Abstract
LPA (lysophosphatidic acid, 1-acyl-2-hydroxy-sn-glycero-3-phosphate), is a growth factor-like lipid mediator that regulates many cellular functions, many of which are unique to malignantly transformed cells. The simple chemical structure of LPA and its profound effects in cancer cells has attracted the attention of the cancer therapeutics field and drives the development of therapeutics based on the LPA scaffold. In biological fluids, LPA is generated by ATX (autotaxin), a lysophospholipase D that cleaves the choline/serine headgroup from lysophosphatidylcholine and lysophosphatidylserine to generate LPA. In the present article, we review some of the key findings that make the ATX–LPA signalling axis an emerging target for cancer therapy.
Keywords: autotaxin, cancer, drug discovery, lysophosphatidic acid (LPA), 4-pentadecylbenzylphosphonic acid (4-PBA), therapy
The many roles of autotaxin and lysophosphatidic acid in malignancy
ATX (autotaxin) is a member of the NPP (nucleotide pyrophosphatase/phosphodiesterase) enzyme family and is also known as NPP2 [1,2]. ATX was originally identified as a secreted phosphatase in melanoma culture supernatant that promoted cancer cell motility [3]. Ten years later, ATX was identified as a lysophospholipase D responsible for the production of LPA (lysophosphatidic acid, 1-acyl-2-hydroxy-sn-glycero-3-phosphate) in blood [4,5]. ATX-KO (knockout) mice die in utero due to angiogenic defects, whereas heterozygous ATX-KOs appear to be normal, but have half the plasma LPA concentration compared with wild-type littermates [6,7].
Many cancers secrete ATX, which contributes to their invasive properties (Figure 1). Ovarian cancer cells produce high levels of LPA [8,9]. Whether plasma LPA levels due to ATX production by cancer cells are elevated and can be used as a cancer biomarker has generated some controversy. The plasma ATX level does not appear to be a cancer marker because it is also elevated in patients with liver disease [10,11], during pregnancy [12] and in patients with acute coronary syndrome [13]. Plasma LPA levels follow the trend of plasma ATX levels. Gene copy numbers are elevated in ovarian cancers in chromosomal region 8q24, which contains the gene encoding ATX [14]. There is consensus in the field that LPA levels are highly elevated in ovarian cancer ascites [8,15], but also in tumour cell effusates from other types of malignancies [8]. The acyl chain composition of plasma LPA and the LPA in tumour cell effusates differ suggesting that LPA does not freely cross the barriers between body compartments [8]. Plasma LPA level has been found elevated in only a select group of malignancies that include pancreatic cancer [16] and follicular lymphoma [17]. Extreme care must be exercised during the collection, handling and storage of blood samples intended for ATX and LPA analysis because LPA production continues ex vivo due in part to the removal of the LPA degradation by cells of the vasculature and the liver, and also due to the continued action of ATX [18] and the release of phospholipases A1 and A2 from platelets [19].
Figure 1. Juxtacrine signalling via the ATX–LPA receptor axis.
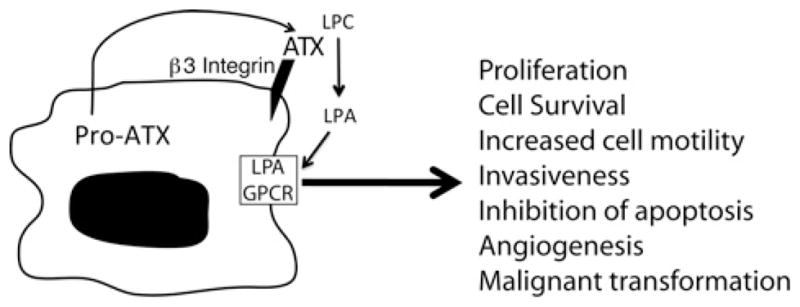
ATX secreted from the tumour cell can be captured at the cell surface through binding to β3 integrin in the vicinity of the LPA GPCR. Activation of the LPA GPCR elicits responses that modulate the biological properties of the cancer cell.
ATX is among the 40 most up-regulated genes in highly metastatic cancers [20]. Ectopic expression of ATX in mice leads to mammary intraepithelial neoplasia, developing into invasive and metastatic tumours [21]. ATX inhibits paclitaxel-induced apoptosis in breast cancer cells [22], and we have shown that LPA renders ovarian cancer cells chemoresistant to cisplatin and adriamycin [23]. ATX becomes overexpressed in patients with recurrent disease after treatment with chemotherapy [24]. In a genome-wide siRNA (small interfering RNA) screen, we identified ATX as a candidate drug-resistance gene in ovarian cancer and showed that an inhibitor of ATX increases paclitaxel sensitivity of resistant cancer cells [25].
LPA receptors in cancer cells
LPA activates two clusters of GPCRs (G-protein-coupled receptors). The EDG (endothelial differentiation gene) cluster includes the LPA1, LPA2 and LPA3 receptors that are close relatives of the EDG family sphingosine 1-phosphate receptors. LPA1 and LPA2 are broadly expressed. Many cancers overexpress multiple subtypes of LPA receptors [26]. LPA1 has been shown to be a regulator of cancer cell motility and metastasis [27] and matrix metalloproteinase expression [28]. LPA2 plays an important role in the invasiveness of ovarian cancer in at least two ways. First, LPA2 promotes production of VEGF (vascular endothelial growth factor), uPA (urokinase-type plasminogen activator) and matrix metalloproteinases [29]. LPA increases VEGF production, and in turn up-regulates ATX production, which increases LPA levels [30], another potential feedforward loop that also promotes angiogenesis. Secondly, LPA-induced uPA production promotes invasiveness of ovarian cancer cell lines [31]. Clinically, high uPA levels indicate poor prognosis in ovarian cancer patients [32].
A second cluster of LPA receptors is found within the purinergic P2Y gene cluster. The better characterized members of this cluster are LPA4 (P2Y9), LPA5 (GPR92) and LPA6 (P2Y5). Mouse embryonic fibroblasts isolated from LPA4-KOs showed increased migration to LPA and developed spontaneous tumours [33], suggesting that LPA4 has a tumour-suppressor role. There are additional related GPCRs that appear to be activated by LPA. In chronological order of discovery, these include GPR87, P2Y10 and GPR35, but their biological function remains to be characterized.
Thus compelling evidence exists that the overexpressed LPA receptors play a fundamental role in carcinogenesis, invasiveness, metastatic potential and therapeutic resistance in many types of carcinomas.
Juxtacrine signalling between the metastatic cancer cell and cells of the endothelial or mesothelial barrier
ATX via LPA production promotes metastasis. During haematogenous metastasis, cancer cells have to cross the vessel wall twice, first when entering the blood stream and secondly when transmigrating it to seed the site of metastasis. For this reason, the role of LPA and ATX in the interaction between the cancer cell and the vascular endothelial cell is of peculiar importance. ATX–LPA signalling takes place in the cancer cell microenvironment (Figure 1). The recent elucidation of the crystal structure of ATX [34,35] reinforces this concept in two ways: first, the interaction of the ATX somatomedin B domain with β3 integrin provides the structural framework for localizing ATX-mediated LPA production to the cancer and endothelial cell surfaces, which express β3 integrin. Secondly, identification of an LPA-binding site within a hydrophobic tunnel of ATX important for releasing LPA at the cell surface juxtaposed to the LPA receptor provides yet another structural framework for juxtacrine signalling. Thus dual targeting of ATX and LPA receptors for the control of metastasis and tumour growth is a logical extension of the juxtacrine signalling concept because one inhibits both targets that are spatially and functionally linked in the cancer cell microenvironment.
Validation of ATX as a drug target for metastasis control
We examined the effect of silencing ATX expression in the B16 melanoma metastasis model syngeneic to the C57BL/6 mouse strain. ATX expression was knocked down using a lentivirally delivered shRNA (small hairpin RNA) construct (ShATX, Figure 2). ATX-KD (knockdown) reduced the expression of ATX mRNA and protein (Figures 2A and 2B) and also reduced the lysophospholipase D activity secreted into the conditioned medium compared with wild-type B16 or Scr (scrambled) shRNA-transduced cells (Figure 2C). We examined the effect of ATX-KD using a transcellular migration assay described previously by Mukai et al. [36]. Briefly, ShATX and Scr shRNA transduced cells (105 cells/ml) were seeded on to a confluent rat mesothelial cell monolayer and increasing concentrations of LPC (lysophosphatidylcholine) or 3 μM LPA were added. After 6 h of incubation, the medium was removed and the monolayer was washed and fixed. The number of B16 melanoma cells that penetrated the mesothelial monolayer was counted under a phase-contrast microscope. LPA treatment increased the transmonolayer invasion of the ShATX and control cells (Figure 2D). Addition of the ATX substrate LPC to Scr shRNA-transduced melanoma cells dose-dependently increased invasion. In contrast, LPC failed to induce invasion of the ShATX-transduced melanoma cells. These observations support the hypothesis that down-regulation of ATX activity in the tumour cells alters their invasive capacity.
Figure 2. Down-regulation of ATX in B16-F10 melanoma cells inhibits invasion in vitro and metastasis in vivo.
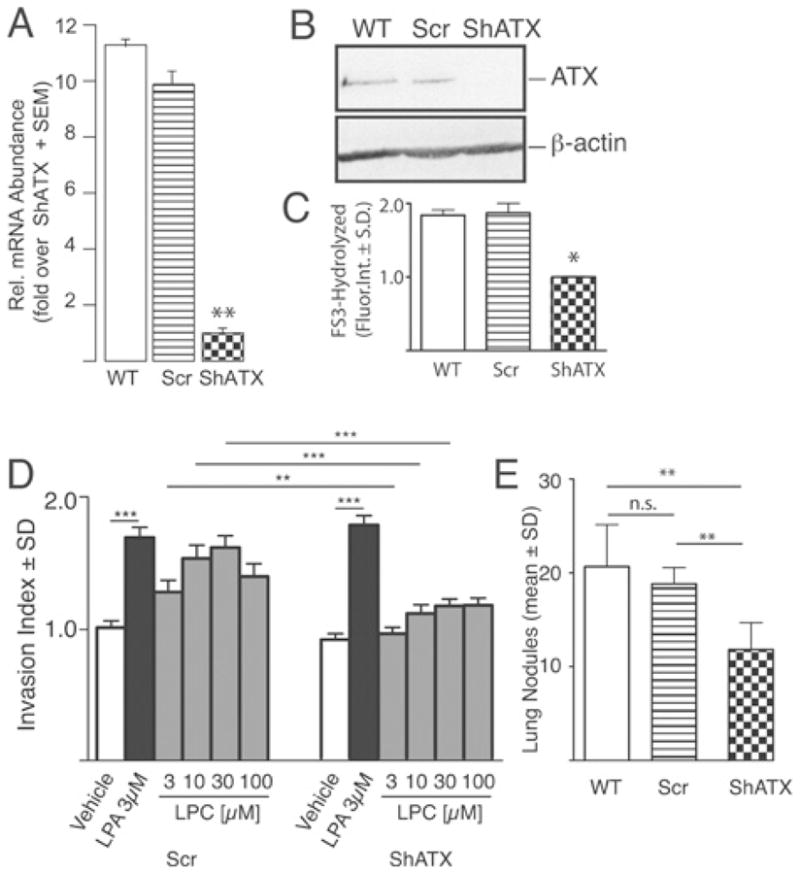
B16 cells were transduced with lentivirus encoding a shRNA to ATX (ShATX) or an Scr construct. mRNA level (A), ATX protein expression (B) and ATX catalytic activity in 48-h conditioned medium measured using FS-3 substrate (C) were all down-regulated in ShATX cells compared with Scr and wild-type (WT) B16-F10 cells. Scr cells showed an LPC dose-dependent increase in invasion of rat mesothelial monolayers, whereas ShATX cells failed to respond to LPC (D). Both cell types responded equally to LPA with increased invasion of the monolayer. When injected into C57BL/6 mice (5×104/cell per mouse in 100 μl) via the tail vein, ShATX cells yielded 50% fewer metastases compared with Scr and WT B16-F10 melanoma cells (E). n.s., not significant; *P < 0.05; **P < 0.01; P < 0.001. Reproduced from [43] Gupte, R., Patil, R., Liu, J., Wang, Y., Lee, S.C., Fujiwara, Y., Fells, J., Bolen, A.L., Emmons-Thompson, K., Yates, C.R., Siddam, A., Panupinthu, N., Pham, T.C., Baker, D.L., Parrill, A.L., Mills, G.B., Tigyi, G. and Miller, D.D. (2011) Benzyl and naphthalene methylphosphonic acid inhibitors of autotaxin with anti-invasive and anti-metastatic activity. ChemMedChem 6, 922–935. Copyright Wiley-VCH Verlag GmbH & Co. KGaA with permission.
To extend these in vitro observations, we injected 5×105 Scr- and ShATX-transduced B16 melanoma cells into C57BL/6 mice via the tail vein. At 3 weeks post-inoculation, animals were killed, the lungs were washed, inflated and fixed with formalin, and the number of lung metastases was determined. Melanoma cells with ATX-KD showed a 50% reduction in the number of metastasis compared with control cells (Figure 2E). This observation reinforces our in vitro findings and supports a role for ATX in metastasis.
Drug discovery targeting ATX
On the basis of the relevance of the ATX–LPA–LPA receptor signalling axis to many types of cancers, it is reasonable to propose drug discovery aimed at ATX and LPA receptors, or the combination of these targets to prevent and inhibit cancer invasion, metastasis and growth. At present, there are no FDA (U.S. Food and Drug Administration)-approved drugs to either of these targets. ATX is continuously turning over with a half-life estimated to be less than 1 h [37]. The half-life of LPA injected into blood is of the order of a few minutes, indicating that this pool of LPA is rapidly turning over [38]. These two properties taken together suggest that ATX has to be inhibited for a long duration in order to significantly affect LPA production. We envisage that the drug candidates targeting ATX and/or LPA receptors will ultimately be used as part of the multimodal treatment of cancers for the prevention of metastasis before or after removal of the primary tumour. We must not ignore the fact that ATX inhibitors have therapeutic utility in controlling inflammatory diseases as well, because of the documented role of ATX in autoimmunity [39], asthma [40], macular degeneration [41] and neuropathic pain [42].
We reported recently the characterization of a new ATX inhibitor 4PBPA (4-pentadecylbenzylphosphonic acid) [43]. 4PBPA has a relatively long (10.5 h) half-life and it reduced plasma LPA levels by 50% over a 24-h period [43]. This inhibitor was tested on the invasion of confluent monolayers of HUVECs (human umbilical vein endothelial cells) by MM1 hepatoma cells in 1% low-serum medium with or without 5 μM LPC (Figure 3A). MM1 cells express ATX, whereas the mesothelium and HUVECs show very low expression, and we were unable to detect ATX activity in HUVEC-conditioned medium [43]. Thus the source of ATX in this system is primarily from the MM1 carcinoma cells. Addition of LPC increased invasion significantly, whereas 4PBPA dose-dependently blocked the LPC-induced invasion of MM1 cells on the HUVEC monolayers. These types of monolayer invasion assays have been considered as excellent in vitro models for cancer invasion because the invading cells must penetrate and migrate below the monolayer, resembling the process that takes place during metastasis [36]. We also examined the effect of 4PBPA or vehicle using the B16 melanoma metastasis model in vivo. Daily injections starting 30 min after melanoma inoculation were continued until day 20. On day 21, mice were killed, the lungs were harvested, and the number of melanoma nodules in the lungs was counted. In this treatment paradigm, 4PBPA reduced the number of metastases from an average of 42 per mouse in the vehicle group to eight per mouse (Figure 3B).
Figure 3. Pharmacological inhibition of ATX blocks tumour cell invasion in vitro and seeding of metastasis in vivo.
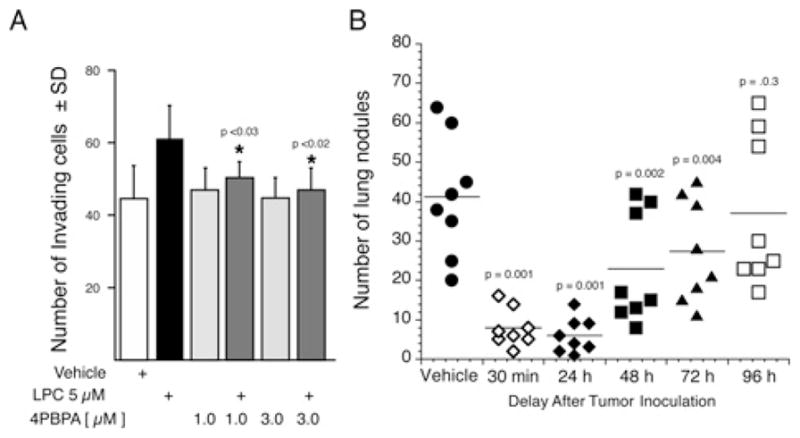
The benzyl-methyl phosphonate ATX inhibitor 4PBPA inhibits the invasion of HUVEC monolayers by MM1 rat hepatocarcinoma cells induced by 5 μM LPC added to the medium (A). 4PBPA does not inhibit tumour growth, but reduces the number of metastases when administered up to 72 h post-inocculation of the tumour cells. 4PBPA (1 mg/kg) was injected daily starting at different times after tumour inoculation (7.5×104 cells/mouse) and continued up to day 20. The number of lung metastases was counted on day 21. Note that 4PBPA yields very effective protection against the establishment of micrometastases even when administration began at 48 h after inoculation of B16-F10 melanoma cells via the tail vein. Reproduced from [43] Gupte, R., Patil, R., Liu, J., Wang, Y., Lee, S.C., Fujiwara, Y., Fells, J., Bolen, A.L., Emmons-Thompson, K., Yates, C.R., Siddam, A., Panupinthu, N., Pham, T.C., Baker, D.L., Parrill, A.L., Mills, G.B., Tigyi, G. and Miller, D.D. (2011) Benzyl and naphthalene methylphosphonic acid inhibitors of autotaxin with anti-invasive and anti-metastatic activity. ChemMedChem 6, 922–935. Copyright Wiley-VCH Verlag GmbH & Co. KGaA with permission.
To examine the role of ATX inhibition on the micro-to-macro metastasis transition, we performed the same experiment by delaying the first treatment with 4PBPA from 30 min to 96-h post-inoculation. The treatment continued with single daily doses of 4PBPA until day 20. The mice were killed on day 21 and the lung metastases were counted. 4PBPA injection starting up to 48 h post-inocculation caused a highly significant reduction in the number of metastases. However, when the treatment started at 72 h post-inocculation, the reduction of metastases, although significant, began to diminish and when treatment began on 96 h after inoculation it was no longer effective (Figure 3B). These results point to an important limitation of ATX inhibition therapy and indicate that blocking of ATX activity only reduces the seeding of metastases, but does not inhibit tumour growth. These results with the melanoma model corroborate similar findings obtained in a breast cancer metastasis model by the Peyruchaud group [44].
Dual targeting of ATX and LPA receptors controls metastasis and tumour growth
LPA exerts feedback inhibition on ATX [45]. This observation leads to the concept that one should be able to find LPA analogues that inhibit ATX without activating any of the LPA receptor subtypes. We obtained proof of this principle through the development of carba-cyclic phosphatidic acid analogues that inhibited ATX without the activation of LPA1–4. Carba-cyclic phosphatidic acid inhibited MM1 carcinoma and A2058 melanoma invasion in vitro and also reduced the number of B16 melanoma metastasis in mice [46–48]. In collaboration with the Prestwich group, we have characterized several lipid analogues of LPA that inhibit ATX and also function as antagonists of multiple LPA receptors [49]. One of the LPA bromophosphonate enantiomers was highly effective in blocking the metastasis and growth of xenotransplanted human breast, colon and liver carcinomas in nude mice [49,50].
The studies outlined in the present review provide the foundation for drug discovery targeting the ATX–LPA–LPA receptor signalling axis. The lack of phenotype in heterozygous ATX-KO mice with 50% lower plasma LPA levels and the initial data using ATX inhibitors over extended periods of time for the treatment of tumour-bearing mice revealed no obvious side effects. There are numerous publications that have appeared in the literature during the last 2 years describing multiple ATX inhibitors. The race is on now to find the one that has ideal pharmacokinetics and bioavailability with minimal side effects. We predict that, within the next few years, many new chemical scaffolds that block ATX and LPA receptors will be identified. Today, at the dawn of personalized medicine, we envisage that combination treatment protocols consisting of a long-acting ATX inhibitor and individual LPA receptor antagonists matching the expression profile of the patient’s malignancy will emerge as valuable modalities of cancer treatment and metastasis control.
Acknowledgments
Funding
This work was supported by the National Institutes of Health [grant number CA092160 (to G.J.T.)]. G.J.T. is a founder and stockholder in RxBio Inc.
Abbreviations used
- ATX
autotaxin
- EDG
endothelial differentiation gene
- GPCR
G-protein-coupled receptor
- HUVEC
human umbilical vein endothelial cell
- KD
knockdown
- KO
knockout
- LPA
lysophosphatidic acid
- LPC
lysophosphatidylcholine
- NPP
nucleotide pyrophosphatase/phosphodiesterase
- 4PBA
4-pentadecylbenzylphosphonic acid
- Scr
scrambled
- shRNA
small hairpin RNA
- siRNA
small interfering RNA
- uPA
urokinase-type plasminogen activator
- VEGF
vascular endothelial growth factor
References
- 1.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 2.Tigyi G. Aiming drug discovery at lysophosphatidic acid targets. Br J Pharmacol. 2010;161:241–270. doi: 10.1111/j.1476-5381.2010.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stracke ML, Krutzsch HC, Unsworth EJ, Arestad A, Cioce V, Schiffmann E, Liotta LA. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992;267:2524–2529. [PubMed] [Google Scholar]
- 4.Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277:39436–39442. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- 5.Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26:5015–5022. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281:25822–25830. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- 8.Baker DL, Morrison P, Miller B, Riely CA, Tolley B, Westermann AM, Bonfrer JM, Bais E, Moolenaar WH, Tigyi G. Plasma lysophosphatidic acid concentration and ovarian cancer. JAMA, J Am Med Assoc. 2002;287:3081–3082. doi: 10.1001/jama.287.23.3081. [DOI] [PubMed] [Google Scholar]
- 9.Sutphen R, Xu Y, Wilbanks GD, Fiorica J, Grendys EC, Jr, LaPolla JP, Arango H, Hoffman MS, Martino M, Wakeley K, et al. Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1185–1191. [PubMed] [Google Scholar]
- 10.Nakagawa H, Ikeda H, Nakamura K, Ohkawa R, Masuzaki R, Tateishi R, Yoshida H, Watanabe N, Tejima K, Kume Y, et al. Autotaxin as a novel serum marker of liver fibrosis. Clin Chim Acta. 2011;412:1201–1206. doi: 10.1016/j.cca.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Aoki J, Hama K, Okudaira S, Tanaka M, Tomiya T, et al. Both plasma lysophosphatidic acid and serum autotaxin levels are increased in chronic hepatitis C. J Clin Gastroenterol. 2007;41:616–623. doi: 10.1097/01.mcg.0000225642.90898.0e. [DOI] [PubMed] [Google Scholar]
- 12.Iwasawa Y, Fujii T, Nagamatsu T, Kawana K, Okudaira S, Miura S, Matsumoto J, Tomio A, Hyodo H, Yamashita T, et al. Expression of autotaxin, an ectoenzyme that produces lysophosphatidic acid, in human placenta. Am J Reprod Immunol. 2009;62:90–95. doi: 10.1111/j.1600-0897.2009.00715.x. [DOI] [PubMed] [Google Scholar]
- 13.Kimura T, Itoh T, Fusazaki T, Matsui H, Sugawara S, Ogino Y, Endo H, Kobayashi K, Nakamura M. Low-density lipoprotein-cholesterol/high-density lipoprotein-cholesterol ratio predicts lipid-rich coronary plaque in patients with coronary artery disease: integrated-backscatter intravascular ultrasound study. Circ J. 2010;74:1392–1398. doi: 10.1253/circj.cj-09-0849. [DOI] [PubMed] [Google Scholar]
- 14.Dimova I, Raitcheva S, Dimitrov R, Doganov N, Toncheva D. Correlations between c-myc gene copy-number and clinicopathological parameters of ovarian tumours. Eur J Cancer. 2006;42:674–679. doi: 10.1016/j.ejca.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Shen Z, Wiper DW, Wu M, Morton RE, Elson P, Kennedy AW, Belinson J, Markman M, Casey G. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA, J Am Med Assoc. 1998:719–723. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- 16.Masuda A, Nakamura K, Izutsu K, Igarashi K, Ohkawa R, Jona M, Higashi K, Yokota H, Okudaira S, Kishimoto T, et al. Serum autotaxin measurement in haematological malignancies: a promising marker for follicular lymphoma. Br J Haematol. 2008;143:60–70. doi: 10.1111/j.1365-2141.2008.07325.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakai Y, Ikeda H, Nakamura K, Kume Y, Fujishiro M, Sasahira N, Hirano K, Isayama H, Tada M, Kawabe T, et al. Specific increase in serum autotaxin activity in patients with pancreatic cancer. Clin Biochem. 2011;44:576–581. doi: 10.1016/j.clinbiochem.2011.03.128. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K, Kishimoto T, Ohkawa R, Okubo S, Tozuka M, Yokota H, Ikeda H, Ohshima N, Mizuno K, Yatomi Y. Suppression of lysophosphatidic acid and lysophosphatidylcholine formation in the plasma in vitro: proposal of a plasma sample preparation method for laboratory testing of these lipids. Anal Biochem. 2007;367:20–27. doi: 10.1016/j.ab.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Bolen AL, Naren AP, Yarlagadda S, Beranova-Giorgianni S, Chen L, Norman D, Baker DL, Rowland MM, Best MD, Sano T, et al. The phospholipase A1 activity of lysophospholipase A-I links platelet activation to LPA production during blood coagulation. J Lipid Res. 2011;52:958–970. doi: 10.1194/jlr.M013326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Euer N, Schwirzke M, Evtimova V, Burtscher H, Jarsch M, Tarin D, Weidle UH. Identification of genes associated with metastasis of mammary carcinoma in metastatic versus non-metastatic cell lines. Anticancer Res. 2002;22:733–740. [PubMed] [Google Scholar]
- 21.Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang F, Yu S, Stephens LC, Cui X, Murrow G, et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 2009;15:539–550. doi: 10.1016/j.ccr.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samadi N, Gaetano C, Goping IS, Brindley DN. Autotaxin protects MCF-7 breast cancer and MDA-MB-435 melanoma cells against Taxol-induced apoptosis. Oncogene. 2009;28:1028–1039. doi: 10.1038/onc.2008.442. [DOI] [PubMed] [Google Scholar]
- 23.ES, Lai YJ, Tsukahara R, Chen CS, Fujiwara Y, Yue J, Yu JH, Guo H, Kihara A, Tigyi G, Lin FT. Lysophosphatidic acid 2 receptor-mediated supramolecular complex formation regulates its antiapoptotic effect. J Biol Chem. 2009;284:14558–14571. doi: 10.1074/jbc.M900185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jazaeri AA, Awtrey CS, Chandramouli GV, Chuang YE, Khan J, Sotiriou C, Aprelikova O, Yee CJ, Zorn KK, Birrer MJ, et al. Gene expression profiles associated with response to chemotherapy in epithelial ovarian cancers. Clin Cancer Res. 2005;11:6300–6310. doi: 10.1158/1078-0432.CCR-04-2682. [DOI] [PubMed] [Google Scholar]
- 25.Vidot S, Witham J, Agarwal R, Greenhough S, Bamrah HS, Tigyi GJ, Kaye SB, Richardson A. Autotaxin delays apoptosis induced by carboplatin in ovarian cancer cells. Cell Signalling. 2010;22:926–935. doi: 10.1016/j.cellsig.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Müller R, Berliner C, Leptin J, Pörtner D, Bialecki W, Kleuser B, Schumacher U, Milićević NM. Expression of sphingosine-1-phosphate receptors and lysophosphatidic acid receptors on cultured and xenografted human colon, breast, melanoma, and lung tumor cells. Tumour Biol. 2010;31:341–349. doi: 10.1007/s13277-010-0043-7. [DOI] [PubMed] [Google Scholar]
- 27.Boucharaba A, Serre CM, Guglielmi J, Bordet JC, Clezardin P, Peyruchaud O. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc Natl Acad Sci USA. 2006;103:9643–9648. doi: 10.1073/pnas.0600979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SY, Jeong KJ, Panupinthu N, Yu S, Lee J, Han JW, Kim JM, Lee JS, Kang J, Park CG, et al. Lysophosphatidic acid augments human hepatocellular carcinoma cell invasion through LPA1 receptor and MMP-9 expression. Oncogene. 2010;30:1351–1359. doi: 10.1038/onc.2010.517. [DOI] [PubMed] [Google Scholar]
- 29.So J, Wang FQ, Navari J, Schreher J, Fishman DA. LPA-induced epithelial ovarian cancer (EOC) in vitro invasion and migration are mediated by VEGF receptor-2 (VEGF-R2) Gynecol Oncol. 2005;97:870–878. doi: 10.1016/j.ygyno.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Ptaszynska MM, Pendrak ML, Bandle RW, Stracke ML, Roberts DD. Positive feedback between vascular endothelial growth factor-A and autotaxin in ovarian cancer cells. Mol Cancer Res. 2008;6:352–363. doi: 10.1158/1541-7786.MCR-07-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pustilnik TB, Estrella V, Wiener JR, Mao M, Eder A, Watt MA, Bast RC, Jr, Mills GB. Lysophosphatidic acid induces urokinase secretion by ovarian cancer cells. Clin Cancer Res. 1999;5:3704–3710. [PubMed] [Google Scholar]
- 32.Murthi P, Barker G, Nowell CJ, Rice GE, Baker MS, Kalionis B, Quinn MA. Plasminogen fragmentation and increased production of extracellular matrix-degrading proteinases are associated with serous epithelial ovarian cancer progression. Gynecol Oncol. 2004;92:80–88. doi: 10.1016/j.ygyno.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Lee Z, Cheng CT, Zhang H, Subler MA, Wu J, Mukherjee A, Windle JJ, Chen CK, Fang X. Role of LPA4/p2y9/GPR23 in negative regulation of cell motility. Mol Biol Cell. 2008;19:5435–5445. doi: 10.1091/mbc.E08-03-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hausmann J, Kamtekar S, Christodoulou E, Day JE, Wu T, Fulkerson Z, Albers HM, van Meeteren LA, Houben AJ, van Zeijl L, et al. Structural basis of substrate discrimination and integrin binding by autotaxin. Nat Struct Mol Biol. 2011;18:198–204. doi: 10.1038/nsmb.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishimasu H, Okudaira S, Hama K, Mihara E, Dohmae N, Inoue A, Ishitani R, Takagi J, Aoki J, Nureki O. Crystal structure of autotaxin and insight into GPCR activation by lipid mediators. Nat Struct Mol Biol. 2011;18:205–212. doi: 10.1038/nsmb.1998. [DOI] [PubMed] [Google Scholar]
- 36.Mukai M, Nakamura H, Tatsuta M, Iwasaki T, Togawa A, Imamura F, Akedo H. Hepatoma cell migration through a mesothelial cell monolayer is inhibited by cyclic AMP-elevating agents via a Rho-dependent pathway. FEBS Lett. 2000;484:69–73. doi: 10.1016/s0014-5793(00)02129-3. [DOI] [PubMed] [Google Scholar]
- 37.Jansen S, Andries M, Vekemans K, Vanbilloen H, Verbruggen A, Bollen M. Rapid clearance of the circulating metastatic factor autotaxin by the scavenger receptors of liver sinusoidal endothelial cells. Cancer Lett. 2009;284:216–221. doi: 10.1016/j.canlet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 38.Albers HM, Dong A, van Meeteren LA, Egan DA, Sunkara M, van Tilburg EW, Schuurman K, van Tellingen O, Morris AJ, Smyth SS, et al. Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation. Proc Natl Acad Sci USA. 2010;107:7257–7262. doi: 10.1073/pnas.1001529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanda H, Newton R, Klein R, Morita Y, Gunn MD, Rosen SD. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat Immunol. 2008;9:415–423. doi: 10.1038/ni1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuzaki S, Ishizuka T, Hisada T, Aoki H, Komachi M, Ichimonji I, Utsugi M, Ono A, Koga Y, Dobashi K, et al. Lysophosphatidic acid inhibits CC chemokine ligand 5/RANTES production by blocking IRF-1-mediated gene transcription in human bronchial epithelial cells. J Immunol. 2010;185:4863–4872. doi: 10.4049/jimmunol.1000904. [DOI] [PubMed] [Google Scholar]
- 41.Im E, Motiejunaite R, Aranda J, Park EY, Federico L, Kim TI, Clair T, Stracke ML, Smyth S, Kazlauskas A. Phospholipase Cγ activation drives increased production of autotaxin in endothelial cells and lysophosphatidic acid-dependent regression. Mol Cell Biol. 2010;30:2401–2410. doi: 10.1128/MCB.01275-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inoue M, Ma L, Aoki J, Chun J, Ueda H. Autotaxin, a synthetic enzyme of lysophosphatidic acid (LPA), mediates the induction of nerve-injured neuropathic pain. Mol Pain. 2008;4:6. doi: 10.1186/1744-8069-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupte R, Patil R, Liu J, Wang Y, Lee SC, Fujiwara Y, Fells J, Bolen AL, Emmons-Thompson K, Yates CR, et al. Benzyl and naphthalene methylphosphonic acid inhibitors of autotaxin with anti-invasive and anti-metastatic activity. ChemMedChem. 2011;6:922–935. doi: 10.1002/cmdc.201000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.David M, Wannecq E, Descotes F, Jansen S, Deux B, Ribeiro J, Serre CM, Gres S, Bendriss-Vermare N, Bollen M, et al. Cancer cell expression of autotaxin controls bone metastasis formation in mouse through lysophosphatidic acid-dependent activation of osteoclasts. PLoS ONE. 2010;5:e9741. doi: 10.1371/journal.pone.0009741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Meeteren LA, Ruurs P, Christodoulou E, Goding JW, Takakusa H, Kikuchi K, Perrakis A, Nagano T, Moolenaar WH. Inhibition of autotaxin by lysophosphatidic acid and sphingosine 1-phosphate. J Biol Chem. 2005;280:21155–21161. doi: 10.1074/jbc.M413183200. [DOI] [PubMed] [Google Scholar]
- 46.Baker DL, Fujiwara Y, Pigg KR, Tsukahara R, Kobayashi S, Murofushi H, Uchiyama A, Murakami-Murofushi K, Koh E, Bandle RW, et al. Carba analogs of cyclic phosphatidic acid are selective inhibitors of autotaxin and cancer cell invasion and metastasis. J Biol Chem. 2006;281:22786–22793. doi: 10.1074/jbc.M512486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uchiyama A, Mukai M, Fujiwara Y, Kobayashi S, Kawai N, Murofushi H, Inoue M, Enoki S, Tanaka Y, Niki T, et al. Inhibition of transcellular tumor cell migration and metastasis by novel carba-derivatives of cyclic phosphatidic acid. Biochim Biophys Acta. 2007;1771:103–112. doi: 10.1016/j.bbalip.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupte R, Siddam A, Lu Y, Li W, Fujiwara Y, Panupinthu N, Pham TC, Baker DL, Parrill AL, Gotoh M, et al. Synthesis and pharmacological evaluation of the stereoisomers of 3-carba cyclic-phosphatidic acid. Bioorg Med Chem Lett. 2010;20:7525–7528. doi: 10.1016/j.bmcl.2010.09.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Xu X, Gajewiak J, Tsukahara R, Fujiwara Y, Liu J, Fells JI, Perygin D, Parrill AL, Tigyi G, Prestwich GD. Dual activity lysophosphatidic acid receptor pan-antagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo. Cancer Res. 2009;69:5441–5449. doi: 10.1158/0008-5472.CAN-09-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu X, Prestwich GD. Inhibition of tumor growth and angiogenesis by a lysophosphatidic acid antagonist in an engineered three-dimensional lung cancer xenograft model. Cancer. 2010;116:1739–1750. doi: 10.1042/BST20110608. Received 12 May 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


