6.8 | BMD: FN, LS, TH, trochanter [DXA, Lunar prodigy] | Multivariate regression, β for association folate-BMD β (SE) + P value |
LS: 0.007 (0.002) P = 0.808L
TH: 0.006 (0.001) P = 0.834L |
||
|
| ||||||||
| Rumbak et al. 2012 [40] | Cross-sectional Croatia Low risk |
131 (0%) 54.0 ± 4.9 |
22.4 ± 7.5 | BMD: FN, LS, TH, radius [DXA, Lunar-prodigy] |
Stepwise multivariate regression, β for association folate-BMD β + P value |
Premenopausal: LS: 3.31 (4.73) P = 0.490m, 2 FN: 1.32 (4.90) P = 0.791m, 2 TH: 2.87 (4.35) P = 0.516m, 2 Postmenopausal: LS: −3.75 (3.47) P = 0.284m, 2 FN: −1.32 (3.15) P = 0.679m, 2 TH: 0.66 (3.89) P = 0.862m, 2 |
||
*Serum/plasma folate concentrations were converted to nmol/L if applicable, using the following equation: 1 ng/ml = 2.266 nmol/L. Subsequent outcomes were also converted. Where possible, subgroups were combined. BMD sites—LS: Lumbar Spine, FN: Femoral Neck, TH: Total Hip #data presented in article as μmol/L, this is presumably a typing error and should be nmol/L.
1 β (SE) as calculated from data provided by author; 2 β (SE) as calculated from presented data.
aadjusted for age, BMI, smoking, coffee intake, physical activity, vit D use, educational level, estrogen use in women; badjusted for sex, age, height, weight, estrogen use in women; cadjusted for age, gender, education, osteoporosis drug, serum creatinine, tHcy; dAdjusted for duration of menopause, smoking, BMI, B12, tHcy; eadjusted for age, BMI, logtHcy, logB12, creatinine clearance, smoking, alcohol intake;
fAdjusted for age, weight, weight change; gAdjusted for smoking, BMI, creatinin, coffee intake, physical activity, use of estrogen therapy; hadjusted for age, BMI, alkaline phosphatase; iadjusted for years since menopause, BMI, alkaline phosphatase, creatinine; jadjusted for age, B12, tHcy, PTH, CTx, Ca, Cr; kAdjusted for age, sex, ethnicity, BMI, smoking, physical activity, creatinin, alcohol, coffee, energy, calcium, vitamin D zinc intake; Ladjusted for age, BMI, tHcy, B12; madjusted for Age, BMI, smoking, alcohol, physical activity, tHcy, B12.
3.1.3. Homocysteine
Eleven longitudinal observational studies examined the association between homocysteine status and fracture incidence [3–5, 25–29] (Table 3). A meta-analysis of eight studies, including 11511 elderly people with 3 to 12.6 years of follow-up and 1353 cases, showed a significantly increased fracture risk with increasing plasma homocysteine (μmol/L) (summary estimate RR 1.04, (95% CI: 1.02 to 1.07). Heterogeneity between studies was large (I 2 = 60.6%, P = 0.0002) (Figure 3). When hip fractures (3 studies; [24, 28, 29]) and total fractures (5 studies; [3, 26, 27, 30, 31]) were analyzed separately, the relation remained significant, 1.06 (95% CI: 1.03 to 1.08, I 2 = 0%, P = 0.72) and 1.04 (95% CI: 1.00 to 1.08, I 2 = 65.0%, P = 0.011).
Table 3.
Studies regarding the association between homocysteine and bone health.
| Author Year |
Study characteristics Duration of follow-up (when applicable) Country Risk of bias |
Population characteristics: N (%men) Age (y) ± SD |
Homocysteine status (μmol/L) Mean ± SD |
Outcome | Association type | Results | ||
|---|---|---|---|---|---|---|---|---|
| Dhonukshe-Rutten et al. 2005 [3] | Cohort (3y) The Netherlands High risk |
1253 (48%) 75.5 ± 6.6 |
geometric mean (10–90 percentile) ♀: 13.0 (8.6–19.7) ♂: 14.9 (10.2–22.8) |
Fracture (verified by physician or radiograph) | β (SE) for association tHcy-fracture |
♀: 0.07 (0.05)a, 2
♂: 0.11 (0.05)a, 2 |
||
|
| ||||||||
| Enneman et al. 2012 [30] | Cohort (7 y) The Netherlands Moderate risk |
503 (0%) 68.5 (61.3–74.9) Median (range) |
Median (range) 9.3 (3.5–29.7) |
Fracture (verified by physician) | β (SE) for association tHcy-fracture | 0.05 (0.02)b, 2 | ||
|
| ||||||||
| Gerdhem et al. 2007 [29] | Cohort (7 y) Sweden Low risk |
996 (0%) 75 |
Median (IQR) 14.1 (11.6–17.3) |
Hip fracture (verified by radiograph) | β (SE) for association tHcy-hip fracture | 0.07 (0.03)c, 2 | ||
|
| ||||||||
| Gjesdal et al. 2007 [24] | Cohort (12.6 y) Norway Low risk |
4761 (45%) 65–67 at baseline |
♀: 11.6 ± 4.2 ♂: 13.1 ± 5.8 |
Hip fracture (verified by hospital discharge diagnoses) | β (SE) for association tHcy-hip fracture |
♀: 0.05 (0.02)d, 2
♂: 0.03 (0.03)d, 2 |
||
|
| ||||||||
| Leboff et al. 2009 [28] |
Nested case-control USA Moderate risk |
800 (0%) 70.8 ± 6.2 |
11.2 ± 4.1 |
Hip fracture (verified by radiograph) | β (SE) for association tHcy-Hip fracture | 0.07 (0.03)e, 2 | ||
|
| ||||||||
| HR (95% CI) for hip fracture risk by quartiles of tHcy. Mean tHcy per quartile: | ||||||||
| McLean et al. 2004 [4] |
Cohort (♀ 15 y; ♂12.3 y) USA Moderate risk |
1999 (41%) 70.0 ± 7.0 |
♀: 12.1 ± 5.3 ♂: 13.4 ± 9.1 |
Hip fracture (verified by review medical records) | ♀: Q1: 7.6 ± 1.0 Q2: 9.9 ± 0.7 Q3: 12.2 ± 0.7 Q4: 18.6 ± 6.4 |
♂: 8.5 ± 0.9 11.0 ± 0.6 13.4 ± 0.9 20.8 ± 15.7 |
♀: 1: 1.00 (reference) 2: 0.78 (0.45–1.33) 3: 1.07 (0.64–1.78) 4: 1.92 (1.18–3.10) |
♂: 1.00 (reference) 1.57 (0.54–5.14) 2.07 (0.70–6.09) 3.84 (1.38–10.70) |
| HR (95% CI) for each increase of 1 SD in log-transformed tHcy concentration |
♀/♂ Test for trend: P < 0.01 ♂ HR per SD 1.59 (1.31–1.94)f ♀ HR per SD 1.26 (1.08–1.47)f |
|||||||
|
| ||||||||
| McLean et al. 2008 [25] | Cohort (16 y) USA Low risk |
979 (41%) 75.3 ± 4.9 |
73.7% normal (≤14 μmol/l) 26.3% high (>14) |
Hip fracture (verified by review medical records) | HR (95% CI) for high plasma tHcy (≥14 μmol/L) versus normal tHcy | Normal 1.00 High 1.69 |
(reference) (1.12–2.55)g |
|
|
| ||||||||
| Van Meurs et al. 2004 [5] | Cohort (4.7 y) The Netherlands High risk |
2406 (47%) 73.9 ± 7.8 |
14.3 ± 5.8 |
Fracture (verified by physician) | RR (95% CI) for fracture for each increment of 1 SD in the natural log-transformed tHcy value. | 1.4 (1.2–1.6)h | ||
|
| ||||||||
| Périer et al. 2007 [27] | Cohort (10 y) France Moderate risk |
671 (0%) 61.6 ± 8.4 |
10.6 ± 3.5 | Fracture (verified by radiograph or surgical report) | β (SE) for association tHcy-fracture | 0.02 (0.02)i, 3 | ||
|
| ||||||||
| Ravaglia et al. 2005 [26] | Cohort (4 y) Italy Moderate risk |
702 (47%) 73.0 ± 6.0 |
Geometric mean (95% CI) 12.7 (11.3–15.1) |
Fracture (verified by review medical records) | β (SE) for association tHcy-fracture | 0.09 (0.05)j, 2 | ||
|
| ||||||||
| Zhu et al. 2009 [31] | Cohort (5 y) Australia Moderate risk |
1213 (0%) 75.2 ± 2.7 |
12.1 ± 4.6 | Fracture (verified by radiograph) | β (SE) for association tHcy-fracture | −0.002 (0.006)k, 2 | ||
|
| ||||||||
| Baines et al. 2007 [41] | Cross-sectional Great Britain High risk |
328 (0%) 67.5 (40–85) mean (range) |
12.3 ± 5.4 |
BMD: os calcis/heel bone [PIXI, GELunar] | Stepwise multivariate linear regression β (SE) + P value for association log tHcy-BMD | −1.548 (0.607) P = 0.011L | ||
|
| ||||||||
| Bozkurt et al. 2009 [32] | Cross-sectional Turkey High risk |
178 (0%) 53.5 ± 8.0 |
10.4 ± 3.0# | BMD: FN/LS [DXA] | Logistic regression for FN, LS and FN + LS combined. β (SE) + P value for association hcy level under the median value-BMD |
LS: −0.8 (0.5) P = 0.140 FN: −0.5 (0.6) P = 0.408 LS + FN: −1.3 (0.6) P = 0.032m |
||
|
| ||||||||
| Bucciarelli et al. 2010 [33] | Cross-sectional Italy Moderate risk |
446 (0%) 65.1 ± 9.4 |
(geometric mean ± SD) 10.6 ± 1.3 |
BMD: FN, LS, TH [DXA, Prodigy, GE, Lunar] | Multivariate linear regression β for association log tHcy-total femur BMD. β (SE) P value | −0.050 (0.025) P = 0.048n, 2 | ||
|
| ||||||||
| Cagnacci et al. 2008 [34] | Cohort Italy Moderate risk |
117 (0%) 54.4 ± 0.5 |
(Mean ± SE) 10.7 ± 0.5 |
BMD: LS [DXA: Lunar DPX] |
Regression analysis for Hcy-BMD change β (SE) + P value | −0.825 (1.09) P = 0.449o | ||
|
| ||||||||
| Cagnacci et al. 2003 [8] | Cross-sectional Italy Moderate risk |
161 (0%) 53.3 ± 1.0 |
10.5 ± 0.9 |
BMD: LS [DXA: Lunar DPX] |
Regression analysis, β for association Hcy-BMD | β = −0.002p, 1 | ||
|
| ||||||||
| Gerdhem et al. 2007 [29] | Cohort (cross sect data) Sweden Low risk |
996 (0%) 75 |
Median (IQR) 14.1 (11.6–17.3) |
BMD: FN, LS, TH [DXA: Lunar DPX-L] |
t-test for difference in BMD (P value) between highest quartile of hcy versus all others |
FN: Q4 versus LS: Q4 versus TH: Q4 versus |
Q1–3: P = 0.032 Q1–3: P = 0.821 Q1–3: P = 0.001 |
|
|
| ||||||||
| Gjesdal et al. 2006 [10] |
Cross-sectional Norway Moderate risk |
5329 (43%) middle aged: 47–50 Older: 71–75 |
♀: 10.2 ± 4.5 ♂: 11.8 ± 3.9 |
BMD: TH [DXA, Lunar EXPERT-XL] |
Multivariate regression, β for association tHcy-BMD (P value) for middle aged and elderly women. (Data men not shown) OR (95% CI) for low BMD per category tHcy status + P for trend: |
Mid. aged women: β = 0.004 (P < 0.001) elderly women: β = 0.003 (P < 0.001)q |
||
|
1: <9.0 μmol/L 2: 9.0–11.9 μmol/L 3: 12.0–14.9 μmol/L 4: ≥15 μmol/L |
♀: 1: 1.00 (reference) 2: 1.14 (0.90–1.44) 3: 1.30 (0.95–1.79) 4: 2.19 (1.48–3.25) P for trend <0.001 |
♂: 1.00 (reference) 1.01 (0.74–1.37) 1.12 (0.79–1.60) 1.02 (0.66–1.56) P for trend = 0.72q |
||||||
|
| ||||||||
| Golbahar et al. 2004 [9] | Cross-sectional Iran Moderate risk |
271 (0%) 60.8 ± 6.8 |
geometric mean (95% CI) 13.7 (7–14) |
BMD: FN, LS [DXA, Lunar DPX-L] | β for association tHcy-BMD β (SE) |
FN: −0.012 (0.023)2
LS: −0.010 (0.024)2 |
||
|
| ||||||||
| Haliloglu et al. 2010 [36] | Cross-sectional Turkey Moderate risk |
120 (0%) 54.4 ± 1.1 |
Osteoporotic: 15.0 ± 4.6 Osteopenic: 14.2 ± 3.7 Normal: 11.2 ± 2.6 |
BMD: LS [DXA, Lunar DPX-L] |
ANOVA for difference in tHcy status per BMD group |
tHcy was sign. higher in the osteoporotic group versus normal group (P < 0.05) | ||
|
| ||||||||
| Krivosikova et al. 2010 [37] | Cross-sectional Slovakia High risk |
272 (0%) 41.3 ± 19.8 |
(μmol/L) 14.6 ± 5.5 |
BMD: FN, LS, trochanter, TH [DXA, Lunar DPX-L] |
Stepwise multivariate linear regression, β for association tHcy-BMD. β (SE) P value |
FN: −0.093 (0.06) P = 0.100r, 2
LS: 0.003 (0.07) P = 0.965r, 2 TH: −0.134 (0.06) P = 0.033r, 2 |
||
|
| ||||||||
| Morris et al. 2005 [7] | Cross-sectional USA Low risk |
1550 (47%) 68 |
Osteoporosis: 11.5 (10.3–12.7) Osteopenia: 10.2 (9.5–10.8) Normal: 10.0 (9.6–10.5) Geometric mean (95% CI) |
BMD: Trochanter, intertrochanter, FN, Ward's triangle, TH [DXA, Hologic QDR-1000] |
OR (95% CI) for mean BMD in relation to quartile categories of tHcy status + P for trend Category median (μmol/L): Q1: 6.9 Q2: 8.9 Q3: 10.8 Q4: 14.8 |
Q1: 1.0 (reference) Q2: 0.9 (0.4–1.9) Q3: 2.0 (0.7–5.1) Q4: 2.0 (0.8–4.9) P for trend = 0.09 sDose response analysis: subjects with tHcy level >20 μmol/L had sign lower BMD than subj with tHcy level <10 μmol/L |
||
|
| ||||||||
| Ouzzif et al. 2012 [39] | Cross-sectional Morocco Moderate risk |
188 (0%) 57.8 ± 8.5 |
12.4 ± 4.1 | BMD: FN, LS, TH, trochanter [DXA, Lunar prodigy] | Multivariate regression, β for association tHcy-BMD β (SE) + P value |
LS: −0.089 (0.003) P = 0.200t
TH: −0.155 (0.002) P = 0.021t |
||
|
| ||||||||
| Périer et al. 2007 [27] | Cohort (cross-sect data) France Moderate risk |
671 (0%) 61.6 ± 8.4 |
10.6 ± 3.5 |
BMD: FN, LS,TH [DXA, Hologic QDR-2000] | β for association tHcy-BMD β (SE) |
LS: −0.000065 (0.004) FN: −0.006 (0.004) TH: −0.006 (0.004)2 |
||
|
| ||||||||
| Rumbak et al. 2012 [40] | Cross-sectional Croatia Low risk |
131 (0%) 54.0 ± 4.9 |
9.9 ± 2.0 | BMD: FN, LS, TH, radius [DXA, Lunar-prodigy] |
Stepwise multivariate regression, β for association tHcy-BMD. β (SE) for premenopausal and postmenopausal women |
Premenopausal womenu, 2: LS: 0.20 (0.14) P = 0.176 FN: 0.17 (0.15) P = 0.253 TH: 0.20 (0.14) P = 0.170 Postmenopausal womenu, 2: LS: 0.12 (0.15) P = 0.439 FN: 0.20 (0.15) P = 0.181 TH: 0.12 (0.14) P = 0.391 |
||
|
| ||||||||
| Zhu et al. 2009 [31] | Cohort (5 y) Australia Moderate risk |
1213 (0%) 75.2 ± 2.7 |
12.1 ± 4.6 | BMD: TH [DXA, Hologic Acclaim 4500A] | Change in hip BMD from 1 to 5 years per tertile of tHcy (μmol/L) ANOVA | Tertile 1 and 3 differ significantly (P < 0.05) | ||
BMD sites—LS: Lumbar Spine, FN: Femoral Neck #data presented in article as nmol/L, this is presumably a typing error and should be μmol/L.
1data as provided by author on our request, 2 β (SE) as calculated from presented data, 3 β (SE) as calculated from data provided by author on our request.
aadjusted for age, BMI, smoking status, recurrent falling, serum creatinine; badjusted for age and BMI; cadjusted for serum creatinine (natural log), B12 level, folic acid level, BMI, smoking, walking speed, BMD, LnPTH; dadjusted for age, BMI, smoking, coffee intake, physical activity, vit D use, educational level, estrogen use in women; ecase-control matched for age and ethnicity. Adjusted for BMI, parental history of hip fracture, treated diabetes, alcohol use, smoking, history of stroke, total calcium intake; fadjusted for sex, age, height, weight, smoking status, caffeine intake, alcohol intake, education level, estrogen use in women; gadjusted for sex, age, height, weight, estrogen use in women; hadjusted for age, sex, BMI, changes in BMI before entry in the study, smoking, fall history, serum creatinine; iadjusted for age, prevalent fractures, BMD, calcium intake, physical activity, vitamin D level, creatinine, albumin, estradiol; jadjusted for age, gender, education, serum creatinine, osteoporosis drugs; kadjusted for age, weight, hip BMD, prevalent fracture, calcium treatment; Ladjusted for weight, cysteine, smoking and height; mAdjusted for duration of menopause, smoking, BMI, folic acid levels, homocysteine levels; nadjusted for age, BMI, logFolate, logB12, creatinine clearance; oAdjusted for age, weight, weight change; pAdjusted for BMI, smoking, age; qAdjusted for smoking, BMI, creatinine, coffee intake, physical activity, use of estrogen therapy; radjusted for age, B12, folate, PTH, CTx, Ca, Cr; sadjusted for age, sex, ethnicity, BMI, smoking, physical activity, creatinin, alcohol, coffee, energy, calcium, vitamin D zinc intake; tadjusted for age, BMI, folate, B12; uadjusted for age, BMI, smoking, alcohol intake, physical activity, duration of menopause, HRT, levels of hcy, vitB12 and folate.
Figure 3.
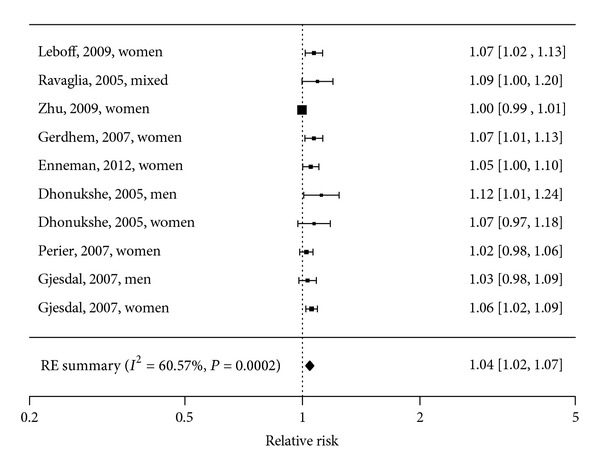
Forest plot of the association between homocysteine and risk of fracture: Meta-Analysis of 8 observational studies.
Three studies that were not included in the meta-analysis also showed significant associations between homocysteine levels and fracture risk. These studies were not included because the necessary data could not be retrieved from the articles; either homocysteine levels were log-transformed [4, 5] or data was not shown for population homocysteine status [25]. Regardless the type of analysis, women and men in the highest homocysteine quartile had a 1.7 to 3.8 higher RR or HR than those in the lowest or the lowest three quartiles [4, 5, 25].
3.2. Bone Mineral Density
In the studies included in this review BMD was measured at various sites in the body (e.g., lumbar spine, femoral neck, radius, hip, and total body). As BMD differs per site in the body, we pooled results per biomarker (serum/plasma vitamin B12, folate, and homocysteine) and per site for the three sites generally measured (FN, LS, or total hip), thus resulting in 9 meta-analyses. Betas of the individual studies are shown in Tables 1, 2, and 3. The studies included in the meta-analyses took only women into account. Only five studies regarding BMD included a male population [6, 7, 10, 35, 38], and these studies were not comparable quantitatively because differences in the presentation of results or differences in the measured BMD sites.
3.2.1. Vitamin B12
Pooled analysis showed no association between serum/plasma vitamin B12 levels and BMD in women; FN: β = 0.00, 95% CI: −0.13 to 0.14, I 2 = 0%, P = 0.40 [9, 37, 40]; LS: β = −2.25, 95% CI: −7.98 to 3.49, I 2 = 99.5%, P < 0.0001 [9, 37, 39, 40]; total hip β = −2.23, 95% CI: −10.38 to 5.92, I 2 = 97.7%, and P = 0.0001 [33, 37, 39, 40]. The studies that could not be included in the meta-analyses showed diverse results; in six out of eight studies low serum/plasma vitamin B12 was significantly associated with low BMD at at least one site [6, 7, 11, 32, 35, 38]. Two studies did not observe an association between vitamin B12 status and BMD [34, 36]. Morris et al. addressed MMA levels as well as a marker for vitamin B12 status and observed a lower BMD with higher serum MMA concentrations [7].
3.2.2. Folate
Pooled analysis showed no association between serum/plasma folate and BMD in women; FN: β = 0.00, 95% CI: −0.03 to 0.03, I 2 = 0.00%, P = 0.88 [9, 37, 40]; LS: β = 0.01, 95% CI: 0.00 to 0.01, I 2 = 0%, P = 0.77 [9, 37, 39, 40]; total hip: β = 0.00, 95% CI: 0.00 to 0.01, I 2 = 78.5%, P = 0.0003 [10, 33, 37, 39, 40].
From the studies that could not be compared in a meta-analysis, three studies showed significant associations between folate status and BMD or change in BMD over time [8, 10, 34]. Five studies did not observe an association between folate status and BMD [7, 32, 36, 38, 41].
3.2.3. Homocysteine
Pooled analyses showed no association between serum/plasma homocysteine levels and BMD in women; FN: β = −0.01, 95% CI: −0.04 to 0.02, I 2 = 31.5%, P = 0.21 [9, 27, 37, 40]; LS: β = −0.01, 95% CI: −0.08 to 0.05, I 2 = 98.4%, P < 0.0001 [9, 27, 37, 39, 40]; total hip: β = −0.03, 95% CI: −0.08 to 0.02, I 2 = 99.9%, P < 0.0001 [10, 27, 33, 37, 39, 40]. The studies that could not be pooled showed diverse results. In five studies a high homocysteine level was significantly associated with low BMD or change in BMD over time at at least one site [7, 29, 31, 32, 41]. Three studies did not observe a significant association between homocysteine status and BMD or change in BMD [8, 34, 36].
3.3. Intervention Studies
Up until now, only one RCT (N = 47) which met our inclusion criteria studied the efficacy of B-vitamin supplementation on BMD [42]. This study shows some evidence that BMD may be increased with high doses of B-vitamin supplementation in people with hyperhomocysteinemia (tHcy > 15 μmol/L). However, this outcome was only found in a subanalysis of 8 hyperhomocysteinemic subjects [42].
4. Discussion
Our meta-analyses showed a significant association of homocysteine levels with fracture risk and a weak though significant inverse association of vitamin B12 levels with fracture risk. We could not draw a conclusion regarding folate levels and fracture risk, as too few studies investigated this association. Meta-analyses regarding vitamin B12, folate and homocysteine levels and BMD in women found no associations. Results from studies regarding BMD that could not be included in the meta-analyses are not univocal.
To our knowledge this systematic review with meta-analyses is the most extensive systematic review on the association of vitamin B12, folate and homocysteine with bone health until now. Previous non-systematic literature reviews on the association between folate, vitamin B12, and homocysteine with bone health reported similar results, that is, conflicting evidence with suggestions towards the association of homocysteine levels with fracture [43–45]. These reviews did not report a systematic literature search strategy and did not provide a quantitative cumulative result. In our review the most recent published articles have been taken into account. The search strategy we used was systematic and extensive, and we used well-defined in- and exclusion criteria.
One recent systematic review included a meta-analysis on the association between tHcy and fractures [20]. This meta-analysis is different in design than ours, as it is not a dose-response meta-analysis. To overcome the variation in cut-off levels for low vitamin B12 and folate status and high homocysteine status, and to allow comparison and subsequent combination of individual studies in the performed meta-analyses, we expressed results of individual studies in a standardized format. We assumed a linear, continuous dose-response association between markers of vitamin B12 and folate with fracture rather than a threshold effect. This assumption is generally used in meta-analyses. Furthermore, in some of the key articles addressing the association of homocysteine levels with fractures this association is present [4, 5].
A common concern in meta-analyses is heterogeneity between studies. In our meta-analyses we experienced various levels of statistical heterogeneity (no heterogeneity to large heterogeneity). The heterogeneity may be explained by the differences in mean age of the study populations (41–78 years), differences in mean status of vitamin B12 (190–549 pmol/L), folate (5.2–24.9 nmol/L) and homocysteine (9.3–16.5 μmol/L), differences in sex distribution of the study populations, duration of follow-up (3–16 years), and level of adjustment for confounders. Although most included studies adjusted for a wide range of confounders for fracture risk or BMD, residual confounding by other unmeasured or inadequately measured factors cannot be ruled out. For example, low vitamin D status is a risk factor for fracture [46]. From the studies included in our meta-analyses for fracture three out of nine adjusted for vitamin D status [24, 25, 27]. Outcomes do not seem to differ between studies that corrected for vitamin D status and studies that did not. Homocysteine levels are increased with renal dysfunction, often measured by serum or urine creatinine levels. Five out of eight studies in the meta-analysis regarding homocysteine and fracture risk corrected for creatinine levels [3, 24, 26, 27, 29], and outcomes did not seem to differ.
As almost all studies were performed in countries without mandatory folate fortification or were performed before the fortification era in the USA and Australia, we do not consider folate fortification as a source of heterogeneity in our analyses.
The majority of studies included were longitudinal and cross-sectional observational studies. We could only include one intervention study, which had a very small study population (N = 47). One intervention study which found a beneficial effect of vitamin B12 and folic acid supplementation on fracture risk could not be included in our systematic review, because this study investigated a population of hemiplegic patients following stroke [47]. The generalizability of these findings is confined to a highly selective patient population with a high percentage of vitamin D deficiency and a high fracture risk. As evidence from intervention studies is lacking, currently no causal effect between vitamin B12, folate and homocysteine levels and bone health can be established. Consequently, it is yet unknown whether extra vitamin B12 and folate intake through supplementation could reverse the observed negative effects of vitamin B12 and folate deficiency and elevated homocysteine levels. Further evidence from an intervention study is expected soon, as a large intervention study on the effect of vitamin B12 and folic acid supplementation on fracture risk, BMD, and bone turnover markers is currently carried out with results expected in 2013 [48].
As the quality of included studies determines the quality of the review and meta-analysis, we assessed the overall risk of bias of each individual study using standardized procedures largely based on guidance from the Cochrane Collaboration [49], resulting in one of the following judgments: low, moderate, or high risk of bias. Twenty out of the 28 included studies were evaluated as having moderate (n = 15) or high risk (n = 5) of bias. These studies did take one or more of the predefined confounders into account, that is, age, sex, smoking, physical activity, and body weight, or the study was funded or cofunded by a commercial organization. Due to the limited number of studies included in the meta-analyses, we were not able to study the effect of the overall risk of bias, nor of its single components on the pooled effect measures. There seems to be no difference in the outcomes of studies with low risk of bias compared to studies with moderate or high risk of bias, and we therefore assume that the quality of the included studies had no effect on the outcome of this review.
The intake of folate and vitamin B12 are a determinant of folate, vitamin B12, and homocysteine status. To deal with potential malabsorption of vitamin B12 [50] and reduced bioavailability of folate [51], the use of biomarkers for vitamin B12 and folate status is preferred over measures of intake when studying associations with bone health in elderly people.
In studies addressing folate status, serum or plasma folate was measured, which is considered as an appropriate marker for folate status in epidemiological studies [52]. Homocysteine is a nonspecific marker for both folate and vitamin B12 status [53], which makes it a relevant biomarker in this review. Regarding the metabolic interactions between vitamin B12, folate, and homocysteine combined with the variety in data presented in the studies, we were not able to investigate the possibility that a low vitamin B12 or folate status in combination with a high homocysteine level might result in a higher fracture risk in comparison to a low vitamin B12 or folate status or homocysteine level alone. In most studies regarding vitamin B12 status, status was assessed with serum or plasma vitamin B12. Other, more sensitive markers for vitamin B12 deficiency, like MMA and HoloTC [54], were addressed only in a few studies. We could therefore not draw conclusions about the association between these biomarkers and outcomes on bone health.
There are several suggested mechanisms for the association between vitamin B12, folate, homocysteine, and bone health. Homocysteine may interfere with collagen cross-linking. Cross-links are important for the stability and strength of the collagen network. Interference in cross-link formation would cause an altered bone matrix, resulting in more fragile bones [55]. As collagen cross-links do not alter BMD, this may explain why a more convincing result is found regarding fractures than BMD, as suggested for example by Van Meurs et al. [5]. Vitamin B12 deficiency has been associated with impaired functional maturation of osteoblasts [56]. Some in vitro studies support the hypothesis of a possible favorable effect of vitamin B12 supplementation, although results are equivocal. Vitamin B12 has been shown to stimulate osteoblast proliferation and alkaline phosphatase activity [57] but Herrmann et al. were not able to show any significant and consistent effect of vitamin B12 or folic acid on osteoblast activity [58]. Recent publications show evidence of osteoclast stimulation in the presence of high homocysteine and low vitamin B12 concentrations [59–61]. Vitamin B12 and folate are not the only B-vitamins involved in the homocysteine metabolism. Various micronutrients, such as vitamin B2 (riboflavin), vitamin B6 (pyridoxine), and choline also affect homocysteine levels [16, 17, 62], and may consequently affect bone health. Given that vitamin B12 and folate are the main factors influencing homocysteine levels, and therefore the primary focus in a homocysteine lowering intervention [63], our review focused on vitamin B12, folate, and homocysteine.
Considerations for Future Research and Conclusions
The mechanisms involved in the association between biomarkers of B-vitamins and bone health are still unclear and therefore more fundamental research is required to establish the potential mechanisms. Subsequently, both observational and intervention studies should preferably not focus on just one biomarker in relation to the homocysteine metabolism, but take a biomarker profile into account, including serum/plasma vitamin B12, MMA, HoloTC, folate, and homocysteine levels. Evidence is needed from well-designed, large intervention studies to establish a causal relationship between markers of B-vitamins and bone health.
This systematic review with meta-analyses shows that elevated homocysteine levels are associated with increased fracture risk. Vitamin B12 status may be associated with fracture risk and evidence for an association between folate status and fracture risk is scarce. Vitamin B12, folate, and homocysteine levels are probably not associated with BMD, but results are not univocal.
Supplementary Material
The electronic databases MEDLINE, EMBASE, and Cochrane Library Central were searched, using search terms in “MeSH” terms and “title” and “abstract” on study designs in humans, vitamin B12, folate, homocysteine, and intake or status. The fullMedline search strategy is available in Appendix 1.
Acknowledgments
The work reported herein has been carried out within the EURRECA Network of Excellence (http://www.eurreca.org/) which is financially supported by the Commission of the European Communities, specific Research, Technology and Development (RTD) Programme Quality of Life and Management of Living Resources, within the Sixth Framework Programme, Contract no. 036196. This review does not necessarily reflect the Commission's views or its future policy in this area. The original conception of the systematic review was undertaken by the EURRECA Network and coordinated by partners based at Wageningen University (WU), the Netherlands and the University of East Anglia (UEA), United Kingdom. Susan Fairweather-Tait (UEA), C. P. G. M. de Groot (WU), P. van't Veer (WU), Kate Ashton (UEA), Amélie Casgrain (UEA), A. E. J. M. Cavelaars (WU), Rachel Collings (UEA), R. A. M. Dhonukshe-Rutten (WU), E. L. Doets (WU), Linda Harvey (UEA), and Lee Hooper (UEA) designed and developed the review protocol and search strategy. The authors thank Silvia Bell, Iris Iglesias (University of Zaragosa, Spain), Maria Plada (University of Athens, Greece), Nathalie van Borrendam, and Margreet Smit (Wageningen University, the Netherlands) for assistance in article selection and data extraction. Furthermore The authors thank Dr. RAM Dhonukshe-Rutten, Department of Human nutrition, Wageningen University, The Netherlands, Dr. A. Cagnacci, Department of Obstetrics, Gynecology and Pediatrics, Obstetrics and Gynecology Unit, Policlinico of Modena, Modena, Italy, R. R. McLean, Dsc, MPH, Institute for Aging Research, Hebrew SeniorLife, Boston, MA, USA, Harvard Medical School, Boston, MA, USA, and Dr. E. Sarnay-Rendu, INSERM, France, for providing the authors with additional data regarding their articles.
References
- 1.Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. The American Journal of Medicine. 1993;94(6):646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 2.Kanis JA, Johnell O. Requirements for DXA for the management of osteoporosis in Europe. Osteoporosis International. 2005;16(3):229–238. doi: 10.1007/s00198-004-1811-2. [DOI] [PubMed] [Google Scholar]
- 3.Dhonukshe-Rutten RAM, Pluijm SMF, De Groot LCPGM, Lips P, Smit JH, Van Staveren WA. Homocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation, and fractures in healthy elderly people. Journal of Bone and Mineral Research. 2005;20(6):921–929. doi: 10.1359/JBMR.050202. [DOI] [PubMed] [Google Scholar]
- 4.McLean RR, Jacques PF, Selhub J, et al. Homocysteine as a predictive factor for hip fracture in older persons. The New England Journal of Medicine. 2004;350(20):2042–2049. doi: 10.1056/NEJMoa032739. [DOI] [PubMed] [Google Scholar]
- 5.van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM, et al. Homocysteine levels and the risk of osteoporotic fracture. The New England Journal of Medicine. 2004;350(20):2033–2041. doi: 10.1056/NEJMoa032546. [DOI] [PubMed] [Google Scholar]
- 6.Tucker KL, Hannan MT, Qiao N, et al. Low plasma vitamin B12 is associated with lower BMD: The Framingham osteoporosis study. Journal of Bone and Mineral Research. 2005;20(1):152–158. doi: 10.1359/JBMR.041018. [DOI] [PubMed] [Google Scholar]
- 7.Morris MS, Jacques PF, Selhub J. Relation between homocysteine and B-vitamin status indicators and bone mineral density in older Americans. Bone. 2005;37(2):234–242. doi: 10.1016/j.bone.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Cagnacci A, Baldassari F, Rivolta G, Arangino S, Volpe A. Relation of homocysteine, folate, and vitamin B12 to bone mineral density of postmenopausal women. Bone. 2003;33(6):956–959. doi: 10.1016/j.bone.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Golbahar J, Hamidi A, Aminzadeh MA, Omrani GR. Association of plasma folate, plasma total homocysteine, but not methylenetetrahydrofolate reductase C667T polymorphism, with bone mineral density in postmenopausal Iranian women: a cross-sectional study. Bone. 2004;35(3):760–765. doi: 10.1016/j.bone.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Gjesdal CG, Vollset SE, Ueland PM, et al. Plasma total homocysteine level and bone mineral density: The Hordaland Homocysteine Study. Archives of Internal Medicine. 2006;166(1):88–94. doi: 10.1001/archinte.166.1.88. [DOI] [PubMed] [Google Scholar]
- 11.Stone KL, Bauer DC, Sellmeyer D, Cummings SR. Low serum vitamin B-12 levels are associated with increased hip bone loss in older women: a prospective study. Journal of Clinical Endocrinology and Metabolism. 2004;89(3):1217–1221. doi: 10.1210/jc.2003-030074. [DOI] [PubMed] [Google Scholar]
- 12.de Bree A, van der Put NM, Mennen LI, et al. Prevalences of hyperhomocysteinemia, unfavorable cholesterol profile and hypertension in European populations. European Journal of Clinical Nutrition. 2005;59(4):480–488. doi: 10.1038/sj.ejcn.1602097. [DOI] [PubMed] [Google Scholar]
- 13.Wouters-Wesseling W, Wouters AEJ, Kleijer CN, Bindels JG, de Groot CPGM, van Staveren WA. Study of the effect of a liquid nutrition supplement on the nutritional status of psycho-geriatric nursing home patients. European Journal of Clinical Nutrition. 2002;56(3):245–251. doi: 10.1038/sj.ejcn.1601319. [DOI] [PubMed] [Google Scholar]
- 14.Eussen SJPM, De Groot LCPGM, Clarke R, et al. Oral cyanocobalamin supplementation in older people with vitamin B 12 deficiency: a dose-finding trial. Archives of Internal Medicine. 2005;165(10):1167–1172. doi: 10.1001/archinte.165.10.1167. [DOI] [PubMed] [Google Scholar]
- 15.Green R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. American Journal of Clinical Nutrition. 2011;94(2):666S–672S. doi: 10.3945/ajcn.110.009613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selhub J, Jacques PF, Wilson PWF, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. Journal of the American Medical Association. 1993;270(22):2693–2698. doi: 10.1001/jama.1993.03510220049033. [DOI] [PubMed] [Google Scholar]
- 17.Jacques PF, Bostom AG, Wilson PWF, Rich S, Rosenberg IH, Selhub J. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. American Journal of Clinical Nutrition. 2001;73(3):613–621. doi: 10.1093/ajcn/73.3.613. [DOI] [PubMed] [Google Scholar]
- 18.Clarke R. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. British Medical Journal. 1998;316(7135):894–898. [PMC free article] [PubMed] [Google Scholar]
- 19.Kuzminski AM, Del Giacco EJ, Allen RH, Stabler SP, Lindenbaum J. Effective treatment of cobalamin deficiency with oral cobalamin. Blood. 1998;92(4):1191–1198. [PubMed] [Google Scholar]
- 20.Yang J, Hu X, Zhang Q, Cao H, Wang J, Liu B. Homocysteine level and risk of fracture: a meta-analysis and systematic review. Bone. 2012;51(3):376–382. doi: 10.1016/j.bone.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Matthys C, van't Veer P, de Groot L, et al. EURRECAs approach for estimating micronutrient requirements. International Journal for Vitamin and Nutrition Research. 2011;81(4):256–263. doi: 10.1024/0300-9831/a000071. [DOI] [PubMed] [Google Scholar]
- 22.Souverein OW, Dullemeijer C, van't Veer P, van der Voet H. Transformations of summary statistics as input in meta-analysis for linear dose-response models on a logarithmic scale: a methodology developed within EURRECA. BMC Medical Research Methodology . 2012;12(1, article 57) doi: 10.1186/1471-2288-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Gjesdal CG, Vollset SE, Ueland PM, Refsum H, Meyer HE, Tell GS. Plasma homocysteine, folate, and vitamin B12 and the risk of hip fracture: the hordaland homocysteine study. Journal of Bone and Mineral Research. 2007;22(5):747–756. doi: 10.1359/jbmr.070210. [DOI] [PubMed] [Google Scholar]
- 25.McLean RR, Jacques PF, Selhub J, et al. Plasma B vitamins, homocysteine, and their relation with bone loss and hip fracture in elderly men and women. Journal of Clinical Endocrinology and Metabolism. 2008;93(6):2206–2212. doi: 10.1210/jc.2007-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravaglia G, Forti P, Maioli F, et al. Folate, but not homocysteine, predicts the risk of fracture in elderly persons. Journals of Gerontology A. 2005;60(11):1458–1462. doi: 10.1093/gerona/60.11.1458. [DOI] [PubMed] [Google Scholar]
- 27.Périer MA, Gineyts E, Munoz F, Sornay-Rendu E, Delmas PD. Homocysteine and fracture risk in postmenopausal women: The OFELY study. Osteoporosis International. 2007;18(10):1329–1336. doi: 10.1007/s00198-007-0393-1. [DOI] [PubMed] [Google Scholar]
- 28.Leboff MS, Narweker R, Lacroix A, et al. Homocysteine levels and risk of hip Fracture in postmenopausal women. Journal of Clinical Endocrinology and Metabolism. 2009;94(4):1207–1213. doi: 10.1210/jc.2008-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerdhem P, Ivaska KK, Isaksson A, et al. Associations between homocysteine, bone turnover, BMD, mortality, and fracture risk in elderly women. Journal of Bone and Mineral Research. 2007;22(1):127–134. doi: 10.1359/jbmr.061003. [DOI] [PubMed] [Google Scholar]
- 30.Enneman AW, van der Velde N, de Jonge R, et al. The association between plasma homocysteine levels, methylation capacity and incident osteoporotic fractures. Bone. 2012;50(6):1401–1405. doi: 10.1016/j.bone.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Zhu K, Beilby J, Dick IM, Devine A, Soós M, Prince RL. The effects of homocysteine and MTHFR genotype on hip bone loss and fracture risk in elderly women. Osteoporosis International. 2009;20(7):1183–1191. doi: 10.1007/s00198-008-0804-y. [DOI] [PubMed] [Google Scholar]
- 32.Bozkurt N, Erdem M, YIlmaz E, et al. The relationship of homocyteine, B12 and folic acid with the bone mineral density of the femur and lumbar spine in Turkish postmenopausal women. Archives of Gynecology and Obstetrics. 2009;280(3):381–387. doi: 10.1007/s00404-009-0936-0. [DOI] [PubMed] [Google Scholar]
- 33.Bucciarelli P, Martini G, Martinelli I, et al. The relationship between plasma homocysteine levels and bone mineral density in post-menopausal women. European Journal of Internal Medicine. 2010;21(4):301–305. doi: 10.1016/j.ejim.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Cagnacci A, Bagni B, Zini A, Cannoletta M, Generali M, Volpe A. Relation of folates, vitamin B12 and homocysteine to vertebral bone mineral density change in postmenopausal women: a five-year longitudinal evaluation. Bone. 2008;42(2):314–320. doi: 10.1016/j.bone.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 35.Dhonukshe-Rutten RAM, Lips M, De Jong N, et al. Vitamin B-12 status is associated with bone mineral content and bone mineral density in frail elderly women but not in men. Journal of Nutrition. 2003;133(3):801–807. doi: 10.1093/jn/133.3.801. [DOI] [PubMed] [Google Scholar]
- 36.Haliloglu B, Aksungar FB, Ilter E, et al. Relationship between bone mineral density, bone turnover markers and homocysteine, folate and vitamin B12 levels in postmenopausal women. Archives of Gynecology and Obstetrics. 2010;281(4):663–668. doi: 10.1007/s00404-009-1297-4. [DOI] [PubMed] [Google Scholar]
- 37.Krivosikova Z, Krajčovičová-Kudláčková M, Spustová V, et al. The association between high plasma homocysteine levels and lower bone mineral density in Slovak women: the impact of vegetarian diet. European Journal of Nutrition. 2010;49(3):147–153. doi: 10.1007/s00394-009-0059-1. [DOI] [PubMed] [Google Scholar]
- 38.Naharci I, Bozoglu E, Karadurmus N, et al. Vitamin B12 and folic acid levels as therapeutic target in preserving bone mineral density (BMD) of older men. Archives of Gerontology and Geriatrics. 2012;54(3):469–472. doi: 10.1016/j.archger.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 39.Ouzzif Z, Oumghar K, Sbai K, Mounach A, Derouiche EM, El Maghraoui A. Relation of plasma total homocysteine, folate and vitamin B12 levels to bone mineral density in Moroccan healthy postmenopausal women. Rheumatology International. 2012;32(1):123–128. doi: 10.1007/s00296-010-1551-x. [DOI] [PubMed] [Google Scholar]
- 40.Rumbak I, Ziic V, Sokolic L, Cvijetic S, Kajfe R, Colic Baric I. Bone mineral density is not associated with homocysteine level, folate and vitamin B12 status. Archives of Gynecology and Obstetrics. 2012;285(4):991–1000. doi: 10.1007/s00404-011-2079-3. [DOI] [PubMed] [Google Scholar]
- 41.Baines M, Kredan MB, Davison A, et al. The association between cysteine, bone turnover, and low bone mass. Calcified Tissue International. 2007;81(6):450–454. doi: 10.1007/s00223-007-9089-y. [DOI] [PubMed] [Google Scholar]
- 42.Herrmann M, Umanskaya N, Traber L, et al. The effect of B-vitamins on biochemical bone turnover markers and bone mineral density in osteoporotic patients: a 1-year double blind placebo controlled trial. Clinical Chemistry and Laboratory Medicine. 2007;45(12):1785–1792. doi: 10.1515/CCLM.2007.352. [DOI] [PubMed] [Google Scholar]
- 43.Herrmann M, Peter Schmidt J, Umanskaya N, et al. The role of hyperhomocysteinemia as well as folate, vitamin B6 and B12 deficiencies in osteoporosis—a systematic review. Clinical Chemistry and Laboratory Medicine. 2007;45(12):1621–1632. doi: 10.1515/CCLM.2007.362. [DOI] [PubMed] [Google Scholar]
- 44.McLean RR, Hannan MT. B vitamins, homocysteine, and bone disease: epidemiology and pathophysiology. Current Osteoporosis Reports. 2007;5(3):112–119. doi: 10.1007/s11914-007-0026-9. [DOI] [PubMed] [Google Scholar]
- 45.Levasseur R. Bone tissue and hyperhomocysteinemia. Joint Bone Spine. 2009;76(3):234–240. doi: 10.1016/j.jbspin.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Lips P, van Schoor NM. The effect of vitamin D on bone and osteoporosis. Best Practice & Research. 2011;25(4):585–591. doi: 10.1016/j.beem.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Sato Y, Honda Y, Iwamoto J, Kanoko T, Satoh K. Effect of folate and mecobalamin on hip fractures in patients with stroke: a randomized controlled trial. Journal of the American Medical Association. 2005;293(9):1082–1088. doi: 10.1001/jama.293.9.1082. [DOI] [PubMed] [Google Scholar]
- 48.van Wijngaarden JP, Dhonukshe-Rutten RA, van Schoor NM, et al. Rationale and design of the B-PROOF study, a randomized controlled trial on the effect of supplemental intake of vitamin B12 and folic acid on fracture incidence. BMC Geriatrics. 2011;11(1, article 80) doi: 10.1186/1471-2318-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5. 1. 0. The Cochrane Collaboration; 2011. [Google Scholar]
- 50.Allen LH. How common is vitamin B-12 deficiency? The American Journal of Clinical Nutrition. 2009;89(2):693S–696S. doi: 10.3945/ajcn.2008.26947A. [DOI] [PubMed] [Google Scholar]
- 51.McNulty H, Pentieva K. Folate bioavailability. Proceedings of the Nutrition Society. 2004;63(4):529–536. doi: 10.1079/pns2004383. [DOI] [PubMed] [Google Scholar]
- 52.Yetley EA, Coates PM, Johnson CL. Overview of a roundtable on NHANES monitoring of biomarkers of folate and vitamin B-12 status: measurement procedure issues. American Journal of Clinical Nutrition. 2011;94(1):297S–302S. doi: 10.3945/ajcn.111.017392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carmel R, Green R, Rosenblatt DS, Watkins D. Update on cobalamin, folate, and homocysteine. Hematology. 2003:62–81. doi: 10.1182/asheducation-2003.1.62. [DOI] [PubMed] [Google Scholar]
- 54.Hoey L, Strain JJ, McNulty H. Studies of biomarker responses to intervention with vitamin B-12: a systematic review of randomized controlled trials. American Journal of Clinical Nutrition. 2009;89(6):1981S–1996S. doi: 10.3945/ajcn.2009.27230C. [DOI] [PubMed] [Google Scholar]
- 55.Saito M, Fujii K, Marumo K. Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls. Calcified Tissue International. 2006;79(3):160–168. doi: 10.1007/s00223-006-0035-1. [DOI] [PubMed] [Google Scholar]
- 56.Carmel R, Lau KHW, Baylink DJ, Saxena S, Singer FR. Cobalamin and osteoblast-specific proteins. The New England Journal of Medicine. 1988;319(2):70–75. doi: 10.1056/NEJM198807143190202. [DOI] [PubMed] [Google Scholar]
- 57.Kim GS, Kim CH, Park JY, Lee KU, Park CS. Effects of vitamin B12 on cell proliferation and cellular alkaline phosphatase activity in human bone marrow stromal osteoprogenitor cells and UMR106 osteoblastic cells. Metabolism: Clinical and Experimental. 1996;45(12):1443–1446. doi: 10.1016/s0026-0495(96)90171-7. [DOI] [PubMed] [Google Scholar]
- 58.Herrmann M, Umanskaya N, Wildemann B, et al. Accumulation of homocysteine by decreasing concentrations of folate, vitamin B12 and B6 does not influence the activity of human osteoblasts in vitro. Clinica Chimica Acta. 2007;384(1-2):129–134. doi: 10.1016/j.cca.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 59.Herrmann M, Widmann T, Colaianni G, Colucci S, Zallone A, Herrmann W. Increased osteoclast activity in the presence of increased homocysteine concentrations. Clinical Chemistry. 2005;51(12):2348–2353. doi: 10.1373/clinchem.2005.053363. [DOI] [PubMed] [Google Scholar]
- 60.Vaes BLT, Lute C, Blom HJ, et al. Vitamin B12 deficiency stimulates osteoclastogenesis via increased homocysteine and methylmalonic acid. Calcified Tissue International. 2009;84(5):413–422. doi: 10.1007/s00223-009-9244-8. [DOI] [PubMed] [Google Scholar]
- 61.Vaes BLT, Lute C, van der Woning SP, et al. Inhibition of methylation decreases osteoblast differentiation via a non-DNA-dependent methylation mechanism. Bone. 2010;46(2):514–523. doi: 10.1016/j.bone.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 62.Holm PI, Ueland PM, Vollset SE, et al. Betaine and folate status as cooperative determinants of plasma homocysteine in humans. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(2):379–385. doi: 10.1161/01.ATV.0000151283.33976.e6. [DOI] [PubMed] [Google Scholar]
- 63.Clarke R, Armitage J. Vitamin supplements and cardiovascular risk: review of the randomized trials of homocysteine-lowering vitamin supplements. Seminars in Thrombosis and Hemostasis. 2000;26(3):341–348. doi: 10.1055/s-2000-8101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The electronic databases MEDLINE, EMBASE, and Cochrane Library Central were searched, using search terms in “MeSH” terms and “title” and “abstract” on study designs in humans, vitamin B12, folate, homocysteine, and intake or status. The fullMedline search strategy is available in Appendix 1.


