Abstract
We have previously shown that green and black tea extracts increase the phosphorylation of AMP-activated protein kinase (AMPK) and HMG-CoA reductase in rat hepatoma cells in culture, concomitant with a decrease in cholesterol synthesis. In the present study, we evaluated the ability of a single oral dose of green or black tea extract to promote the phosphorylation of AMPK, liver kinase B1 (LKB1, an AMPK-kinase), and HMG-CoA reductase in mouse liver. Green tea extract administered by gavage at 50 and 100 mg/kg caused a 2- to 3-fold increase in hepatic AMPK phosphorylation at 3 and 6 hours after dosing and a 1.5- to 2-fold increase in LKB1 phosphorylation at these same time points. The phosphorylation of HMG-CoA reductase at these and later time points was not significantly increased. Black tea administered by gavage at up to 250 mg/kg was ineffective in increasing hepatic AMPK phosphorylation. Both green and black tea extracts increased LKB1 phosphorylation in hepatoma cells in culture at 15 μg/mL, and black tea also increased the phosphorylation of protein kinase A in hepatoma cells. These results suggest that compounds in both tea extracts activate AMPK by activating its upstream kinase, LKB1, and that black tea may do so by first activating protein kinase A, a known kinase for LKB1. Only green tea, at 50 and 100 mg/kg, was able to activate AMPK and LKB1 in mouse liver after oral dosing, suggesting that the polymerized catechins present in black tea do not reach the liver in sufficient concentration to affect AMPK activity.
Keywords: Mouse, Liver, Green tea, Black tea, AMP-kinase, Hepatoma cell, LKB1
1. Introduction
Green and black teas have been shown to lower blood cholesterol levels in clinical trials and to decrease cholesterol synthesis in vitro and in animal studies [1-5]. The mechanism(s) by which these substances lower cholesterol and inhibit its synthesis remains to be fully identified, but current evidence suggests several possible actions that may be relevant: most often noted is the ability of both teas to decrease cholesterol absorption in the gut and to promote its excretion [6-8]. There is also evidence that the polyphenols and related substances in tea can decrease cholesterol synthesis directly by inhibiting hydroxymethylglutaryl (HMG)-CoA reductase [5,9] and indirectly by promoting the phosphorylation (inactivation) of HMG-CoA reductase by activation of AMP-activated protein kinase (AMPK) by phosphorylation [5]. We have recently published data supporting both of these mechanisms to decrease cholesterol synthesis in a cell culture model [5], but there are limited data on the ability of tea taken orally to act on hepatic AMPK and HMG-CoA reductase in vivo. To test the hypothesis that orally administered tea can activate AMPK in the liver (the principal site of synthesis of circulating cholesterol), we gavaged mice with green and black tea extracts with the objectives of measuring the change in phosphorylation of hepatic AMPK, liver kinase B1 (LKB1), and HMG-CoA reductase at sequential time points after dosing. The rationale for looking at LKB1 phosphorylation is that this kinase is considered to be upstream of AMPK and likely plays a role in activating AMPK; the reason for looking at phosphorylation of HMG-CoA reductase is that this enzyme is one of several downstream targets for AMPK and provides a significant mechanism by which its inactivation by phosphorylation might lower serum cholesterol levels. Our results indicate that a single oral dose of green but not black tea can increase the phosphorylation of AMPK and LKB1, but this single dose is not sufficient to alter the phosphorylation state of HMG-CoA reductase.
2. Methods and materials
2.1. Materials
Green and black tea extract powders were provided gratis by Naturex (South Hackensack, NJ). The green tea extract contained greater than 95% total polyphenols, greater than 90% catechins, greater than 70% epigallocatechin gallate; the black tea extract contained not less than 60% total polyphenols and not less than 15% epigallocatechin gallate. Extracts were prepared fresh for use in deionized water (mouse studies) or sterile deionized water (cell culture studies). Dulbecco’s modified Eagle’s medium, penicillin-streptomycin-glutamine, fetal bovine serum, and trypsin were purchased from Invitrogen (Carlsbad, CA). Acadesine (AICAR), 1,1-dimethylbiguanide hydrochloride (metformin), dibutyryl-cAMP, and protease inhibitor cocktail were obtained from Sigma (St Louis, MO). HALT phosphatase inhibitor and the BCA protein assay kit were purchased from Roche Diagnostics (Indianapolis, IN) and Pierce/Thermo Scientific (Rockford, IL), respectively. Antibodies to total and phosphorylated AMPK, total and phosphorylated HMG-CoA reductase, and total and phosphorylated LKB1 were purchased from Upstate/Millipore (Billerica, MA), as was human recombinant platelet-derived growth factor-BB (PDGF). Antibodies to total and phosphorylated protein kinase A (PKA) and protein kinase Cζ (PKCζ) were purchased from Cell Signaling Technology (Danvers, MA). Secondary antibodies conjugated to horseradish peroxidase were purchased from Pierce/Thermo Scientific. Nitrocellulose membranes were from Bio-Rad (Hercules, CA). McA-RH7777 rat hepatoma cells were obtained from American Type Culture Collection (Manassas, VA).
2.2. Cell culture, treatments, and preparation of lysates
McA-RH7777 rat hepatoma cells were cultured in standard media (Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum) as previously described [10]. Cells were treated for 3 hours with green or black tea extract dissolved in sterile water at a final concentration of 15 μg/mL. AICAR, metformin, and dibutyryl-cAMP were added in water as indicated for the same period at 1 mM final concentration; PDGF was added at 20 ng/mL. Following treatment, cells were lysed in phosphate-buffered saline by freeze thawing in the presence of protease and phosphatase inhibitors, lysates were cleared by low speed centrifugation, protein was quantified, and samples were assayed by electrophoresis and immunoblotting as described below.
2.3. Animal treatments and preparation of tissue
Experiments involving the use of animals were performed following protocols approved by the Institutional Animal Care and Use Committee of the University of Kentucky and were in accordance with all policies for the use and care of laboratory research animals as stipulated by the National Institutes of Health. Seven-to-eight-week-old female C57BL/6J mice (~15-17 g) were purchased from Jackson laboratories (Bar Harbor, ME) and maintained in a temperature-, humidity-, and light-controlled facility with free access to water and food for 1 week before experimentation. After an overnight fast, mice in groups of 3 and 4 were gavaged with up to 150 μL of green or black tea extract dissolved in water at 50, 100, or 250 mg/kg. Control animals received an equal volume of distilled water. Access to food was restored, and at 0, 3, 6, 12, and 24 hours, the mice were euthanized by CO2 asphyxiation, and the liver was removed and promptly frozen in liquid nitrogen and stored at −80°C until use. Homogenates were prepared from 25 mg of tissue in 4 volumes of Tris-NaCl (RIPA) buffer as described [10] in the presence of protease and phosphatase inhibitors and cleared by low-speed centrifugation (18300g, 10 minutes, 4°C). Protein concentration was determined by BCA assay, and samples were assayed by electrophoresis and immunoblotting as described below.
2.4. Gel electrophoresis and immunoblotting
Thirty micrograms of protein from cell lysate or liver homogenate was fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 8% gels and electro-blotted to nitrocellulose as described [10]. The membranes were incubated overnight at 4° with gentle shaking with antibodies to total or phosphorylated AMPK, LKB1, HMG-CoA reductase, protein kinase A, or PKCζ. Immunoblots were developed with a secondary antibody conjugated to horseradish peroxidase for 1 hour at room temperature and the chemiluminescent image (Supersignal West Pico Chemiluminescent Substrate; Pierce/Thermo Scientific) captured by autoradiography on a Kodak Image Station. Phosphorylated protein levels were adjusted for background and total enzyme content, determined with an antibody to the nonphosphorylated protein, and are reported as relative values with the zero-time untreated value being set at 100.
2.5. Statistical analyses
Results are presented as the means ± SE with significance determined by 1-way analysis of variance (ANOVA) and Dunnett’s post hoc test, setting P < .05, using GraphPad Prism 5.0. Power analysis was not used to determine animal number, as these were preliminary feasibility studies.
3. Results
3.1. Tea extracts activate LKB1
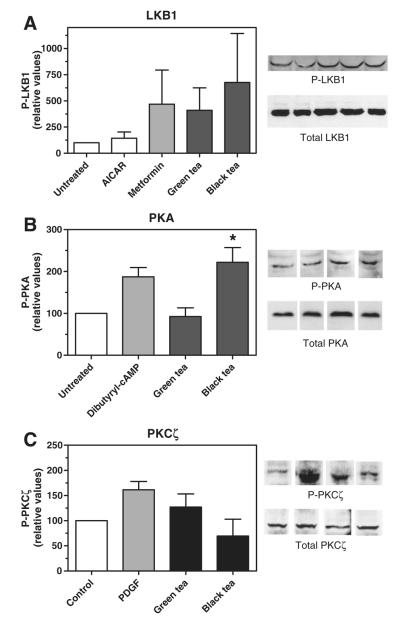
We previously showed that treatment of hepatoma cells with green or black tea extract increased the amount of phosphorylated (activated) AMPK in these cells within 30 to 90 minutes of treatment [5]. The principal kinase responsible for AMPK phosphorylation is LKB1, which is also activated by phosphorylation. To determine if tea extracts activated LKB1, we measured the phosphorylation of this upstream kinase by immunodetection using an antibody specific for the phosphorylated form. As shown in Fig. 1, LKB1 phosphorylation was increased by 3- to 5-fold after treatment of hepatoma cells with 15 μg/mL of either tea extract; this increase, although not statistically significant, was similar to that obtained with metformin, an established activator of LKB1 phosphorylation [11]. AICAR, which increases the activation of AMPK by acting as an AMP mimetic [12], had no effect on LKB1 phosphorylation, as expected. Several kinases have been reported to phosphorylate LKB1, including PKA [13] and PKCζ [11]. Treatment with black tea extract increased the phosphorylation of PKA by 2.5-fold and was statistically significant; the increase was similar to that seen with dibutyryl-cAMP, an established activator of PKA. Green tea extract did not affect PKA phosphorylation but modestly increased the phosphorylation of PKCζ, similar to that seen with PDGF, a reported activator of this kinase [14,15]. Neither increase was statistically significant. These results suggest that both teas activate AMPK, at least, in part, by increasing the phosphorylation and activity of LKB1, and black tea may activate LKB1 via PKA.
Fig. 1.
Activation of LKB1,PKA, and PKCζ by green and black tea extracts. McA-RH7777 rat hepatoma cells were treated with 15 μg/mL of green or black tea extract or 1 mM AICAR, metformin, dibutyryl-cAMP or 20 ng/mL of PDGF for 3 hours and levels of phosphorylated LKB1 (A), PKA (B), or PKCζ (C) were determined by immunodetection; representative immunoblot images are shown on the right. Means and SEs (n = 3); statistical significance determined by ANOVA with Dunnett’s post hoc test, P < .05.
3.2. Green but not black tea activates hepatic AMPK and LKB1 in vivo
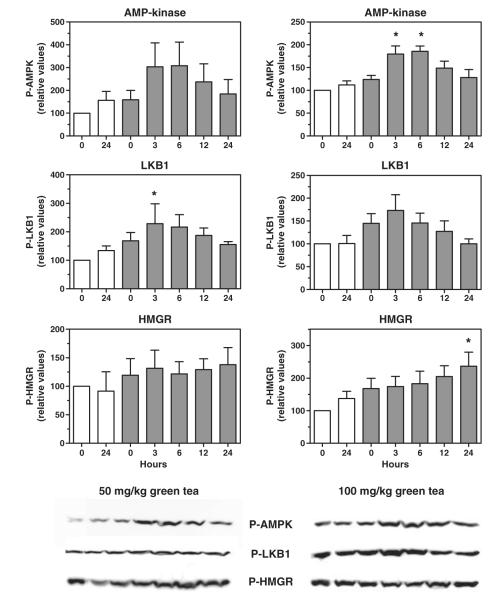
Although tea polyphenols readily activate AMPK in a cell culture model, their absorption from the gut after oral administration is poor [16]. To test the ability of orally administered tea extract to activate AMPK, LKB1, and HMG-CoA reductase in the liver, we administered by gavage a single oral dose of green tea extract to mice and measured the amount of phosphorylated enzyme at various time points after dosing. As shown in Fig. 2, green tea extract increased the amount of phosphorylated AMPK in the liver at 3 and 6 hours after dosing, with the increase reaching statistical significance at the 100 mg/kg dose. Liver kinase B1 phosphorylation was also increased, peaking at 3 hours with both doses. Phosphorylation of HMG-CoA reductase was not noticeably increased by either oral dose, suggesting that a more prolonged period of administration is needed to affect the activity of this enzyme. In contrast to green tea, black tea extract was unable to increase the phosphorylation of AMPK at doses from 50 to 250 mg/kg (Fig. 3). This is consistent with the known very poor absorption of the polymerized polyphenols (theaflavins and thearubigins) that are abundant in black tea [16]. Because AMPK phosphorylation was not increased, we did not examine the phosphorylation of LKB1 or HMG-CoA reductase.
Fig. 2.
Activation of hepatic AMPK and LKB1 by a single oral dose of green tea extract in mice. Mice received 50 mg/kg (left) or 100 mg/kg (right) of green tea extract by gavage and levels of phosphorylated AMPK, LKB1, and HMG-CoA reductase (HMGR) in liver homogenates were determined at various time points (0-24 hours) after dosing by immunodetection; representative immunoblot images are shown below the graphs. Open bars indicate control animals (gavaged with water). Means and SEs (n = 3); statistical significance determined by ANOVA with Dunnett’s post hoc test, P < .05.
Fig. 3.
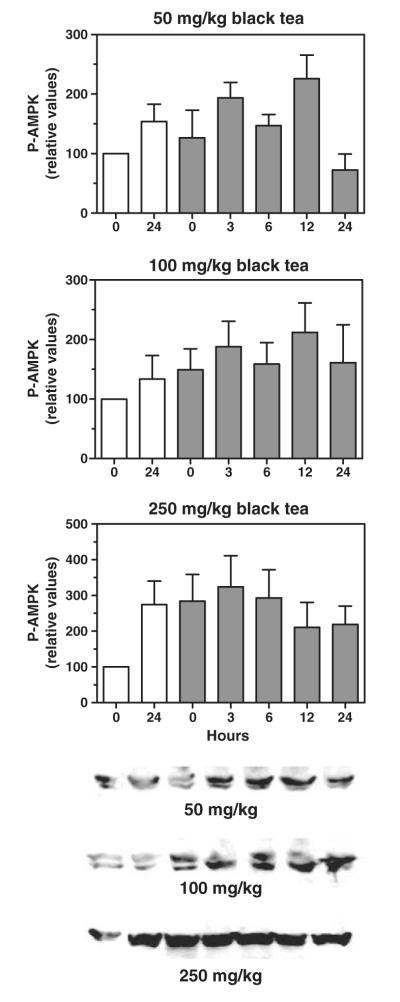
Lack of activation of hepatic AMPK by a single oral dose of black tea extract in mice. Mice received 50 mg/kg (upper), 100 mg/kg (middle), or 250 mg/kg (lower) of black tea extract by gavage and levels of phosphorylated AMPK in liver homogenates were determined at various time points (0-24 hours) after dosing by immunodetection; representative immunoblot images are shown below the graphs. Open bars indicate control animals (gavaged with water). Means and SEs (n = 4); statistical significance determined by ANOVA with Dunnett’s post hoc test, P < .05.
4. Discussion
Epidemiological studies first suggested that tea consumption might lead to lower blood lipid levels [17,18], and several subsequent large clinical trials have provided support for this postulate [1,2]. Although a large part of this effect is attributed to the ability of tea polyphenols to decrease lipid absorption in the intestine and promote sterol excretion, direct effects of these compounds on hepatic lipid and cholesterol synthesis cannot be excluded and are supported by studies carried out in animals and in vitro [3,5,9]. We tested this idea by administering a single oral dose of green or black tea extract to mice and measuring the phosphorylation (a proxy for activation) of AMPK, the key regulatory kinase for energy metabolism. AMP-activated protein kinase phosphorylates and thereby inactivates HMG-CoA reductase, resulting in a decrease in cholesterol synthesis. Inhibition of this enzyme by the statin drugs is the most widely used means to decrease serum cholesterol levels. Interestingly, metformin, a widely used drug for type 2 diabetes, is well known to modestly lower cholesterol levels; because metformin is believed to act by activation of AMPK [11], the possibility that tea polyphenols might similarly lower cholesterol levels by activation of AMPK is an appealing possibility.
Our studies reveal that a single, albeit very large dose of green tea extract, is able to activate AMPK in the liver, perhaps by first activating LKB1, the principal AMPK-activating kinase. This confirms our hypothesis, based on earlier in vitro studies, that oral intake of tea should increase the phosphorylation of AMPK. Black tea, however, in similar and greater doses was not able to significantly increase AMPK phosphorylation. We attribute this lack of activation to the poor absorption of the more complex polyphenols present in black tea and the much lower content of low-molecular-weight catechins that make up the bulk of polyphenols present in green tea. A 50 mg/kg dose of green tea extract would be expected to contain approximately 45 mg/kg of polyphenols as catechins, based on our supplier’s composition sheet. Assuming an average cup of green tea contains approximately 100 mg of catechins, a 70-kg man would need to drink 35 cups of tea to get an equivalent dose. As current recommendations for green tea extract as a nutritional supplement suggest a dose of approximately 500 mg, equivalent to only 5 cups of tea per day, our studies would suggest that this level of consumption is unlikely to impact hepatic lipid synthesis, although it may affect cholesterol absorption in the gut. Nonetheless, because our tea extract was administered as a single dose, it is unclear as to whether more prolonged administration in smaller doses, as tea is normally consumed, and on a long-term basis, might have a greater impact on lipid synthesis. Moreover, regular modest tea consumption may have additional health benefits not addressed here, including decreasing cholesterol absorption in the intestine.
Notable limitations to the present studies are the small number of animals tested in each group and the imprecision of the immunoquantitation studies. Immunoquantitation by immunoblotting, as done here, is well recognized as being difficult to standardize between experiments. The signal intensity varies greatly and, indeed, often exponentially, as a result of the signal amplification inherent in the assay. Although most researchers include appropriate controls in each experiment and then normalize values to these controls (as done here) before combining results, there is nonetheless large variability between experiments. This limits the ability to make statistically significant conclusions, and in our experience, increasing the number of experiments typically does not improve the precision of the results. With regard to the small number of animals tested, because there were (and are) no previous studies addressing the ability of orally administered tea extracts to activate hepatic AMPK, we did not have a sense of how much tea to administer or when to assay for effects on hepatic enzymes. As a result, these experiments cast a very wide net, testing several doses for effects over a 24-hour period. Although each data point represents only 3 or 4 animals, each experiment represents at least 15 animals over a 24-hour period. We believe that this gives a good preliminary picture of the likelihood that a given tea extract can affect hepatic enzyme activities involved in cholesterol, lipid, and glucose metabolism.
Acknowledgment
This work was supported by the US National Institutes of Health National Center for Complementary and Alternative Medicine grant AT-005235.
Abbreviations
- AMPK
AMP-activated protein kinase
- ANOVA
analysis of variance
- HMG
hydroxymethylglutaryl
- LKB1
liver kinase B1
- PDGF
platelet-derived growth factor BB
- PKA
protein kinase A
- PKCζ
protein kinase Cζ
REFERENCES
- [1].Davies MJ, Judd JT, Baer DJ, Clevidence BA, Paul DR, Edwards AJ, et al. Black tea consumption reduces total and LDL cholesterol in mildly hypercholesterolemic adults. J Nutr. 2003;133:3298S–302S. doi: 10.1093/jn/133.10.3298S. [DOI] [PubMed] [Google Scholar]
- [2].Maron DJ, Lu GP, Cai NS, Wu ZG, Li YH, Chen H, et al. Cholesterol-lowering effect of a theaflavin-enriched green tea extract: a randomized controlled trial. Arch Intern Med. 2003;163:1448–53. doi: 10.1001/archinte.163.12.1448. [DOI] [PubMed] [Google Scholar]
- [3].Bursill CA, Abbey M, Roach PD. A green tea extract lowers plasma cholesterol by inhibiting cholesterol synthesis and upregulating the LDL receptor in the cholesterol-fed rabbit. Atherosclerosis. 2007;193:86–93. doi: 10.1016/j.atherosclerosis.2006.08.033. [DOI] [PubMed] [Google Scholar]
- [4].Kim A, Chiu A, Barone MK, Avino D, Wang F, Coleman CI, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011;111:1720–9. doi: 10.1016/j.jada.2011.08.009. [DOI] [PubMed] [Google Scholar]
- [5].Singh DK, Banerjee S, Porter TD. Green and black tea extracts inhibit HMG-CoA reductase and activate AMP kinase to decrease cholesterol synthesis in hepatoma cells. J Nutr Biochem. 2009;20:816–22. doi: 10.1016/j.jnutbio.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ikeda I, Yamahira T, Kato M, Ishikawa A. Black-tea polyphenols decrease micellar solubility of cholesterol in vitro and intestinal absorption of cholesterol in rats. J Agric Food Chem. 2010;58:8591–5. doi: 10.1021/jf1015285. [DOI] [PubMed] [Google Scholar]
- [7].Vermeer MA, Mulder TP, Molhuizen HO. Theaflavins from black tea, especially theaflavin-3-gallate, reduce the incorporation of cholesterol into mixed micelles. J Agric Food Chem. 2008;56:12031–6. doi: 10.1021/jf8022035. [DOI] [PubMed] [Google Scholar]
- [8].Koo SI, Noh SK. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. J Nutr Biochem. 2007;18:179–83. doi: 10.1016/j.jnutbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cuccioloni M, Mozzicafreddo M, Spina M, Tran CN, Falconi M, Eleuteri AM, et al. Epigallocatechin-3-gallate potently inhibits the in vitro activity of hydroxy-3-methyl-glutaryl-CoA reductase. J Lipid Res. 2011;52:897–907. doi: 10.1194/jlr.M011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Banerjee S, Ghoshal S, Porter TD. Activation of AMP-kinase by policosanol requires peroxisomal metabolism. Lipids. 2011;46:311–21. doi: 10.1007/s11745-011-3540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xie Z, Dong Y, Scholz R, Neumann D, Zou MH. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 2008;117:952–62. doi: 10.1161/CIRCULATIONAHA.107.744490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Day P, Sharff A, Parra L, Cleasby A, Williams M, Horer S, et al. Structure of a CBS-domain pair from the regulatory gamma1 subunit of human AMPK in complex with AMP and ZMP. Acta Crystallogr D Biol Crystallogr. 2007;63:587–96. doi: 10.1107/S0907444907009110. [DOI] [PubMed] [Google Scholar]
- [13].Collins SP, Reoma JL, Gamm DM, Uhler MD. LKB1, a novel serine/threonine protein kinase and potential tumour suppressor, is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vivo. Biochem J. 2000;345(Pt 3):673–80. [PMC free article] [PubMed] [Google Scholar]
- [14].Carlin S, Yang KX, Donnelly R, Black JL. Protein kinase C isoforms in human airway smooth muscle cells: activation of PKC-zeta during proliferation. Am J Physiol. 1999;276:L506–12. doi: 10.1152/ajplung.1999.276.3.L506. [DOI] [PubMed] [Google Scholar]
- [15].Holmstrom TE, Mattsson CL, Falting JM, Nedergaard J. Differential signalling pathways for EGF versus PDGF activation of Erk1/2 MAP kinase and cell proliferation in brown preadipocytes. Exp Cell Res. 2008;314:3581–92. doi: 10.1016/j.yexcr.2008.09.007. [DOI] [PubMed] [Google Scholar]
- [16].Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52(Suppl. 1):S139–51. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- [17].Kono S, Shinchi K, Ikeda N, Yanai F, Imanishi K. Green tea consumption and serum lipid profiles: a cross-sectional study in northern Kyushu, Japan. Prev Med. 1992;21:526–31. doi: 10.1016/0091-7435(92)90060-u. [DOI] [PubMed] [Google Scholar]
- [18].Imai K, Nakachi K. Cross sectional study of effects of drinking green tea on cardiovascular and liver diseases. Br Med J. 1995;310:693–6. doi: 10.1136/bmj.310.6981.693. [DOI] [PMC free article] [PubMed] [Google Scholar]