Abstract
Mitochondria play important roles in human physiological processes, and therefore, their dysfunction can lead to a constellation of metabolic and nonmetabolic abnormalities such as a defect in mitochondrial gene expression, imbalance in fuel and energy homeostasis, impairment in oxidative phosphorylation, enhancement of insulin resistance, and abnormalities in fatty acid metabolism. As a consequence, mitochondrial dysfunction contributes to the pathophysiology of insulin resistance, obesity, diabetes, vascular disease, and chronic heart failure. The increased knowledge on mitochondria and their role in cellular metabolism is providing new evidence that these disorders may benefit from mitochondrial-targeted therapies. We review the current knowledge of the contribution of mitochondrial dysfunction to chronic diseases, the outcomes of experimental studies on mitochondrial-targeted therapies, and explore the potential of metabolic modulators in the treatment of selected chronic conditions. As an example of such modulators, we evaluate the efficacy of the administration of L-carnitine and its analogues acetyl and propionyl L-carnitine in several chronic diseases. L-carnitine is intrinsically involved in mitochondrial metabolism and function as it plays a key role in fatty acid oxidation and energy metabolism. In addition to the transportation of free fatty acids across the inner mitochondrial membrane, L-carnitine modulates their oxidation rate and is involved in the regulation of vital cellular functions such as apoptosis. Thus, L-carnitine and its derivatives show promise in the treatment of chronic conditions and diseases associated with mitochondrial dysfunction but further translational studies are needed to fully explore their potential.
The aim of our work is to review the current understanding of mitochondrial functions and dysfunctions, which may provide updated information on optimal strategies to modulate mitochondrial metabolism for clinical applications. As an example of the use of nutrients in the therapy of conditions or diseases having mitochondrial dysfunction as a common pathophysiological mechanism, we focus our attention on the metabolic compound L-carnitine, which has well-established roles in mitochondrial metabolism.
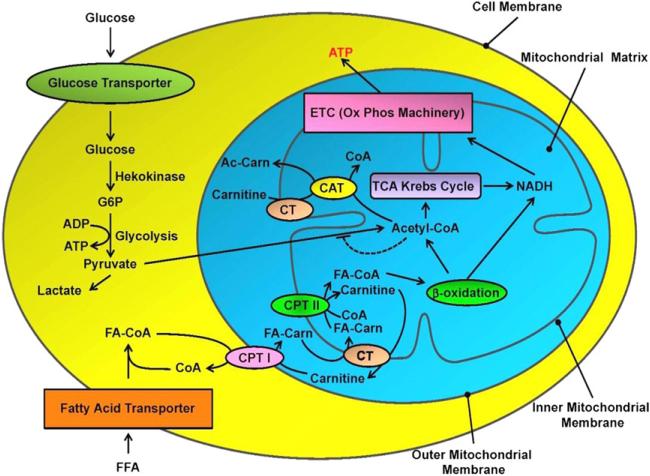
Mitochondria are intracellular double-membrane organelles that coordinate numerous metabolic reactions (Fig 1). They serve as the major site of adenosine-5′-triphosph (ATP) production by pyruvate and fatty acid (FA) metabolism through the Krebs cycle and the process of oxidative phosphorylation (OXPHOS).1 The metabolism of FA and glucose involves the breakdown of these molecules by beta-oxidation and glycolysis, followed by the entry of the resulting intermediary metabolites into the Krebs cycle. Although these processes produce high energy compounds, the majority of the ATP in the mitochondria is produced by the OXPHOS pathway. In this pathway, reduced intermediates, produced through glycolysis and the Krebs cycle, are shuttled through the electron transport chain, resulting in a potential gradient across the mitochondrial inner membrane. This will eventually result in protons pumped into the mitochondria through ATP synthase and production of ATP. In general, the breakdown of one molecule of glucose through aerobic metabolism produces 38 ATP molecules, whereas a 16 carbon FA (like palmitate) results in the generation of 129 ATPs.2
Fig 1.
Cardiac metabolism of fatty acid and glucose. For more detail, please refer to text. ETC = electron transport chain; G6P = glucose-6-phosphate; FFA = free fatty acid; CPT = carnitine palmitoyltransferase; CT = carnitine transporter; CAT = carnitine acetyltransferase.
Mitochondria are a major source of the cellular production of reactive oxygen species (ROS).3 ROS refers to molecules and free radicals (chemicals with an unpaired electron) that are derived from oxygen.4 The metabolic efficiency of mitochondria is closely related to the degree of free-radical production. ROS are highly reactive and can initiate reactions that can lead to irreversible damage of proteins or lipids, causing cellular dysfunction and cytotoxicity. Thus, elevated ROS production (especially in the mitochondria) is believed to contribute to the development of several disorders.
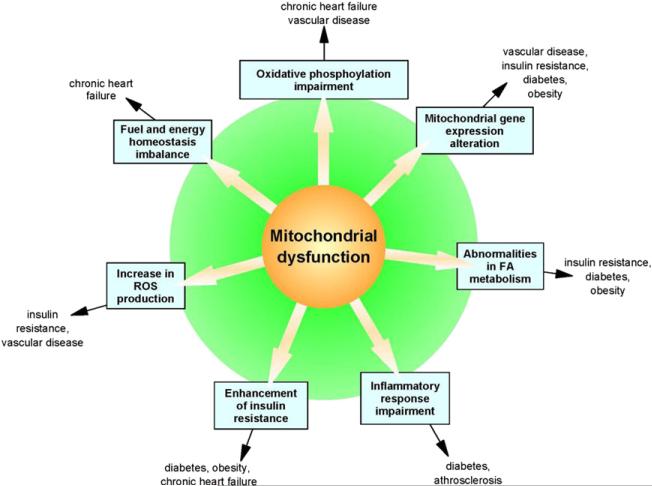
Recent data suggest a complex interplay between insulin responsiveness of skeletal muscle, liver and glucose-stimulated insulin secretion by pancreatic β cells, and the modulation of energy production by mitochondria.5 As a result, the role of mitochondrial dysfunction in the development of insulin resistance has been established as an intersection of pathophysiological mechanisms that lead to a common metabolic disorder in patients with diabetes, obesity, and chronic heart failure (Fig 2). These patients, as well as those with an unbalanced nutrient supply and the elderly, are examples of patients who may benefit from personalized metabolic therapy using small molecules including nutrients as metabolic modulators. In this review, we highlight the role of mitochondria and metabolic processes in several chronic disorders and discuss how the administration of L-carnitine or its analogues acetyl L-carnitine and propionyl L-carnitine, two L-carnitine esters formed respectively with the acetyl and propionyl moiety, can potentially lead to improvement in these diseases.
Fig 2.
Mitochondrial dysfunction is at the basis of a constellation of metabolic abnormalities that significantly contribute to chronic conditions and diseases.
MODELS OF MITOCHONDRIAL DYSFUNCTION
Insulin resistance and associated disorders
One well-substantiated disorder connecting mitochondrial function or dysfunction with human pathology is insulin resistance and its associated disorders. Insulin resistance is observed in liver, adipose tissue, and skeletal muscle in type 2 diabetes, with an about 30% reduction in glucose disposal, higher endogenous glucose production, and increased non-esterified FA concentrations in plasma.6 It is also pivotal in the pathogenesis of the metabolic syndrome,7 a clustering of hypertension, diabetes mellitus, dyslipidemia, and chronic inflammation, frequently observed in obese subjects and in subjects with a family history of diabetes mellitus, renal, or cardiovascular disease.8 Insulin resistance is associated with inactivity and caloric excess, and thus, it is positively and progressively associated with the degree of obesity, regardless of the energy source.9 It has been hypothesized that genetically determined and/or inactivity-mediated alterations in mitochondrial oxidative activity may directly impact adaptive responses to over nutrition, causing an imbalance between oxidative activity and nutrient load, ultimately leading to sustained accumulation of lipid oxidative metabolites that can mediate insulin resistance and insulin secretory dysfunction.6 In fact, multiple studies suggest that human insulin resistance is accompanied by impaired mitochondrial OXPHOS, likely attributable to the accumulation of triglycerides or toxic lipids (such as ceramide or diacylglycerol) and reduced mitochondrial density.8,10-12
Abnormal regulation of fatty acid metabolism plays a pivotal role in the pathogenesis of insulin resistance in the skeletal muscle.13 Under fasting conditions, FA serve as the principal fuel source for energy production in skeletal muscle. Insulin resistance is manifested by decreased insulin-stimulated glucose uptake, which may result from impaired insulin signaling and multiple post-receptor intracellular defects, including impaired glucose transport, glucose phosphorylation, and reduced glucose oxidation and glycogen synthesis. Insulin resistance induced by a high saturated fat diet along with excess calories may also cause an inability of intramuscular injected insulin to diffuse throughout the interstitial space.14 Thus, skeletal muscle insulin resistance after fat feeding can be explained by impaired access of insulin to the tissues, independent of muscle cell insulin resistance. Persistent trafficking of FA from perimuscular adipocytes to intramyocellular storage pools may also contribute to reduced glucose utilization in the obese state.15 Skeletal muscle cells cultured from extremely obese donors exhibited reduced mitochondrial content, which could in turn contribute to the diminished capacity to oxidize lipid and the preferential partitioning of lipid toward intramuscular storage.16 Furthermore, increased intra-myocellular fat content and fatty acid metabolites, such as fatty acyl coenzyme A (CoA) and diacylglycerol, likely play a pivotal role in the development of insulin resistance in skeletal muscle.17 Therefore, obesity and insulin resistance are associated with a number of metabolic abnormalities of FA, which may play a major role in these disorders.7,8,17
Aging is another well-known risk factor for insulin resistance and type 2 diabetes. Studies in the elderly consistently find that decreased insulin sensitivity is associated with a defect in mitochondrial function in muscle.10 Aging mitochondria become progressively inefficient, and acute damage can trigger the permeabilization of mitochondrial membranes to initiate apoptosis or necrosis. Mitophagy represents a mitochondria-specific autophagic turnover of cellular constituents, which eliminates dysfunctional or damaged mitochondria, thus, counteracting degeneration, dampening inflammation, and preventing unwarranted cell loss. Decreased expression of genes that regulate autophagy or mitophagy, which can occur with aging, may cause degenerative diseases in which deficient quality control results in inflammation and the death of cell populations. It is suggested that a combination of mitochondrial dysfunction and insufficient autophagy may contribute to multiple aging-associated pathologies.18
Insulin resistance is also associated with vascular dysfunction. Common vascular complications associated with diabetes are microvascular disease (retinopathy and nephropathy) and macrovascular disease (coronary artery disease, peripheral vascular disease, and stroke). The common feature in these disorders is atherosclerosis. Decreased vasodilation that occurs in insulin resistance is mostly due to a reduction of nitric oxide (NO) generation and bioavailability.19 NO is produced in endothelial cells by endothelial NO synthase (eNOS). eNOS becomes uncoupled and produces ROS when excess oxidative stress is present inside the cell or when there is limited substrate supply.20 Furthermore, mitochondrial derived ROS can interact with NO to generate peroxynitrite, which can disrupt eNOS activity. Folic acid has been shown to improve endothelial reactivity in diabetic dogs by scavenging peroxynitrite and preventing eNOS uncoupling.21 Therefore, uncoupling of eNOS and reduced bioavailability of NO will result in impaired vascular reactivity in diabetic patients. Accordingly, insulin-resistant individuals with obesity are more prone to endothelial dysfunction and subsequent development of hypertension,22 and studies in endothelial cells from diabetic patients have shown the mitochondrial dynamics to be altered and to exhibit increased mitochondrial ROS, impaired eNOS activation, and loss of NO bioavailability.22
Chronic heart failure
Heart failure accounts for more than 3 million hospitalizations in North America and Europe. Although in-hospital mortality is relatively low, and patients appear to respond to standard therapies, in terms of signs and symptoms, the event rate can be as high as 15% for mortality and 30% for rehospitalization within 60 to 90 days post-discharge.23 This unacceptably high event rate occurs in spite of implementation of evidence based therapies for heart failure that include beta blockers, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, mineralocorticoid antagonists, and devices such as the implantable cardioverter defibrillator and cardiac resynchronization.23 In addition, trials conducted to date with newer compounds (ie, vasopressin antagonist, nesiritide, adenosine antagonist, vasodilators) have not shown a decrease in the postdischarge event rate.24
The primary feature of heart failure is myocardial dysfunction (systolic or diastolic) and accordingly, improving myocardial function is the most important target for therapy. The heart is a unique organ since it pumps more than 7200 liters per day, generates over 6 kg of ATP per day, and renews the structural elements every 30 days.25 Available data suggests that patients with decreased systolic or diastolic function have myocytes that are alive, but not functioning. Thus, the concept that the failing heart is an energy-starved engine that has run out of fuel is decades old2 but not entirely accurate based on current understanding. In heart failure, the issue is not that the heart does not have enough “fuel,” but often it may be that it cannot use the “fuel” because of lack of micronutrients.25 The metabolic abnormalities in heart failure are characterized by mitochondrial dysfunction coupled with altered substrate utilization, decreased oxidative phosphorylation, and decreased high-energy phosphate content.26 Metabolic changes associated with heart failure are reviewed in a recent review paper from our group.27 Furthermore, heart failure is associated with a chronic increase in ROS production within the mitochondria, which leads to cellular injury and mitochondrial DNA damage. These deficiencies may be related to excessive activation of neuroendocrine apparatus, but also to deficiencies created by some therapies. For example, high doses of furosemide can cause a vitamin B1 deficiency,28 and statins can deplete the coenzyme Q10 levels.
Several studies have also suggested changes in mitochondrial morphology, biogenesis, and biochemical properties. Transgenic mice expressing the mutant form of troponin T (which display features of heart failure) have increased number of small mitochondria and a loss of membranes and cristae.29 Similar results were obtained in canine models of chronic heart failure.30 Endomyocardial biopsy samples from human and hypertrophic or dilated cardiomypathy revealed giant mitochondria and decreased matrix density and increased mitochondrial number.31
In addition to changes in mitochondrial number, a reduction in the entire pathway of mitochondrial biogenesis (including PGC1α and electron transport chain) has been reported.32 However, other studies have also suggested an increase in mitochondrial proliferation in cardiomyopathy.33 There also appears to be differences in response of the subpopulations of mitochondria to heart failure. Subsarcolemmal mitochondria (mitochondria that exist beneath the sarcolemma) oxidize substrates at a lower rate than mitochondria that are encapsulated within the contractile apparatus (interfibrillar mitochondria).34 It is believed that cardiac SSM are more susceptible to ischemic damage, whereas interfibrillar mitochondria are more sensitive to diabetic insult.35,36
Targeting mitochondrial dysfunction with metabolic therapy to improve substrate utilization, oxidative-phosphorylation, and the availability of high energy phosphates, has emerged as a promising approach for the treatment of heart failure. However, although it is evident that micronutrients can improve cardiac function, to the best of our knowledge, a well conducted randomized trial evaluating clinical outcomes has not been completed. Proof-of-principle clinical studies may use the myocardial phosphocreatine/ATP ratio to monitor the early energetic response of the heart to metabolic therapy, and this approach may provide a surrogate marker of long-term prognostic effects.
ROLE OF L-CARNITINE IN METABOLIC THERAPY
The use of macromolecules and nutrients that are able to correct a relative or absolute deficiency of critical metabolic components or that increase the levels of critical substrates and enzymes resulting in enhanced cellular processes, constitutes the basis of metabolic medicine. A number of compounds used alone or in combination have been evaluated for their potential clinical benefits. Among them, administration of L-carnitine or its analogues has been extensively studied with the objective of ameliorating metabolic abnormalities associated with mitochondrial dysfunction.
Carnitine synthesis and distribution
L-carnitine (3-hydroxy-4-(trimethylazaniumyl) butanoate) is intrinsically involved in mitochondrial metabolism and function. The carnitine pool of a healthy human weighing 70 kg is about 15 to 20 g. This pool, the largest part of which (>95%) is located in skeletal muscle, is maintained by both endogenous synthesis and dietary intake, predominately from meat and dairy. Carnitine is synthesized in vivo from L-lysine and L-methionine, mostly in liver and kidney. Concentrations of carnitine and acylcarnitines change under altered dietary conditions. During starvation and after eating a high fat diet, the proportion of carnitine that is acetylated in liver and kidney increases significantly and, in contrast, a high carbohydrate diet causes very low levels of acetyl-L-carnitine in the liver. In humans, there appears to be a delayed decrease in plasma carnitine and a rapid increase in both long- and short-chain acylcarnitines during fasting or diabetic ketosis, suggesting its overall importance in maintaining energy homeostasis.37 Although in healthy subjects endogenous synthesis is adequate to maintain carnitine levels, addition of carnitine in the diet may be required during certain stages of the life cycle and in disorders, such as aging and diabetes. Thus, carnitine is considered a “conditionally essential” nutrient and the term “functional carnitine deficiency” has been proposed to define abnormal clinical presentations correctable by carnitine administration.38
Carnitine is required for the transport of long-chain acyl CoA into the mitochondrial matrix where the enzymes for β-oxidation are located. Therefore, any deficiency in carnitine availability or in the carnitine-dependent transport system of mitochondria results in the curtailment of FA oxidation. Besides this obligatory role in lipid metabolism, the flow of substrates along several metabolic pathways, including glycolysis and gluconeogenesis, is modulated by the action of various carnitine-dependent transferases that catalyze the reversible exchange of acyl moieties between CoA and carnitine. Thus, the utilization of substrates for oxidative processes requires optimal activities of carnitine dependent transferases.
But the role of carnitine goes beyond the oxidation of fatty acids. The CoA-carnitine relationship is pivotal for energy metabolism. CoA is required for β-oxidation, for the catabolism of several amino acids, for the detoxification of organic acids and xenobiotics, for pyruvate dehydrogenase,39 for α-ketoglutarate dehydrogenase,40,41 and thus, for the tricarboxylic acid cycle. A reduced availability of carnitine induces a decrease of matrix free coenzyme A (CoASH) and a parallel increase of the acyl CoA/CoASH ratio both of which are inhibitory in the aforementioned mitochondrial dehydrogenases. Consequently, not only the oxidation of fatty acids,42 but also the utilization of carbohydrates, the catabolism of several amino acids and the detoxification of organic acids and xenobiotics become impaired.43
By means of carnitine acetyltransferase various short-chain acyl CoA are transformed into the corresponding short-chain acylcarnitines within the matrix and then exported into the cytosol. Unlike acyl CoAs, acylcarnitines, especially short-chain acylcarnitines, are capable of diffusing across cellular membranes and may be eliminated in the urine. The urinary excretion of specific acylcarnitines is relevant for the diagnosis of several inborn errors of metabolism.44
Carnitine administration has been suggested as beneficial in a number of disorders characterized by low carnitine concentrations or impaired FA oxidation, including diabetes, sepsis, and cardiomyopathy.45 Since carnitine is readily excreted, supplemental ingestion is well tolerated.46,47 Much of our knowledge on the biochemistry and the therapeutic efficacy of carnitine derives from clinical data obtained in patients suffering from carnitine deficiencies or various mitochondrial pathologies. From a clinical standpoint, when carnitine availability is reduced or the activities of carnitine-dependent transferases are impaired, fatty acid oxidation is prevented, paving the way to life-threatening alterations of skeletal and cardiac muscle.
A secondary deficiency of carnitine is observed in several inborn errors of metabolism44 where a large proportion of the available carnitine is esterified to buffer the accumulation of specific acyl CoA induced by the enzymatic defect. The transfer of the nonmetabolizable acyls from CoA to carnitine has two major advantages: (1) CoASH is made available for other essential oxidative pathways; and (2) acyl CoAs are mostly compartmentalized within the mitochondrial matrix and cannot cross membranes, whereas the corresponding carnitine esters not only can escape mitochondria, but they are also released in the blood stream and are eventually excreted in the urines. The detection of specific acylcarnitines in plasma or urine can be pathognomonic of several metabolic errors, such as isovaleric or propionic acidemia.48,49 In the most severe cases, the endogenous pool of free carnitine becomes insufficient to cope with the required acyl transfer. The resulting secondary carnitine deficiency or insufficiency mimics the metabolic alterations described for the primary carnitine-related defects. Increased urinary excretion of a given acylcarnitine can reflect mitochondrial dysfunction because of an accumulation of CoA ester that curtails the availability of unesterified CoA. However, the level of urinary acyl-carnitine is of limited usefulness in detecting mitochondrial defects involved in diseases that are not related to specific metabolic pathways. Furthermore, it would be difficult to diagnose the occurrence of mitochondrial dysfunction in tissues from levels of carnitine or free/esterified carnitine in urine as these parameters are modified by nutrient consumption, physical activity, and various pathologies independent of, or in the absence of, mitochondrial dysfunction.
Besides these direct links between carnitine and mitochondrial disorders, the majority of mitochondrial defects are likely to alter carnitine status, thus, worsening the clinical outcome. For instance, any respiratory chain defect reduces the availability of oxidized coenzymes resulting in decreased oxidation rates and accumulation of metabolic intermediates. Under these conditions, a large proportion of the available carnitine is going to be esterified for the disposal of acetyl CoA no longer degradable by TCA cycle. On the other hand, the reduced rate of β-oxidation is followed by the accumulation of long chain acyl CoA and, consequently, of the corresponding carnitine esters.
Manipulation of the carnitine pool of skeletal muscle at rest, both physiologically and pharmacologically, has provided insight into the regulation of skeletal muscle fat and carbohydrate oxidation, both at rest and during exercise, and in the interchange between anaerobic and oxidative energy provision at the onset of exercise. The demonstration that carnitine availability impacts muscle function and that its concentration can be readily increased or manipulated, has stimulated renewed interest in the role of carnitine in skeletal muscle energy metabolism.46
The functions of carnitine in both energy metabolism and phospholipid turnover indicate a general role of this compound in the maintenance of cell viability. The involvement of carnitine in the cellular defense against apoptosis might be contributed by inhibitory effects on ceramide synthesis50 and caspase activities.51 Surprisingly, the anti-apoptotic role of carnitine appears to be largely independent of the actions of the various carnitine transferases. In cardiac myocytes exposed to doxorubicin, carnitine administration reduced the degree of apoptotic death by preventing the increase in intracellular levels of ceramide, a powerful endogenous promoter of apoptosis.50 The inhibition on ceramide production is the result of 2 different actions of carnitine. In fact the subtraction of palmitoyl CoA, which is diverted from ceramide synthesis to oxidative metabolism,52 is reinforced by the inhibitory effect on acid sphingomyelinase, which generates ceramide in response to a host of apoptotic stimuli. In addition, carnitine has been shown to inhibit the activity of caspases 3 and 8,51 which act as initiator and executioner of apoptosis, respectively. Not only is the action of carnitine specific, but it is also reversed by palmitoylcarnitine. Since during the apoptosis of Jurkat cells carnitine levels decrease as opposed to the increase in palmitoylcarnitine, the free/esterified carnitine ratio has been suggested to play a relevant role in the cell commitment to apoptosis.
Measurement of total and free carnitine
The accurate and robust measurement of free and total carnitine in biologic fluids represents a critical issue for the diagnosis and daily management of carnitine deficiency syndromes and some inborn errors of metabolism, such as defects in fatty acid oxidation and the organic acidemias. For years, a radioenzymatic assay53 has been regarded as the standard method for carnitine measurement, in which free carnitine is measured by enzymatically converting it to radioactively labeled acetylcarnitine. Total carnitine is measured after hydrolysis of acylcarnitines. However, this approach is laborious and time consuming and not amenable to a high throughput format. Consequently, liquid chromatography tandem mass spectrometry (LC-MS-MS) methods have been developed that provide significantly increased throughput, specificity, and sensitivity.54,55 This approach permits the evaluation of acylcarnitine species of various carbon chain lengths in several biologic specimen types, including plasma, dried blood and bile spots, and urine. In spite of the easy management of these MS/MS automated methods within the clinical chemistry laboratory, some of the MS/MS methods have limitations attributable to unintended hydrolysis of acylcarnitines, which result in the overestimation of carnitine concentration. Furthermore, isobaric non-acylcarnitine compounds may yield false positive results, and constitutional isomers cannot be distinguished. However, the newer HPLC-mass spectrometry methods have largely overcome these limitations.55,56
Cytoprotective effects of carnitine
Acetyl-L-carnitine administration has been shown to reduce oxidative stress and mitochondrial alterations counteracting neurologic disorders associated with aging.57 The cytoprotective effects of carnitine are likely to be contributed by a decrease in oxidative stress. Although this antioxidant role has been reported in numerous studies,58 the underlying mechanism has not yet been elucidated.59,60 Carnitine and its esters are devoid of direct antioxidant properties. Therefore, the main mechanism causing a decrease in oxidative stress is likely to be linked to the positive effects on mitochondrial metabolism and function. More recently, propionyl-L-carnitine administration has been shown to decrease the expression of the ROS generating enzyme nicotinamide adenine dinucleotide phosphate oxidase.61 Although the link between this carnitine ester and protein expression has not been clarified, the decrease in ROS formation appears to underlie the improvement in postischemic flow recovery and revascularization induced by propionyl-L-carnitine.
Carnitine as a potential metabolic modulator in obesity, insulin resistance and type 2 diabetes
Carnitine supplementation may be of additional benefit when treating obesity, potentially because of its effects in improving glucose intolerance and total energy expenditure.62 Capaldo et al observed that whole body glucose use is acutely increased by L-carnitine infusion in insulin-resistant patients with type 2 diabetes mellitus.63 Subsequent studies showed that this effect is mediated by increased glucose storage and oxidative glucose use,64 possibly through improved carnitine-mediated lipid metabolism. Besides its role in β-oxidation, carnitine also regulates key enzymes involved in glycolysis, which may explain its beneficial impact on glucose uptake and utilization.46,65
These and other findings have provided the rationale for a prospective, controlled trial to assess whether acetyl-L-carnitine, a derivative product of carnitine, could achieve a sustained improvement of insulin-dependent glucose disposal.66 The study included insulin-resistant subjects with the presence of other cardiovascular risk factors, such as obesity and hypertension. Subjects with normal or near-normal insulin sensitivity served as controls. Oral administration of acetyl-L-carnitine for 6 months significantly reduced arterial blood pressure (BP), increased plasma adiponectin levels, and improved the overall cardiovascular risk profile. In those who were more insulin resistant at inclusion (glucose disposal rate <7.9 mg/kg), treatment also ameliorated insulin sensitivity and glucose tolerance, an effect that was associated with a reduced prevalence of the metabolic syndrome. Consistent with previous reports in other clinical settings,67,68 treatment with acetyl-L-carnitine was remarkably well tolerated in all patients. This report provides evidence that chronic oral supplementation with acetyl-L-carnitine ameliorates insulin sensitivity even in the absence of overt diabetes. It should be noted, however, that this was an off-on-off treatment study without a placebo group; as each patient served as his own control. This approach minimizes the likelihood that the results are because of chance even though the treatment group was relatively small (32 patients). The fact that acetyl-L-carnitine was associated with a substantial reduction in systolic BP but minimal changes in diastolic BP suggest that the observed changes were because of acetyl-L-carnitine rather than some “trial effect.” This pilot study provides a sound rational for future adequately powered nutraceutical trials to assess the potential beneficial effects of acetyl-L-carnitine on target organs of diabetes mellitus and hypertension.
Carnitine as a potential metabolic modulator in hypertension and vascular dysfunction
L-carnitine and its derivatives have been reported to improve endothelial cell function in animal models of hypertension and diabetes.69,70 Essential hypertension is strongly associated with visceral obesity and insulin resistance,71 and insulin resistant individuals with obesity are more prone to endothelial dysfunction and subsequent development of arterial hypertension.72 Thus, enhancement of insulin sensitivity might explain, at least in part, the reduction in arterial BP achieved by acetyl-L-carnitine therapy.66 Other mechanisms, however, should be advocated, since enhancement of insulin sensitivity was appreciable only in subjects who were more severely insulin resistant, whereas BP was consistently reduced in all subjects, regardless of their glucose disposal rate at inclusion. Of note, BP reduction was mirrored by a trend toward an increase in total and high-molecular weight adiponectin levels that reached statistical significance in the study population as a whole and in subjects with higher glucose disposal rate at inclusion. Enhanced adiponectin bioavailability, possibly associated with enhanced NO production,73,74 may play a role in acetyl-L-carnitine–induced BP reduction.75,76 However, the mechanism of acetyl-L-carnitine–mediated increase in plasma adiponectin levels is unknown. A plausible speculation is that improved oxidation of free FA in skeletal muscle with a secondary increase in adiponectin expression may be involved.77
Studies performed in rats have also shown that supplementation with alpha-lipoic-acid and acetyl-L-carnitine was able reduce oxidative stress and improve mitochondrial function.78 In addition, results of studies in 2 different rat models of arterial hypertension have shown that treatment with L-carnitine significantly modulates risk factors for vascular disease. L-carnitine caused a significant reduction of systolic and diastolic BP, a decrease in the systemic oxidative stress and prevented the reduction of NO levels in the hypertensive animals.79,80 The ability of L-carnitine to affect these functions may be relevant in the management of arterial hypertension associated with vascular disease in humans.
Carnitine therapy in end-stage renal disease
The kidney maintains plasma-L-carnitine levels by selective reabsorption. The preferential retention of L-carnitine over acyl-L-carnitine by the kidney is lost in patients with end-stage renal disease. Patients undergoing maintenance hemodialysis usually have concomitant carnitine insufficiency in plasma and tissues due to impaired carnitine biosynthesis, reduced protein uptake, and increased carnitine removal by dialysis.81 Carnitine insufficiency in chronic kidney disease is associated with erythropoietin—resistant anemia, proinfammatory systemic disease, cardiomyopathy, and muscle weakness.82 Consequently, carnitine therapy has been used and studied extensively in end-stage renal disease. Although a large number of clinical studies have shown that carnitine administration can alleviate many of these dialysis-related symptoms in hemodialysis patients, controversy regarding its use persists because of conflicting results. Different results of these clinical studies may be related to the lack of randomization and of control groups, heterogeneity in dose, duration and method of carnitine administration, non-standard measures of symptom improvement, different laboratory or clinical endpoints, variations in patient population, and patient response.81 It is clear, therefore, that large, robust, randomized controlled clinical trials are needed to better evaluate the use of L-carnitine in the management of chronic renal disease. However, it has been suggested that L-carnitine may have to be administered at doses that achieve supra-physiological concentrations in plasma and target tissues to fully exploit its pharmacologic potential.83 High concentrations of L-carnitine have been shown to improve RBC membrane stability, inhibit apoptosis, and reduce proinflammatory indices. Future clinical trials should address the issue of the appropriate concentration and exposure of L-carnitine that needs to be achieved in particular subgroups of patients on hemodialysis undergoing carnitine therapy.
Carnitine as a potential metabolic modulator in chronic heart failure
Reduction of key compounds required for energy metabolism, including L-carnitine has been well documented in chronic heart failure.25 Treatment with propionyl-L-carnitine in a small study of patients with congestive heart failure (18 treated and 12 placebo) has been shown to increase peak heart rate (12%), exercise capacity (21%), and peak oxygen consumption (45 %).84 Also after 1 month of oral therapy, there was a small but significant reduction in pulmonary artery pressure and ventricular and atrial size but no change in other hemodynamic measures. As these observed changes in the heart were of small magnitude and were observed after only 1 month of treatment, larger studies with a longer treatment period are needed to better evaluate both the magnitude of the changes in the heart and the durability of the therapeutic effect. Although the magnitude of the effect of propionyl-L-carnitine on the heart appears to be quite small, it is premature to conclude that this may limit its usefulness for the treatment of congestive heart failure. L-carnitine has been reported to improve cardiac mitochondrial function, attenuate mitochondrial membrane depolarization and swelling, and inhibit cytochrome c release and apoptosis, resulting from excess of FA.85 There are also data suggesting that carnitine improves hemodynamic and echocardiographic properties of the heart.84 Thus, these therapies have the potential to improve the symptoms and prognosis of patients with the life-threatening chronic heart failure.2,25 However, large scale clinical trials will be required to establish or refute a role for carnitine and other metabolic modulators in treatment of chronic heart failure.
Restoration of age-induced mitochondrial defects by treatment with acetyl-L-carnitine
Aging decreases the rate of mitochondrial oxidative phosphorylation, increases the capacity of mitochondria to produce ROS, and impairs the mitochondrial oxidation of specific substrates.86 As a result, these age-induced alterations in oxidative phosphorylation impair energy production as well as increase the production of toxic reactive oxygen intermediates. Acetyl-L-carnitine supplementation restores the age-related decrease in mitochondrial content and function and reverses the defective reaction of aged mitochondria to challenge.87 Feeding old rats acetyl-L-carnitine reverses the age-related decline in carnitine levels and improves β oxidation.88 The proposed mechanism of the restoration of age-induced mitochondrial defects is as follows: the supplemented acetyl-L-carnitine is transported into the cardiac mitochondria where the acetyl group promotes the production of acetyl-CoA and activates carnitine acetyl-transferace; the acetyl-CoA increases the acetylation status of the mitochondrial proteins and histone; and non-histone nuclear proteins, such that DNA tran-scription and translation is increased, resulting in increased mitochondrial protein synthesis.87 Propyl derivatives of carnitine also have the potential to enhance mitochondrial protein synthesis by a similar mechanism. Recent studies by Gadaleta and colleagues have shown that acety-L-carnitine supplementation activates the peroxisme proliferator-activated receptor signaling cascade of mitochondrial biogenesis and decreases the oxidized peroxiredoxin content in old rat lever.89 This same group has also reported that acetyl-L-carnitine treatment reversed the age-related alterations of 10 mitochondrial proteins associated with mito-chondrial cristae morphology, oxidative phosphor-ylation and antioxidant systems, the urea cycle, and purine biosynthesis.90
Acetylation/deacetylation mechanisms
Within the mitochondria, the acetylation/deacetylation mechanisms represent some biochemical key-processes where acyl-carnitine and some members of the sirtuins family make contact. Sirtuins are nicotinamide adenine dinuleotide-dependent protein deacetylases and were initially found to slow aging in lower organisms and more recently shown to mediate many of the effects of calorie restriction on metabolism and longevity in mammals.91 Among the 7 so far studied mammalian sirtuins (SIRT1 to SIRT7) at least 3 sirtuins (SIRT3, SIRT4, and SIRT5) localize to mitochondria.92 The dependence of SIRT3 enzymatic activity on nicotinamide adenine dinuleotide+ suggests that SIRT3 could serve as a metabolic sensor and couples the energy status of the cell with the level of mitochondrial protein acetylation. In SIRT3 deficiency, long-chain acylcarnitine species, but not medium- or short-chain acylcarnitines, accumulate in the liver, and are suggestive of incomplete oxidation of long-chain fatty acids.93 The decreased level of SIRT-3 in skeletal muscle in states of diabetes and obesity is presupposed to be a component of the pathogenesis of type 2 diabetes, which can induce altered mitochondrial function, increase ROS production and oxidative stress, and lead to insulin resistance.94 Disruption of the Aug II type 1 receptor has been shown to promote longevity in mice and is associated with increased mitochondrial number and upregulation of SIRT-3.95 Thus, acyl-carnitine and sirtuins together affect mitochondria acetylation/deacetylation and thereby have the potential to regulate the cellular redox state and energy homeostasis in insulin resistance and diabetes.
Carnitine as a potential metabolic modulator in dyslipoproteinemia
Prospective epidemiologic studies and meta-analyses of prospective studies have provided evidence that elevated lipoprotein(a) [Lp(a)] levels are associated with an increased risk of myocardial infarction and ischemic heart disease.96 Furthermore, Mendelian randomization studies have established a casual role for Lp(a) in cardiovascular disease.97,98 Lp(a) is a peculiar plasma macromolecule composed of a single low density lipoprotein-like molecule containing apolipoprotein B-100 and one molecule of a polymorphic protein called apo(a).99 Lp(a) levels are largely genetically determined and relatively resistant to drug and lifestyle interventions. Niacin is the only lipid lowering drug that affects Lp(a) levels but therapeutic doses can lead to flushing and poor compliance.100 Treatment of patients who have elevated Lp(a) with L-carnitine (2g/day) has been shown to significantly reduce Lp(a) levels in these patients,101,102 providing further evidence of the benefit of L-carnitine for patients with cardiovascular disease and Lp(a) elevation. However, more studies are needed to rigorously explore the effect of carnitine on patients exhibiting a wide range of Lp(a) levels and apo(a) sizes and to evaluate the clinical benefits of Lp(a) lowering.
SUMMARY
Altered mitochondrial dynamics lead to a constellation of metabolic abnormalities: impairment in oxidative phosphorylation, defects in mitochondrial gene expression, imbalance in fuel and energy homeostasis, increased ROS production, enhancement in insulin resistance, and abnormalities in FA metabolism. As a consequence, mitochondrial dysfunctions contribute to insulin resistance, obesity, diabetes, vascular disease, and chronic heart failure.
Changes in diet and physical activity have been shown in many investigations to be among the best interventions to prevent and to treat many chronic diseases. One reason is that the benefits are not accompanied by adverse effects, resulting in a risk/benefit ratio much better than usual medication options. Identification of nutrients targeting mitochondria has recently become a widely tested approach to enhance the effect of diet and physical activity. Among these, L-carnitine and its derivatives have shown promising responses in metabolic disorders characterized by mitochondrial dysfunction as well as in the management of aging-associated inefficient energy production. While L-carnitine has the potential to become an important metabolic modulator in numerous conditions, further translational and clinical studies are needed to fully explore its potential to modify pathophysiological mechanisms and to treat diseases associated with mitochondrial dysfunction.
Acknowledgments
The authors wish to express their gratitude to the Giovanni Lorenzini Medical Science Foundation (Milan, Italy and Houston, Texas, USA) for organizing the focus meetings on mitochondria and acknowledge receiving a honorarium for their participation in the meetings.
Abbreviations
- ATP
adenosine-5′-triphosphate
- BP
blood pressure
- CoA
coenzyme A
- CoASH
free coenzyme A
- eNOS
endothelial nitric oxide synthase
- FA
fatty acid
- Lp(a)
lipoprotein(a)
- NO
nitric oxide
- OXPHOS
oxidative-phosphorylation system
- ROS
reactive oxygen species
Footnotes
Conflict of interest: All authors have read the journal's policy on conflicts of interest and have no substantial conflict of interest to disclose relevant to the subject of this review.
REFERENCES
- 1.Chan DC. Mitochondrial dynamics in disease. N Engl J Med. 2007;356:1707–9. doi: 10.1056/NEJMp078040. [DOI] [PubMed] [Google Scholar]
- 2.Neubauer S. The failing heart–an engine out of fuel. N Engl J Med. 2007;356:1140–51. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 3.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–73. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 4.Hulsmans M, Van Dooren E, Holvoet P. Mitochondrial reactive oxygen species and risk of atherosclerosis. Curr Atheroscler Rep. 2012;14:264–76. doi: 10.1007/s11883-012-0237-0. [DOI] [PubMed] [Google Scholar]
- 5.Coletta DK, Mandarino LJ. Mitochondrial dysfunction and insulin resistance from the outside in: extracellular matrix, the cytoskeleton, and mitochondria. Am J Physiol Endocrinol Metab. 2011;301:E749–55. doi: 10.1152/ajpendo.00363.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;13:92–103. doi: 10.1038/nrendo.2011.138. [DOI] [PubMed] [Google Scholar]
- 7.Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:12587–94. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckel RH, Alberti KG, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;375:181–3. doi: 10.1016/S0140-6736(09)61794-3. [DOI] [PubMed] [Google Scholar]
- 9.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–81. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–2. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–50. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 12.Patti ME, Corvera S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev. 2010;31:364–95. doi: 10.1210/er.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. 2010;2010:476279. doi: 10.1155/2010/476279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broad EM, Maughan RJ, Galloway SDR. Effects of exercise intensity and altered substrate availability on cardiovascular and metabolic responses to exercise after oral carnitine supplementation in athletes. Int J Sport Nutr Exerc Metab. 2011;21:385–97. doi: 10.1123/ijsnem.21.5.385. [DOI] [PubMed] [Google Scholar]
- 15.Kovalik JP, Slentz D, Stevens RD, et al. Metabolic remodeling of human skeletal myocytes by cocultured adipocytes depends on the lipolytic state of the system. Diabetes. 2011;60:1882–93. doi: 10.2337/db10-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Eckardt K, Taube A, Eckel J. Obesity-associated insulin resistance in skeletal muscle: role of lipid accumulation and physical inactivity. Rev Endocr Metab Disord. 2011;12:163–72. doi: 10.1007/s11154-011-9168-2. [DOI] [PubMed] [Google Scholar]
- 18.Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333:1109–12. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;2:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- 20.Schulz E, Jansen T, Wenzel P, et al. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal. 2008;10:1115–26. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 21.Antoniades C, Shirodaria C, Warrick N, et al. 5-methyltetra-hydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006;114:1193–201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 22.Shenouda SM, Widlansky ME, Chen K, et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–53. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53:557–73. doi: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 24.Felker GM, Pang PS, Adams KF, et al. International AHFS Working Group. Clinical trials of pharmacological therapies in acute heart failure syndromes: lessons learned and directions forward. Circ Heart Fail. 2010;3:314–25. doi: 10.1161/CIRCHEARTFAILURE.109.893222. [DOI] [PubMed] [Google Scholar]
- 25.Soukoulis V, Dihu JB, Sole M, et al. Micronutrient deficiencies an unmet need in heart failure. J Am Coll Cardiol. 2009;54:1660–73. doi: 10.1016/j.jacc.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 26.van Bilsen M, Smeets PJ, Gilde AJ, van der Vusse GJ. Metabolic remodelling of the failing heart: the cardiac burn-out syndrome? Cardiovasc Res. 2004;61:218–26. doi: 10.1016/j.cardiores.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Ardehali H, Sabbah HN, Burke MA, et al. Targeting myocardial substrate metabolism in heart failure: potential for new therapies. Eur J Heart Fail. 2012;14:120–9. doi: 10.1093/eurjhf/hfr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leslie D, Gheorghiade M. Is there a role for thiamine supplementation in the management of heart failure? Am Heart J. 1996;131:1248–50. doi: 10.1016/s0002-8703(96)90121-0. [DOI] [PubMed] [Google Scholar]
- 29.Tardiff JC, Hewett TE, Palmer BM, et al. Cardiac troponin T mutations result in allele-specific phenotypes in a mouse model for hypertrophic cardiomyopathy. J Clin Invest. 1999;104:469–81. doi: 10.1172/JCI6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharov VG, Goussev A, Lesch M, et al. Abnormal mitochondrial function in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol. 1998;30:1757–62. doi: 10.1006/jmcc.1998.0739. [DOI] [PubMed] [Google Scholar]
- 31.Baandrup U, Florio RA, Roters F, Olsen EG. Electron microscopic investigation of endomyocardial biopsy samples in hypertrophy and cardiomyopathy. A semiquantitative study in 48 patients. Circulation. 1981;63:1289–98. doi: 10.1161/01.cir.63.6.1289. [DOI] [PubMed] [Google Scholar]
- 32.Rosca MG, Hoppel CL. Mitochondria in heart failure. Cardiovasc Res. 2010;88:40–50. doi: 10.1093/cvr/cvq240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sebastiani M, Giordano C, Nediani C, et al. Induction of mitochondrial biogenesis is a maladaptive mechanism in mitochondrial cardiomyopathies. J Am Coll Cardiol. 2007;50:1362–9. doi: 10.1016/j.jacc.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein ES, Benson DW, Fry DE. Subpopulations of human heart mitochondria. J Surg Res. 1986;40:495–8. doi: 10.1016/0022-4804(86)90221-0. [DOI] [PubMed] [Google Scholar]
- 35.Dabkowski ER, Williamson CL, Bukowski VC, et al. Diabetic cardiomyopathy-associated dysfunction in spatially distinct mitochondrial subpopulations. Am J Physiol. 2009;296:H359–69. doi: 10.1152/ajpheart.00467.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan J, Karmazyn M. Relationship between oxidative phosphorylation and adenine nucleotide translocase activity of two populations of cardiac mitochondria and mechanical recovery of ischemic hearts following reperfusion. Can J Physiol Pharmacol. 1989;67:704–9. doi: 10.1139/y89-114. [DOI] [PubMed] [Google Scholar]
- 37.Jones LL, McDonald DA, Borum PR. Acylcarnitines: role in brain. Prog Lipid Res. 2010;49:61–75. doi: 10.1016/j.plipres.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Rebouche CJ. Carnitine function and requirements during the life cycle. FASEB J. 1992;6:3379–86. [PubMed] [Google Scholar]
- 39.Kerbey AL, Randle PJ, Cooper RH, Whitehouse S, Pask HT, Denton RM. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976;154:327–48. doi: 10.1042/bj1540327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huelsmann Wc, Siliprandi D, Ciman M, Siliprandi N. Effect of carnitine on the oxidation of alpha-oxoglutarate to succinate in the presence of acetoacetate or pyruvate. Biochim Biophys Acta. 1964;93:166–8. doi: 10.1016/0304-4165(64)90271-5. [DOI] [PubMed] [Google Scholar]
- 41.Russell RR, III, Taegtmeyer H. Coenzyme A sequestration in rat hearts oxidizing ketone bodies. J Clin Invest. 1992;89:968–73. doi: 10.1172/JCI115679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulz H. Regulation of fatty acid oxidation in heart. J Nutr. 1994;124:165–71. doi: 10.1093/jn/124.2.165. [DOI] [PubMed] [Google Scholar]
- 43.Quistad GB, Staiger LE, Schooley DA. The role of carnitine in the conjugation of acidic xenobiotics. Drug Metab Dispos. 1986;14:521–5. [PubMed] [Google Scholar]
- 44.Chalmers RA, Roe CR, Stacey TE, Hoppel CL. Urinary excretion of L-carnitine and acylcarnitines by patients with disorders of organic acid metabolism: evidence for secondary insufficiency of L-carnitine. Pediatric Res. 1984;18:1326–8. doi: 10.1203/00006450-198412000-00021. [DOI] [PubMed] [Google Scholar]
- 45.Stanley CA. Carnitine deficiency disorders in children. Ann N Y Acad Sci. 2004;1033:42–51. doi: 10.1196/annals.1320.004. [DOI] [PubMed] [Google Scholar]
- 46.Stephens FB, Constantin-Teodosiu D, Greenhaff PL. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol. 2007;581:431–44. doi: 10.1113/jphysiol.2006.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rebouche CJ. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann N Y Acad Sci. 2004;1033:30–41. doi: 10.1196/annals.1320.003. [DOI] [PubMed] [Google Scholar]
- 48.Roe CR, Millington DS, Maltby DA, Kahler SG, Bohan TP. L-carnitine therapy in isovaleric acedemia. J Clin Invest. 1984;74:2290–5. doi: 10.1172/JCI111657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roe CR, Millington DS, Maltby DA, Hoppel CL. L-carnitine enhances excretion of propionyl coenzyme A as propionylcarnitine in propionic acidemia. J Clin Invest. 1984;73:1785–8. doi: 10.1172/JCI111387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrieu-Abadie N, Jaffrezou JP, Hatem S, Laurent G, Levade T, Mercadier JJ. L-carnitine prevents doxorubicin-induced apoptosis of cardiac myocytes: role of inhibition of ceramide generation. FASEB J. 1999;13:1501–10. doi: 10.1096/fasebj.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 51.Mutomba MC, Yuan H, Konyavko M, et al. Regulation of the activity of caspases by L-carnitine and palmitoylcarnitine. FEBS Lett. 2000;478:19–25. doi: 10.1016/s0014-5793(00)01817-2. [DOI] [PubMed] [Google Scholar]
- 52.Paumen MB, Ishida Y, Muramatsu M, Yamamoto M, Honjo T. Inhibition of carnitine palmitoyltransferase I augments sphingolipid synthesis and palmitate-induced apoptosis. J Biol Chem. 1997;272:3324–9. doi: 10.1074/jbc.272.6.3324. [DOI] [PubMed] [Google Scholar]
- 53.Cederbland G, Lindstedt S. A method for the determination of carnitine in the picomole range. Clinica Chimica Acta. 1972;37:235–43. doi: 10.1016/0009-8981(72)90438-x. [DOI] [PubMed] [Google Scholar]
- 54.Osorio JH, Pourfarzam M. Plasma free and total carnitine measured in children by tandem mass spectrometry. Brazilian J Med Biol Res. 2002;35:1265–71. doi: 10.1590/s0100-879x2002001100003. [DOI] [PubMed] [Google Scholar]
- 55.Sowell J, Fuqua M, Wood T. Quantification of total and free carnitine in human plasma by hydrophilic interaction liquid chromatography tandem mass spectrometry. J Chromatogr Sci. 2001;49:463–8. doi: 10.1093/chrsci/49.6.463. [DOI] [PubMed] [Google Scholar]
- 56.Minkler PE, Stoll MSK, Ingalls S, et al. Quantification of carnitine and acylcarnitines in biological matrices by HPLC electro-spray ioniztion–mass spectrometry. Clin Chem. 2008;54:1451–62. doi: 10.1373/clinchem.2007.099226. [DOI] [PubMed] [Google Scholar]
- 57.Liu J, Killilea DW, Ames BN. Age-associated mitochondrial oxidative decay: improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-L- carnitine and/or R-alpha-lipoic acid. Proc Natl Acad Sci U S A. 2002;99:1876–81. doi: 10.1073/pnas.261709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gülçin I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006;78:803–11. doi: 10.1016/j.lfs.2005.05.103. [DOI] [PubMed] [Google Scholar]
- 59.Arduini A. Carnitine and its acyl esters as secondary antioxidants? Am Heart J. 1992;123:1726–7. doi: 10.1016/0002-8703(92)90850-u. [DOI] [PubMed] [Google Scholar]
- 60.Calò LA, Pagnin E, Davis PA, et al. Antioxidant effect of L-carnitine and its short chain esters: relevance for the protection from oxidative stress related cardiovascular damage. Int J Cardiol. 2006;107:54–60. doi: 10.1016/j.ijcard.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 61.Stasi MA, Scioli MG, Arcuri G, et al. Propionyl-L-carnitine improves postischemic blood flow recovery and arteriogenetic revascularization and reduces endothelial NADPH-oxidase 4-mediated superoxide production. Arterioscler Thromb Vasc Biol. 2010;30:426–35. doi: 10.1161/ATVBAHA.109.201533. [DOI] [PubMed] [Google Scholar]
- 62.Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab (Lond) 2010;7:30. doi: 10.1186/1743-7075-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Capaldo B, Napoli R, Di Bonito P, Albano G, Saccà L. Carnitine improves peripheral glucose disposal in non-insulin-dependent diabetic patients. Diabetes Res Clin Pract. 1991;14:191–5. doi: 10.1016/0168-8227(91)90020-e. [DOI] [PubMed] [Google Scholar]
- 64.Mingrone G. Carnitine in type 2 diabetes. Ann N Y Acad Sci. 2004;1033:99–107. doi: 10.1196/annals.1320.009. [DOI] [PubMed] [Google Scholar]
- 65.Foster DW. The role of the carnitine system in human metabolism. Ann N Y Acad Sci. 2004;1033:1–16. doi: 10.1196/annals.1320.001. [DOI] [PubMed] [Google Scholar]
- 66.Ruggenenti P, Cattaneo D, Loriga G, et al. Ameliorating hypertension and insulin resistance in subjects at increased cardiovascular risk: effects of acetyl-L-carnitine therapy. Hypertension. 2009;54:567–74. doi: 10.1161/HYPERTENSIONAHA.109.132522. [DOI] [PubMed] [Google Scholar]
- 67.Mingrone G, Greco AV, Capristo E, et al. L-carnitine improves glucose disposal in type 2 diabetic patients. J Am Coll Nutr. 1999;18:77–82. doi: 10.1080/07315724.1999.10718830. [DOI] [PubMed] [Google Scholar]
- 68.Giancaterini A, De Gaetano A, Mingrone G, et al. Acetyl-L-carnitine infusion increases glucose disposal in type 2 diabetic patients. Metabolism. 2000;49:704–8. doi: 10.1053/meta.2000.6250. [DOI] [PubMed] [Google Scholar]
- 69.Midaoui AE, Elimadi A, Wu L, Haddad PS, de Champlain J. Lipoic acid prevents hypertension, hyperglycemia, and the increase in heart mitochondrial superoxide production. Am J Hypertens. 2003;16:173–9. doi: 10.1016/s0895-7061(02)03253-3. [DOI] [PubMed] [Google Scholar]
- 70.Vasdev S, Gill V, Parai S, Gadag V. Dietary lipoic acid supplementation attenuates hypertension in Dahl salt sensitive rats. Mol Cell Biochem. 2005;275:135–41. doi: 10.1007/s11010-005-1095-7. [DOI] [PubMed] [Google Scholar]
- 71.Ferrannini E, Natali A, Capaldo B, Lehtovirta M, Jacob S, Yki-Järvinen H. Insulin resistance, hyperinsulinemia, and blood pressure: role of age and obesity. European Group for the Study of Insulin Resistance (EGIR). Hypertension. 1997;30:1144–9. doi: 10.1161/01.hyp.30.5.1144. [DOI] [PubMed] [Google Scholar]
- 72.Whaley-Connell A, Sowers JR. Hypertension and insulin resistance. Hypertension. 2009;54:462–4. doi: 10.1161/HYPERTENSIONAHA.109.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng KK, Lam KS, Wang Y, et al. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–94. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- 74.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–6. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 75.Zhu W, Cheng KK, Vanhoutte PM, Lam KS, Xu A. Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci (Lond) 2008;114:361–74. doi: 10.1042/CS20070347. [DOI] [PubMed] [Google Scholar]
- 76.Bełtowski J, Jamroz-Wiśniewska A, Widomska S. Adiponectin and its role in cardiovascular diseases. Cardiovasc Hematol Disord Drug Targets. 2008;8:7–46. doi: 10.2174/187152908783884920. [DOI] [PubMed] [Google Scholar]
- 77.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 78.Hagen TM, Liu J, Lykkesfeldt J, et al. Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc Natl Acad Sci U S A. 2002;99:1870–5. doi: 10.1073/pnas.261708898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miguel-Carrasco JL, Mate A, Monserrat MT, Arias JL, Aramburu O, Vázquez CM. The role of inflammatory markers in the cardioprotective effect of L-carnitine in L-NAME-induced hypertension. Am J Hypertens. 2008;21:1231–7. doi: 10.1038/ajh.2008.271. [DOI] [PubMed] [Google Scholar]
- 80.Mate A, Miguel-Carrasco JL, Monserrat MT, Vázquez CM. Systemic antioxidant properties of L-carnitine in two different models of arterial hypertension. J Physiol Biochem. 2010;66:127–36. doi: 10.1007/s13105-010-0017-7. [DOI] [PubMed] [Google Scholar]
- 81.Hedayati S. Dialysis-related carnitine disorder. Semin Dial. 2006;19:323–8. doi: 10.1111/j.1525-139X.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- 82.Schreiber BD. Debte forum: levocarnitine therapy is rational and justified in selected dialysis patients. Blood Purif. 2006;24:128–34. doi: 10.1159/000089449. [DOI] [PubMed] [Google Scholar]
- 83.Bohomini M. Pharmacological use of L-carnitine in uremic anemia: has its full potential been exploited? Pharmacol Res. 2011;63:157–64. doi: 10.1016/j.phrs.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 84.Anand I, Chandrashekhan Y, De Giuli F, et al. Acute and chronic effects of propionyl-L-carnitine on the hemodynamics, exercise capacity, and hormones in patients with congestive heart failure. Cardiovasc Drugs Ther. 1998;12:291–9. doi: 10.1023/a:1007721917561. [DOI] [PubMed] [Google Scholar]
- 85.Oyanagi E, Yano H, Uchida M, Utsumi K, Sasaki J. Protective action of L-carnitine on cardiac mitochondrial function and structure against fatty acid stress. Biochem Biophys Res Commun. 2011;412:61–7. doi: 10.1016/j.bbrc.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 86.Lesnefsky EJ, Hoppel CL. Oxidative phosphorylation and aging. Aging Res Rev. 2006;5:402–33. doi: 10.1016/j.arr.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 87.Rosca MG, Lemieux H, Hoppel CL. Mitochondria in the elderly: is acetylcarnitine a rejuvenator. Adv Drug Deliv Rev. 2009;61:1332–42. doi: 10.1016/j.addr.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hagen TM, Moreau R, Suh JH, Visioli F. Mitochondrial decay in the agine rat heart: evidence for improvement y. Ann NY Acad Sci. 2002;959:491–507. doi: 10.1111/j.1749-6632.2002.tb02119.x. [DOI] [PubMed] [Google Scholar]
- 89.Pesce V, Nicassio L, Fracasso F, Musicco C, Cantatore P, Gadaleta MN. Acetyl-L-carnitine activates the peroxisome proliferator-activated receptor-γ coactivators PGC-1α/PGCβ-dependent signaling cascade of mitochondrial biogenesis and decreases the oxidized peroxiredoxins content in old rat liver. Rejuvenation Res. 2012;15:136–9. doi: 10.1089/rej.2011.1255. [DOI] [PubMed] [Google Scholar]
- 90.Musicco C, Capelli V, Pesce V, et al. Rat liver mitochondrial proteome: changes associated with aging and acetyl-L-carnitne treatment. J Proteomics. 2011;74:2536–47. doi: 10.1016/j.jprot.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 91.Guarente L. Sirtuins, aging, and metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:81–90. doi: 10.1101/sqb.2011.76.010629. [DOI] [PubMed] [Google Scholar]
- 92.Lombard DB, Ahf W, Cheng HL, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;24:8807–14. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hirschey MD, Shimazu T, Goetzman E, et al. SIR3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–5. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jing E, Emanuelli B, Hirschey MD, et al. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A. 2011;108:14608–13. doi: 10.1073/pnas.1111308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Benigni A, Daniela C, Zoja C, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–30. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Emerging Risk Factors Collaboration. Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–23. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–9. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 98.Clarke R, Peden JF, Hopewell JC, et al. PROCARDIS Consortium. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–28. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 99.Koschinsky ML, Marcovina SM. Structure-function relationships in apolipoprotein(a): insights into lipoprotein(a) assembly and pathogenicity. Curr Opin Lipidol. 2004;15:167–74. doi: 10.1097/00041433-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 100.Benyó Z, Gille A, Kero J, et al. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J Clin Invest. 2005;115:3634–40. doi: 10.1172/JCI23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sirtori CR, Calabresi L, Ferrara S, et al. L-carnitine reduces plasma lipoprotein(a) levels in patients with hyper Lp(a). Nutr Metab Cardiovasc Dis. 2000;10:247–51. [PubMed] [Google Scholar]
- 102.Galvano F, Li Volti G, Malaguarnera M, et al. Effects of simvastatin and carnitine versus simvastatin on lipoprotein(a) and apo-protein(a) in type 2 diabetes mellitus. Expert Opin Pharmacother. 2009;10:1875–82. doi: 10.1517/14656560903081745. [DOI] [PubMed] [Google Scholar]