Abstract
Populations of Drosophila melanogaster face significant mortality risks from parasitoid wasps that use species-specific strategies to locate and survive in hosts. We tested the hypothesis that parasitoids with different strategies select for alternative host defense characteristics and in doing so contribute to the maintenance of fitness variation and produce trade-offs among traits. We characterized defense traits of Drosophila when exposed to parasitoids with different host searching behaviors (Aphaereta sp. and Leptopilina boulardi). We used host larvae with different natural alleles of the gene Dopa decarboxylase (Ddc), a gene controlling the production of dopamine and known to influence the immune response against parasitoids. Previous population genetic analyses indicate that our focal alleles are maintained by balancing selection. Genotypes exhibited a trade-off between the immune response against Aphaereta sp. and the ability to avoid parasitism by L. boulardi. We also identified a trade off between the ability to avoid parasitism by L. boulardi and larval competitive ability as indicated by differences in foraging and feeding behavior. Genotypes differed in dopamine levels potentially explaining variation in these traits. Our results highlight the potential role of parasitoid biodiversity on host fitness variation and implicate Ddc as an antagonistic pleiotropic locus influencing larval fitness traits.
Keywords: adaptation, pleiotropy, parasitism, genetic variation, behavior, polymorphism
The composition of species in communities not only shapes ecological interactions it also influences the genetic characteristics of interacting populations. The evolutionary importance of these interactions has been very well demonstrated in plant-herbivore (Lankau and Strauss 2008, Agrawal 2011, Winde and Wittstock 2011) and host-parasite systems (Gandon and Van Zandt 1998, Lively and Dybdahl 2000, Gandon et al. 2008) where the fitness of the attacking species (herbivore or parasite) is completely dependent on the ability to find and use a suitable host. In these systems we expect strong selection on traits that both reduce the probability of attack and the damaging effects of the attacker. From the perspective of the host, defense against attack is especially challenging when different species must be defended against (Futuyma and Mitter 1996). Futuyma and Mitter outline two potential scenarios that can result from these interactions. If similar defenses are effective against a range of potential attackers, then positive genetic correlations among defense traits are expected. Alternatively, if one defense trait is useful against one attacker, but decreases defense against another, then negative correlations among such traits are expected (Futuyma and Mitter 1996). Thus, the biodiversity of species attacking hosts in a community can have important influences on the genetic characteristics of host species, potentially resulting in correlations among traits.
Genetic correlations are one of the more important genetic characteristics that influence evolution (Rose 1982), Lande and Arnold 1983, Rose 1985, Arnold 1992, Price et al. 1993, Scarcelli et al. 2007, Gratten et al. 2008). When genetically correlated traits influence fitness they can influence phenotypic evolution in at least three ways. First, selection on one trait can cause evolutionary changes in traits that are not the targets of selection (Leroi et al. 2005, Roff and Fairbairn 2007). Indeed, this could explain many correlated patterns of morphological, behavioral and life history diversification among populations and species (Armbruster 1991, Promislow 1995, Schluter 1996, Steppan et al. 2002, Baker and Wilkinson 2003 but see Baer and Lynch 2003). Second, genetic correlations can act as a constraining force in evolution, limiting the adaptive independent evolution of traits, or at a minimum, constrain the rate and/or evolutionary trajectory of interrelated traits (Lande 1982, Arnold 1992, Schluter 1996, Houle 2001, Roff and Fairbairn 2007 but see Conner et al. 2011). Third, correlations can contribute to the maintenance of variation in fitness through antagonistic pleiotropy (Rose 1982, 1985) or through the general effects of balancing selection acting on traits influenced by pleiotropic loci (Turelli and Barton 2004).
While theoretical models of the evolutionary consequences of genetic correlations are well developed, fundamental questions remain that only empirical data can address. Which traits are genetically correlated and so might be expected to exhibit correlated evolutionary changes? What are the fitness effects of allelic variation at pleiotropic loci and how is this variation maintained? What is the nature of selection acting on correlated traits (and by extension pleiotropic genes) and how do the combined effects of selection and correlation influence phenotypic evolution? While numerous studies provide evidence for the importance of pleiotropy in evolution (Williams 1957, Rose and Charlesworth 1981, Harshman and Hoffmann 2000, Bucan and Abel 2002, Featherstone and Brodie 2002, Anholt and Mackay 2004, Hughes and Reynolds 2005, Kenney-Hunt et al. 2006, Roff and Fairbairn 2007), studies of the pleiotropic effects of natural allelic variation at individual genes (Leroi et al. 2005, Carbone et al. 2006, Scarcelli et al. 2007, Tellier et al. 2007, Anderson et al. 2012) are needed to address the above questions (Leroi et al. 2005).
The host parasitoid interactions of Drosophila melanogaster provide an excellent system to address these questions for three main reasons. First, a number of different species of parasitoids use Drosophila melanogaster as hosts in natural populations (Carton et al. 1986, Allemand et al. 2002, Mitsui et al. 2007). Because many parasitoids use different strategies to locate and survive in hosts (Kraaijeveld and Godfray 2009, Lee et al. 2009), this sets up the conditions that could produce trade-offs in host defense traits (Futuyma and Mitter 1996). Second, parasitoids impose a significant mortality risk for Drosophila larvae, with larval mortality from parasitoids exceeding 50% in some populations (Janssen et al. 1988, Fleury et al. 2004). Thus parasitoids are potentially important agents of natural selection. Finally, as Drosophila are model genetic organisms, we have the potential to identify the genes influencing these traits including those that give rise to trade-offs.
In this study we ask if larval defense traits of D. melanogaster exhibit genetic correlations when confronted with two species of parasitoids, Leptopilina boulardi and Aphaereta sp., that use different behaviors to locate their hosts. In addition, we used a set of fly stocks derived from a natural population to examine the potential pleiotropic effects of polymorphism in the gene Dopa decarboxylase, (Ddc) on larval traits. Ddc encodes DOPA decarboxylase (DDC) an important enzyme in the catecholamine biosynthesis pathway that produces two neurotransmitters, dopamine and serotonin (Christensen et al. 1972, Livingstone and Tempel 1983, Coleman and Neckameyer 2005). Dopamine and serotonin are key players in reproductive, developmental, behavioral, and immune processes in vertebrates and invertebrates (Neckameyer 1998, Goodson et al. 2009, Schweitzer and Driever 2009, Strell et al. 2009). De Luca et al. 2003 found evidence for balancing selection at this locus, predominantly between two haplotypes associated with longevity. These haplotypes are formed via three naturally occurring single nucleotide polymorphisms (SNPs), a T/C polymorphism in the promoter region, C/A polymorphism in exon 2 and T/G polymorphism in intron 3. Assuming that selection is not acting directly on life span (Hamilton 1966, we hypothesized that balancing selection is acting on one or more traits that are genetically correlated with life span and that may also be influenced by polymorphism in Ddc.
There is good reason to suspect that polymorphism in Ddc could affect immunological and non-immunological larval traits in Drosophila. Immunological defense against parasitoids involves the encapsulation and melanization of parasitoid eggs by circulating blood cells of Drosophila larva (Vass and Nappi 2000), killing the developing wasp (Vass and Nappi 2000, Nappi and Christensen 2005). Dopamine is a precursor to melanin production in invertebrates (Nappi and Christensen 2005, Hodgetts and O'Keefe 2006) and indeed, variation in Ddc expression affects the melanotic encapsulation response against the eggs of parasitoid wasps (Nappi et al. 1992, Schlenke et al. 2007). As a result we hypothesized that Ddc polymorphism could affect the melanotic encapsulation of wasp eggs, potentially by altering dopamine levels.
The non-immunological defense of fly larvae is to avoid being attacked and many aspects of fly larval behavior, such as foraging, rolling, and digging influence the likelihood of parasitoid attack (Carton and David 1983, Carton and Sokolowski 1992, Kraaijeveld and van Alphen 1995). As a neurotransmitter, dopamine affects locomotory behavior (Pendleton et al. 2002, Pendleton et al. 2005, Jordan et al. 2006, Vermeulen et al. 2006) so we hypothesized that Ddc polymorphism may also contribute to variation in fly larval behaviors under selection by parasitoids.
Artificial selection experiments have revealed genetically based tradeoffs between immunological and non-immunological traits like the ones mentioned above. Flies that have been artificially selected for increased parasitoid resistance against Asobara tabida and L. boulardi have reduced larval competitive ability under low resource conditions compared to unselected control populations (Kraaijeveld and Godfray 1997, Fellowes et al. 1998). Given that immune response and competitive ability are negatively correlated, we hypothesized that if Ddc polymorphism affects the immune response against parasitoids, it may also contribute to the trade-off between melanotic encapsulation ability and larval competitive ability. To test this hypothesis we measured the feeding rate of the different Ddc genotypes because feeding rate has repeatedly been shown to be indicative of competitive ability in Drosophila (Sewell et al. 1975, Burnet et al. 1977, Joshi and Mueller 1988, 1996).
Materials and Methods
FLY LINES
We selected six Chromosome II (CII) extraction lines derived from the natural population of D. melanogaster in Raleigh, NC (creation of lines are described in detail in De Luca et al. 2003). This extraction procedure resulted in homozygous lines that were genetically identical for genes on the first and third chromosomes but which differed by the origin of the 2nd chromosome.
Three of the lines chosen had a Ddc haplotype that was associated with long-lived flies (CAT) and the other three lines had the alternative SNP haplotype associated with shorter lifespan (TCG). We selected these two haplotypes because, of the seven Ddc haplotypes found segregating in this population, these two were at high frequency and were the only two haplotypes found at significantly higher frequencies than expected under neutrality (De Luca et al. 2003). We used the offspring of crosses between these lines to measure several larval fitness traits, two of which (larval foraging and melanotic encapsulation ability) are related to defense against parasitoids that commonly parasitize larval D. melanogaster in Maryland, L. boulardi and Aphaereta sp. (Hodges, unpublished data).
CROSSING DESIGN TO CREATE EXPERIMENTAL GENOTYPES
We carried out six reciprocal homozygote crosses (each line served as both sire and dam) crossing each of the three lines of each Ddc haplotype to each other in a crossing design similar to that of Geiger-Thornsberry and Mackay (2002). We created heterozygous larvae by crossing two of the lines with the long-lived (CAT) Ddc haplotype to two lines with the short-lived (TCG) Ddc haplotype, also in a reciprocal crossing design. We found no significant maternal or paternal (dam or sire) effect for any trait measured, so we used the pooled data from each reciprocal cross for analyses of the data.
We used the offspring of crosses among the lines rather than testing the inbred lines to minimize the influence of inbreeding depression while at the same time allowing us to examine the average effects of different Ddc genotypes on fitness traits in otherwise outbred backgrounds (Geiger-Thornsberry and Mackay 2002).
We performed phenotypic assays with 2nd instar larvae because this is the developmental stage most efficiently parasitized by parasitoids (van Alphen and Drijver 1982).
PARASITOID COLLECTION AND MAINTENANCE
We established laboratory colonies of Aphaereta sp. and L. boulardi from wasps collected at two local sites, Boordy Vineyards (Hyde, MD) and Boyer Farms (Severn, MD). Wasp colonies were maintained using larvae of an inbred D. melanogaster laboratory strain (Samarkand). Adult wasps were fed a 30% honey solution and maintained as large populations (> 300 individuals) in 12” X 12” X 12” plexiglass cages at 18°C on a 12-hour light/dark cycle.
PARASITOID IDENTIFICATION
The identity of L. boulardi was determined morphologically (and confirmed by Matthew Buffington, Smithsonian Institution). To identify Aphaereta sp., we sequenced the D2 region of the 28S ribosomal RNA (rRNA). We obtained the D2 sequence from three different individual wasps using PCR conditions and primers from Gimeno et al. 1997 (Gimeno et al. 1997): 5’AGAGAGAGTTCAAGAGTACGTG3’ (forward primer) and 5’TTGGTCCGTGTTTCAAGACGGG3’ (reverse primer). Sequences were obtained using an ABI Prism 3100 Genetic Analyzer. Sequences were aligned to thirty-four 28S D2 rRNA sequences from Genbank (including those from the Alysiinae, Exothecinae and Opiinae subfamilies and Gnamptodon pumilo) and a 28S sequence of Aphaereta genevensis provided by Peter Mayhew (University of York, York, UK). Sequences were initially aligned using Sequencher 4.6 (Gene Codes Corp., Ann Arbor, Mich.) and further manually aligned using Mega 4.0 (Tamura et al. 2007). We used 381 base pairs of this sequence to construct a pairwise distance matrix among species using Mega 4.0. The D2 sequence from our samples of Aphaereta did not match any of the published sequences. The species most closely related to our Aphaereta sp. was A. minuta (evolutionary distance of 0.6%) and A. genevensis (evolutionary distance of 0.9%). The next closest relative to our Aphaereta sp. was Phaenocarpa sp. (evolutionary distance of 2.3%). Thus, to the best of our knowledge, this species has either not yet been described or its D2 sequence has not been published.
PARASITOID EXPOSURE ASSAY: LABORATORY
For each homozygote and heterozygote Ddc cross, we placed 30 2nd instar F1 larvae on a Petri dish containing 1% agar covered with a thin layer of standard fly food. This food layer allowed the larvae to evade visual inspection by the wasps. We exposed larvae on each plate for a four-hour period to a single female wasp with prior ovipositioning experience. A different female wasp was used for each exposure assay, with only one individual wasp exposed to each plate. Following exposure, D. melanogaster larvae were placed in incubators at 25°C or 29°C to test the effects of temperature on melanotic encapsulation efficiency. We found no effects of temperature on this process so we pooled the data from both temperature treatments for analysis. Two days after exposure we dissected the 3rd instar D. melanogaster larvae, scoring each for the presence of a wasp larva or a melanized encapsulated egg (both indications of parasitism). The ability to avoid parasitism was determined for each replicate plate and defined as the proportion of fly larvae on a plate that had not been parasitized in the four-hour period. The exposure assay was repeated for each cross until at least 30 larvae per cross per temperature had been parasitized. Melanotic encapsulation ability was scored as the proportion of parasitized larvae on a plate that had a melanotic encapsulated wasp egg. A total of 5,519 3rd instar larvae from 208 assays were dissected. In each of these cases the plate was the unit of replication for the statistical analyses (discussed below).
PARASITOID EXPOSURE ASSAY: FIELD
For each homozygote and heterozygote Ddc cross, we placed 30 2nd instar F1 larvae on a Petri dish containing 1% agar covered with a thin layer of slightly decayed homogenized peaches which had been colonized with natural bacteria and yeast from our field site. Plates containing larvae were transported in a Styrofoam container to Boyer Farms and positioned near baits that had been put out three days prior to the field assay to attract flies and wasps. We exposed 10 plates of larvae at a time (one plate of larvae from each cross), allowing a total exposure time of 40 "wasp-minutes" per plate (defined below), measured from the time that the first wasp landed and started probing for hosts on the plate. Preliminary experiments in the field indicated that a single wasp could parasitize about half of the larvae on a plate in 40 minutes (much more quickly than in the laboratory). Often more than one wasp would land on a plate and begin to probe for hosts in the field experiment. To standardize the time of larval exposure to parasitoids on each plate, we recorded the number of wasps parasitizing a single plate and the time that individual wasps began to probe for hosts. The amount of time that a plate was exposed to wasp(s) varied depending on the number of wasps searching on a plate. If only one wasp was searching, then the exposure time was forty minutes. If there were two wasps, then the time was adjusted to 20 minutes, and so on. The maximum number of wasps on a plate observed at one time was three. At the end of an exposure period for a plate, the wasp(s) were aspirated from the plate and placed in ethanol for identification (all were determined to be L. boulardi based on visual inspection and the fact that none of the fly larvae melanotically encapsulated the eggs). Fly larvae were returned to the lab, placed in an incubator for 48 hours at 25°C and then dissected and scored for parasitism and melanotic encapsulation. The total number of replicates ranged from 6 to 10 plates per cross and a total of 2,165 3rd instar larvae were dissected.
Because our laboratory experiments indicated that the Drosophila genotypes differed only in the probability of parasitism from L. boulardi and not Aphaereta sp. (see Results), we confined our field experiment to late summer, when L. boulardi is the dominant parasitoid. All field experiments were carried out from August through October.
DROSOPHILA LARVAL FORAGING BEHAVIOR ASSAY
For each homozygote and heterozygote Ddc cross, we measured foraging behavior of thirty 2nd instar F1 larvae using the method described in Sokolowski et al. (Sokolowski et al. 1997). Briefly, we placed a single larva in the center of a circular well (0.5 mm deep with an 8.25 cm radius) containing a 2:1 water:yeast mixture and then covered the well with a Petri dish. After five minutes of acclimation, we allowed larvae to forage on the plate for five minutes. The foraging path was then traced on the lid. We measured foraging behavior in a 25°C temperature-controlled room. Paths were photographed using a digital imager and path lengths measured using Metamorph software version 7.0.
DROSOPHILA LARVAL FEEDING RATES
For each cross, we measured the feeding rates of thirty 2nd instar F1 larvae using the number of cephalopharyngeal retractions in a 30 second period as an estimate of feeding rate (Joshi and Mueller 1988, Fellowes et al. 1999a). For each larva, we covered a petri dish containing 1% agar with a layer of 10% water:yeast mixture. We gently transferred the larva to the petri dish. After a 30-sec acclimatization period, we counted the number of cephalopharyngeal contractions made by the larva during a 30-second period. We measured feeding rates in a 25°C temperature-controlled room.
HPLC ANALYSIS OF DOPAMINE LEVELS
For each cross, we collected three samples containing 30–40 mg of 2nd instar larvae. Larvae were treated with 5% meta-phosphoric acid (5uL/mg) and then frozen at −80°C. For analysis of dopamine levels samples were allowed to thaw for 5–10 min, sonicated for 10 min while floated over chilled water in a sonicating bath, and vortexed (3 cycles). The samples were then loaded into spinX tubes and centrifuged at 12,900xg for 10 min and then transferred to tapered hplc vials. Dopamine was quantified on a LC-2010CHT HPLC (Shimadzu, Columbia, MD) equipped with an auto-sampler with Peltier temperature control set to 4°C and an 8-channel ESA CoulArray Detector (model 5600 A; ESA Laboratories, Chelmsford, MI) by the method of Takeda (Takeda 1997) with minor modifications. Briefly, samples (20 µL) were separated on a Luna, reverse phase, C18(2), 150x 4.60 mm column (Phenomenex, Torrance, CA) provided with a Phenomenex guard column (ODS, 4-mm length, 3.0-mm ID) with an isocratic mixture of 50 mM sodium phosphate buffer pH 3.2 containing 200 µM sodium octylsulfonate, and methanol (90:10) (Sigma-Aldrich, St. Louis, MO) at a flow rate of 0.6 ml/min. The analyte was typically analyzed at 350 mV and ratios to signals from leading and/or trailing potentials were used to control for interference of possible co-eluting substances. All samples and standards were run in duplicate, and the results were averaged. The collected data were analyzed with ESA CoulArray software (ESA).
DATA ANALYSES
We tested for differences among the genotypes in melanotic encapsulation ability and ability to avoid parasitism using logistic regression as implemented by SAS (SAS V.9.0, SAS Institute, Cary, NC). The phenotype being analyzed was the response variable and the genotype (as referenced by the known genotype at the Ddc locus, CAT/CAT, CAT/TCG, TCG/TCG) was the predictor. The best fit was a logistic regression with binomial errors, a logit link, and overdispersion parameter.
We tested for differences in foraging behavior, feeding rates, and dopamine levels among the genotypes by ANOVA using Proc GLM. We used CONTRAST statements to compare genotypes in post hoc analyses (SAS V.9.0, SAS Institute, Cary, NC). We transformed the foraging path length data to natural logs to satisfy assumptions of ANOVA.
We tested for an association between the genotype of larvae and the number of wasps attacking a plate of larvae (in the field component of this experiment) with Fishers exact test using Proc Freq (SAS V.9.0, SAS Institute, Cary, NC).
Results
ABILITY OF FLY LARVAE TO AVOID PARASITISM DEPENDS ON GENOTYPE
There were no differences among genotypes in their ability to avoid parasitism by Aphaereta sp. (χ2 = 0.46; P = 0.79) (Figure 1). However, there was a significant difference among Ddc genotypes on the ability to avoid parasitism by L. boulardi (χ2 = 8.87; P = 0.01) (Figure 1), with the rank order of avoidance being TCG homozygotes > CAT homozygotes > heterozygote. Larvae homozygous for the TCG polymorphism were 18% better at avoiding attack than the CAT homozygous larvae (CAT, χ2= 3.60; P = 0.06) and 21% better than heterozygote larvae (CAT/TCG, χ2= 8.00; P= 0.005). So in the case of avoidance of parasitism, larvae homozygous for TCG are best able to avoid parasitism in the laboratory as compared with the heterozygotes and CAT homozygotes.
Figure 1.
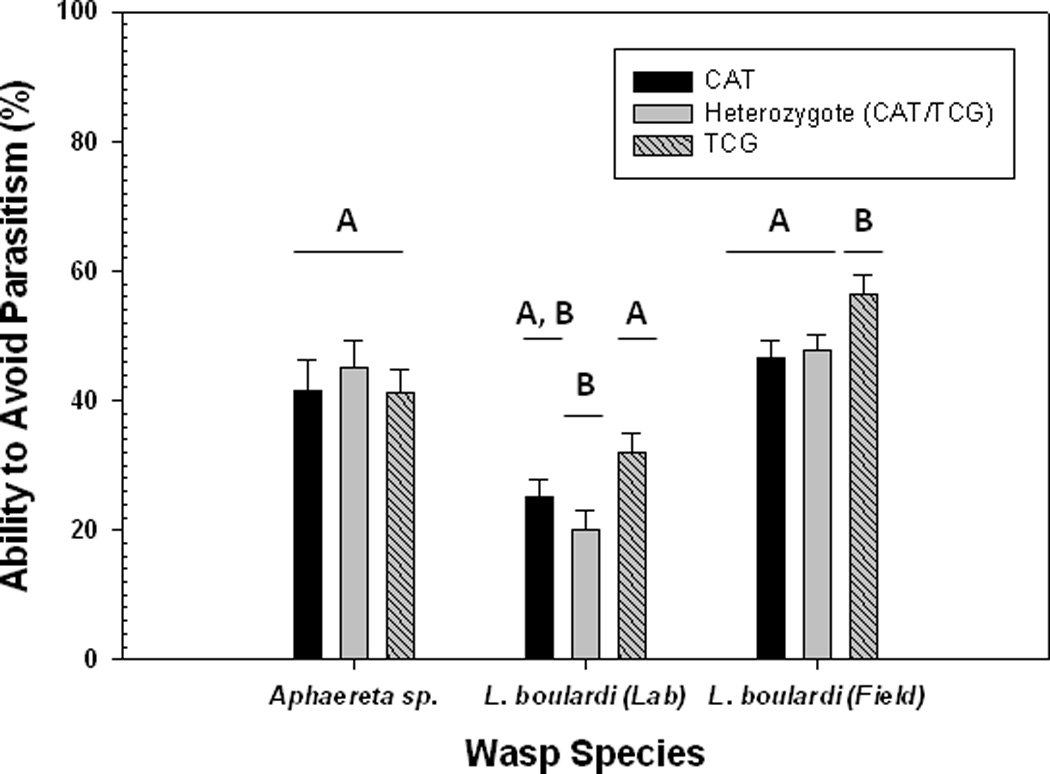
Ability to avoid parasitism by Aphaereta sp., L. boulardi (lab), and L. boulardi (field) for different Ddc genotypes. Shown are mean values (± 1 s.e.m.). Different uppercase letters indicate significant differences between Ddc genotypes.
VARIATION AMONG GENOTYPES IN IMMUNE RESPONSE IS SPECIES-SPECIFIC
We found significant differences in the melanotic encapsulation ability among the genotypes against the parasitoid, Aphaereta sp. (χ2 = 20.39, P < 0.0001) (Figure 2) with the rank order being heterozygotes > CAT homozygotes > TCG homozygotes. This is a large effect, notable because more larvae were able to avoid parasitism by Aphaereta sp. (compared to L. boulardi) and so we had reduced power to detect differences in encapsulation ability in this test. Heterozygotes (CAT/TCG) and homozygous CAT larvae had 17% and 10% higher melanotic encapsulation abilities, respectively, compared to TCG homozygotes. While the average melanotic encapsulation ability was greater in heterozygotes it was not formally significantly different compared with the CAT homozygous larvae (χ2 = 3.25, P = 0.07). However, this is approaching significance, suggesting that there may be heterozygote advantage (at least with respect to this trait) that could contribute to the maintenance of variation (and by extension Ddc).
Figure 2.
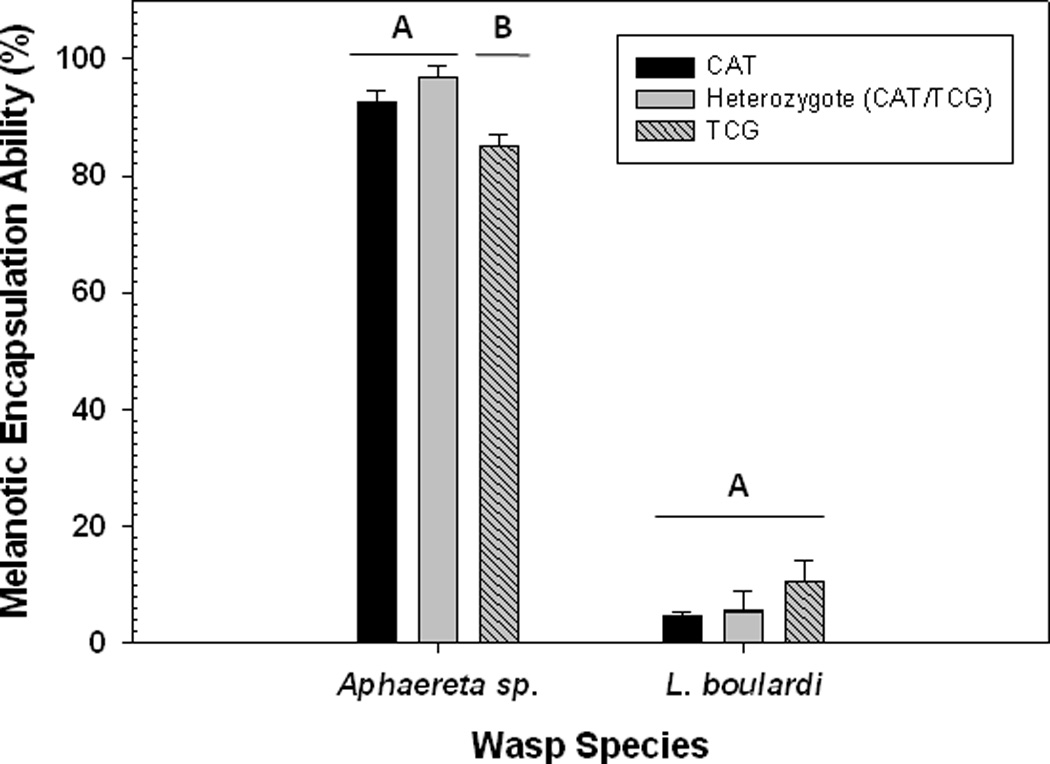
Melanotic encapsulation abilities of 2nd instar larvae with different Ddc genotypes against Aphaereta sp. and L. boulardi. Shown are mean values (± 1 s.e.m.). Different uppercase letters indicate significant differences between Ddc genotypes.
We found no difference among the Ddc genotypes in melanotic encapsulation ability against L. boulardi (χ2 = 4.13, P = 0.13) (Figure 2). In fact very few larvae (< 4%) melanotically encapsulated eggs from this species. This low ability to melanotically encapsulate eggs combined with the fact that fewer larvae were able to avoid being attacked by L. boulardi indicates that this species is probably a more potent agent of selection on larval defense traits than Aphaereta sp. Whether or not this is true will require us to carry out a long-term regional assessment of the prevalence of these parasitiods in field populations.
VARIATION AMONG GENOTYPES INFLUENCES THE ABILITY OF LARVAE TO AVOID PARASITISM BY L. BOULARDI IN NATURAL POPULATIONS: A FIELD TEST OF THE LABORATORY RESULTS
The number of wasps attacking a single plate ranged from 1–3, and more wasps attacking a plate resulted in more hosts being parasitized in spite of our attempts to standardize exposure time (χ2 = 5.72, P = 0.02). A three-way contingency table analysis indicated that the number of wasps attacking a plate did not differ among genotypes (χ2 = 1.3622, P = 0.8507). As a result, the positive relationship between the number of wasps attacking a plate and the number of hosts attacked should not affect our conclusions about differences among genotypes in their ability to avoid attack reported below.
Results in the field were in broad agreement with the laboratory results; there were significant differences among genotypes in their ability to avoid parasitism by L. boulardi (χ2 = 6.86; P = 0.03) (Figure 1), with TCG homozygous larvae being much better at avoiding parasitism than the CAT homozygous larvae (χ2= 5.56; P = 0.02) or heterozygotes (χ2= 5.09; P = 0.02). Dissections of the field-parasitized larvae two days after exposure revealed that none of the larvae were able to melanotically encapsulate the eggs of L. boulardi.
GENOTYPES DIFFER IN LARVAL FORAGING BEHAVIOR
We found significant differences in foraging behavior among genotypes; TCG homozygotes moved 60% more while foraging than CAT homozygous larvae (F1,290 = 88.4, P < 0.0001), and 62% more than heterozygotes (CAT/TCG, F1,290 = 99.7 P < 0.0001) (Figure 3).
Figure 3.
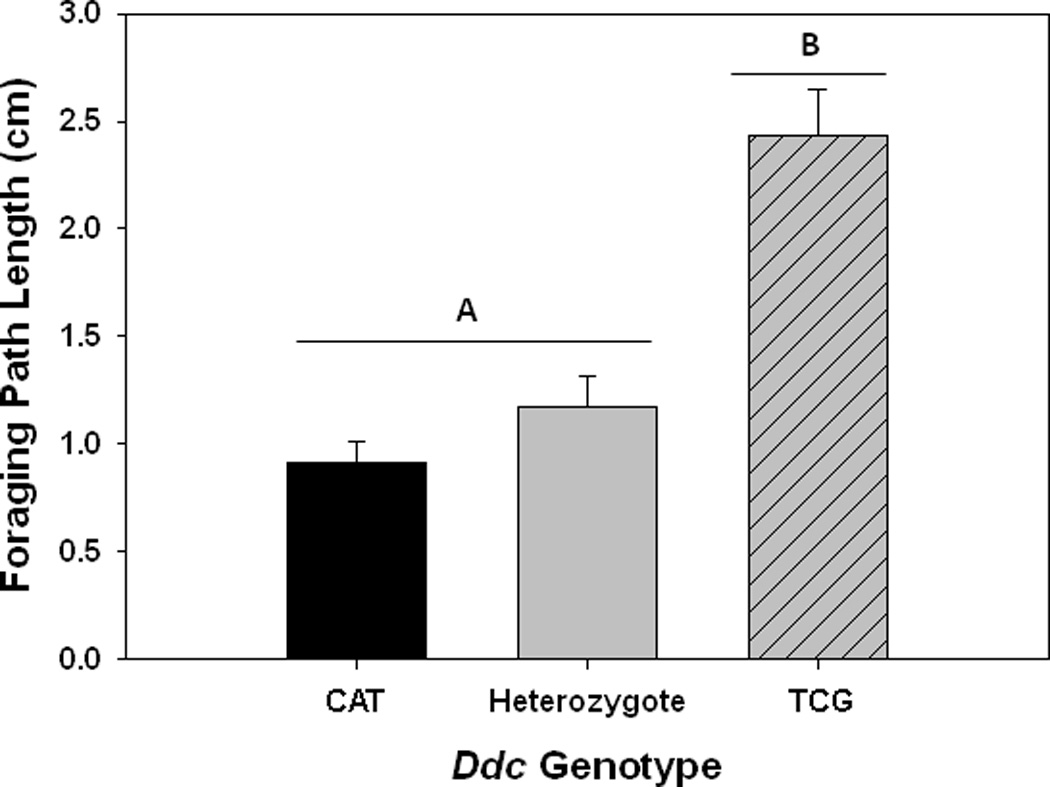
Foraging path lengths of 2nd instar larvae for different Ddc genotypes. Shown are mean values (± 1 s.e.m.). Different uppercase letters indicate significant differences between Ddc genotypes.
GENOTYPES DIFFER IN FEEDING RATE
We found significant differences among genotypes in feeding rates. TCG homozygotes fed 10% faster than CAT homozygous larvae (F1,290 = 57.6, P < 0.0001) and heterozytes (F1,290 = 72.3, P < 0.0001) (Figure 4).
Figure 4.
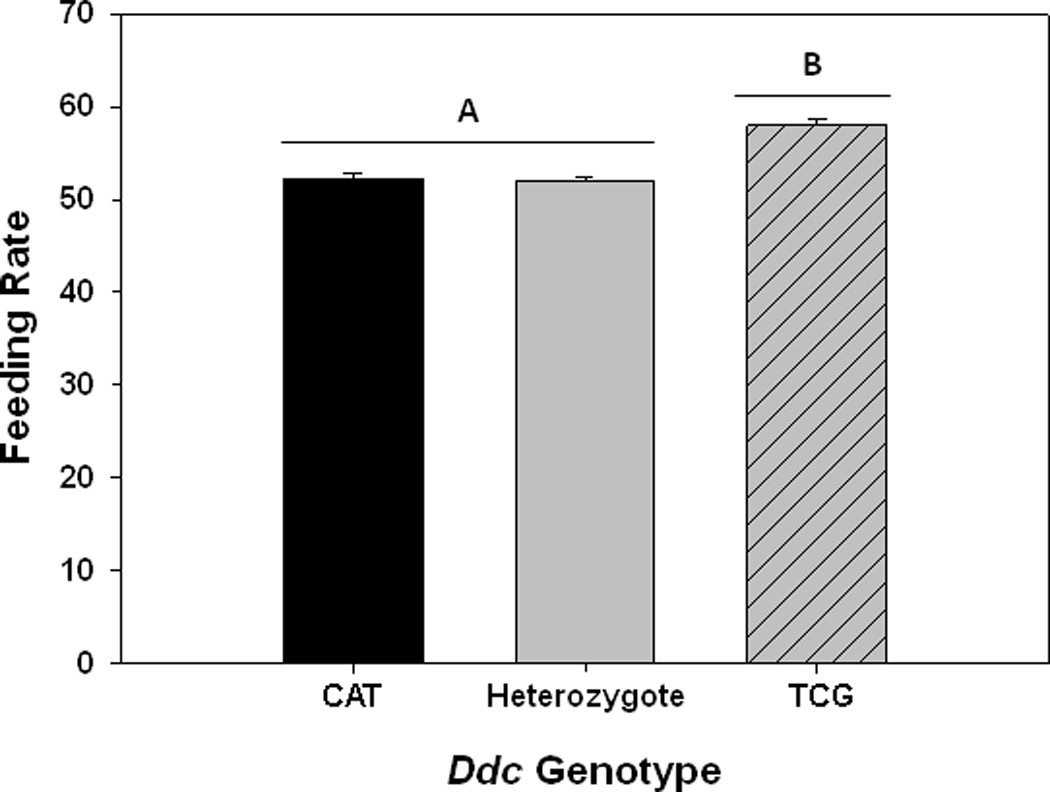
Feeding rates of 2nd instar larvae for different Ddc genotypes. Shown are mean values (± 1 s.e.m.) of the number of cephalopharyngeal retractions in a 30 second period. Different uppercase letters indicate significant differences between Ddc genotypes.
GENOTYPES DIFFER IN DOPAMINE LEVELS
To evaluate the potential that the polymorphism in Ddc contributed to phenotypic differences observed among the genotypes, we measured dopamine levels in 2nd instar larvae from each of the Ddc crosses. Tyrosine hydroxylase (encoded by the pale gene in Drosophila) is thought to control the rate limiting step in the production of dopamine (Wright 1987). However, because the pale gene is on the third chromosome in Drosophila it should be homozygous in our extraction lines and so not contribute to variation in dopamine levels among genotypes.
The rank order of the Ddc haplotypes in the average dopamine levels (Figure 5) matches that of melanotic encapsulation ability of the different genotypes against Aphaereta sp. (Figure 1): heterozygote > CAT homozygotes > TCG homozygotes. This suggests that variation in dopamine levels may contribute to the observed differences in melanotic encapsulation ability.
Figure 5.
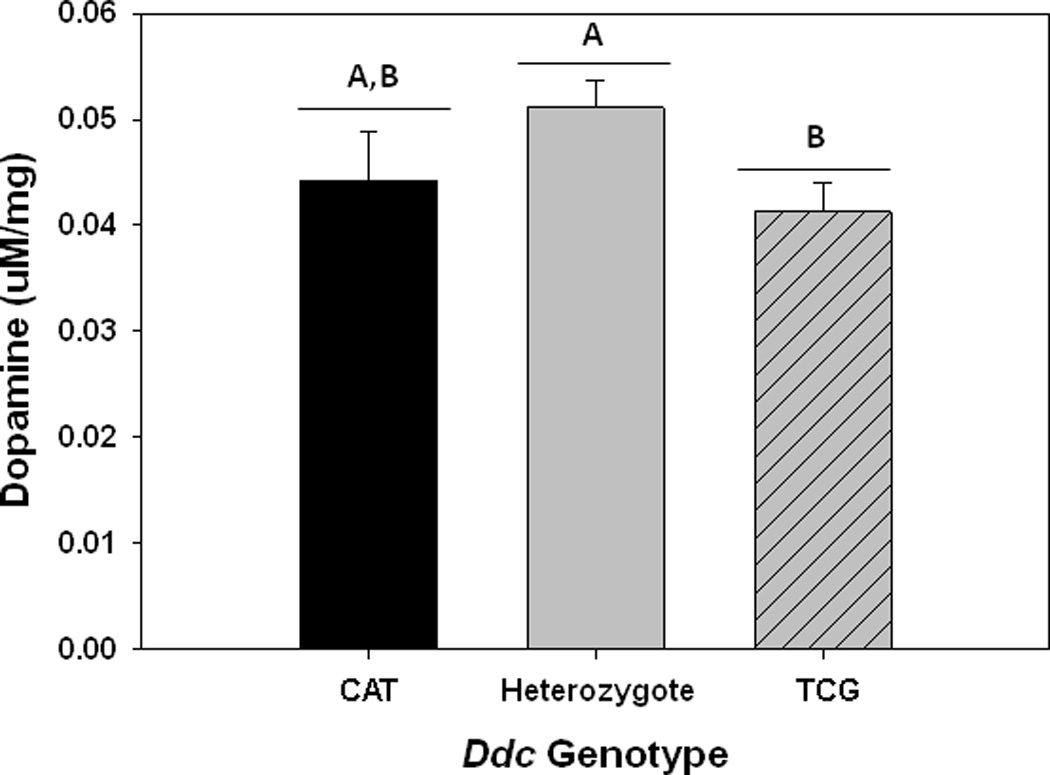
Dopamine levels (uM/mg) of 2nd instar larvae for different Ddc genotypes. Shown are mean values (± 1 s.e.m.). Different uppercase letters indicate significant differences between Ddc genotypes.
Statistically, the analyses were able to separate the genotypes with the most extreme differences in dopamine level (heterozygotes had higher dopamine levels than short-lived homozygotes) but could not distinguish the adjacent genotypes. Heterozygous larvae (CAT/TCG) had significantly higher levels of dopamine than homozygous TCG larvae (F1,20 = 4.93, P = 0.04) but were not significantly different from dopamine levels in CAT homozygotes (F1,20 = 2.46, P = 0.13) (Figure 5). There were no significant differences in dopamine levels between TCG and CAT genotypes (F1,20 = 0.37, P = 0.54) (Figure 5).
Discussion
PARASITOID COMMUNITY COMPOSITION AND THE EVOLUTION OF NEGATIVE GENETIC CORRELATIONS AMONG LARVAL TRAITS
We found that larval defense traits exhibit negative genetic correlations when confronted with two different species of parasitoids, Aphaereta sp. and L. boulardi. Genotypes that have higher melanotic encapsulation abilities against the eggs of Aphaereta sp. face a higher risk of parasitism by L. boulardi. Our results provide support for the predictions of Futuyma and Mitter (1996) that negative correlations between defense traits are expected if different trait values are favored against different potential attackers. However, our results are more complicated than the simplest version of their hypothesis. The most straightforward interpretation is that the tradeoff would involve a single trait. In the context of our work we might have expected that genotypes that were better able to avoid attack by one species of parasitoid would be more vulnerable to attack by a different parasitoid (if each species used a different strategy to locate hosts). Instead what we saw was a trade-off between two different types of defense traits, one immunological (melanotic encapsulation ability) and the other non-immunological (presumably larval behavior). This more complicated scenario of the Futuyma and Mitter hypothesis is expected when traits are connected by pleiotropic loci whose allelic effects produce antagonistic relationships between the traits which is the pattern we observed here.
In our experiment, we found that the genotypes that were more active foragers (those with the TCG Ddc alleles) were better able to avoid parasitism by L. boulardi (which uses mainly ovipositor searching), than the less active foragers (those homozygous for the CAT Ddc polymorphism). These results match the findings of earlier work on the foraging gene (for), showing that less active larvae suffer higher rates of parasitism from L. boulardi (Carton and Sokolowski 1992). In that study, natural polymorphism in the foraging (for) gene in Drosophila produces differences in larval behavior (Sokolowski 1980) and these differences affect the probability of parasitism by different species of parasitoids, depending on the search strategy that parasitoids use to find a host. A potential explanation for our results is that more active larvae are either harder to locate, harder to successfully parasitize, and/or require longer handling times and incur higher energy costs for successful parasitism. Thus, all else being equal, this would reduce the number of larvae that can be parasitized in a given amount of time. Disentangling these mechanisms is a promising area for future work.
We should note that the Ddc and for genes are on the same arm of the second chromosome (2L) but are over 1Mb away from each other, so SNPs in these genes are not likely to be in linkage disequilibrium. However, because the polymorphism(s) in the foraging gene that contribute to the rover-sitter phenotype are not known (Marla Sokolowski pers. comm.) we cannot test this hypothesis.
In our experiment, larvae that were homozygous for the TCG Ddc polymorphism moved more while foraging but were not parasitized at a higher rate by Aphaereta, a genus characterized as a vibrotaxis searcher (Vet and van Alphen 1985). These results seem at odds with the relationship between larval foraging behavior and searching behavior of parasitoids established in the studies of the for gene. In previous studies of the rover-sitter phenotypes of the for gene, larvae with the rover phenotype faced a higher risk of parasitism from parasitoids that use vibrotaxis searching to locate their hosts (Ganaspis xanthopoda; Asobara tabida) (Sokolowski and Turlings 1987, Kraaijeveld and van Alphen 1995, Hughes and Sokolowski 1996). While behavioral observations indicate that the species of Aphaereta used in our experiment uses vibrotaxis, it is possible that this species uses additional cues to locate hosts (e.g., kairomones: Vet et al. 1993).
We also found that larvae that were more active foragers were less able to encapsulate the eggs of Aphaereta sp. This result also differs from the positive correlation between larval Drosophila foraging behavior and melanotic encapsulation ability found in a study of the rover and sitter phenotypes of the for gene (Hughes and Sokolowski 1996). Hughes and Sokolowski (1996) found that rovers were better able to melanotically encapsulate the eggs of Asobara tabida than sitters. However, the phenotypic association between these traits is not always observed (Green et al. 2000) and the ability of Drosophila to encapsulate eggs of parasitoids varies among parasitoid species (reviewed in (Kraaijeveld and Godfray 2009). Considering this and the fact that encapsulation ability and foraging behavior are likely to be polygenic traits, potentially influenced by pleiotropic and non-pleiotropic loci, we need more detailed information on the genetic basis of variation in these traits to resolve the influence of pleiotropy on their evolution.
In our study larvae with the CAT allele are better at melanotically encapsulating the eggs of Aphaereta sp. but have lower feeding rates than larvae homozygous for the TCG allele. This result is consistent with earlier work (Fellowes et al. 1999b) and so supports a trade-off between melanotic encapsulation ability against parasitoids and larval competitive ability.
Pleiotropic Effects of Natural Polymorphism in Ddc?
We found evidence that Ddc is a pleiotropic locus that contributes to tradeoffs among larval fitness traits. Three independent studies have identified Ddc as a locus influencing the melanotic encapsulation defense against parasitoids (Nappi et al. 1992, Orr and Irving 1997, Schlenke et al. 2007) although the QTL region identified by Orr and Irving was later fine mapped to a small region with large effect on this trait that did not contain Ddc (Hita et al. 1999). In addition, in our study genotypes with alternative haplotypes at the SNP locus exhibit differences in dopamine levels that match the patterns of melanotic encapsulation abilities. These independent lines of evidence provide further support for the hypothesis of antagonistic pleiotropy influenced by Ddc.
An important caveat to this conclusion is that Ddc is surrounded by a dense cluster of 17 other functionally-related genes with many of the genes, such as Catsup, Dox-A2, and amd, involved in catecholamine metabolism (Stathakis et al. 1995, Wright 1996, Stathakis et al. 1999). It is possible that the Ddc polymorphism is linked to polymorphisms in a nearby gene that produce the phenotypic variation observed in our experiments. Further studies are needed to validate the effects of the SNPs in Ddc that we observed on the phenotypes evaluated in this study.
Antagonistic Pleiotropy and the Maintenance of Variation at Ddc
The potential antagonistic pleiotropic effects of polymorphism at Ddc have important implications for the evolution of these traits in natural populations of D. melanogaster. Could the antagonistic effects that we measured be enough to maintain variation at Ddc? Based on the models of Curtsinger et al. 1994 and Hedrick 1999, it is not likely. Our experimental design allowed us to evaluate evidence for two hypotheses that could contribute to the maintenance of genetic variation, heterozygote advantage and antagonistic pleiotropy. While antagonistic pleiotropy itself can maintain polymorphism, the conditions under which it does so are restrictive (Curtsinger et al. 1994, Hedrick 1999, Van Dooren 2006). One condition that facilitates the maintenance of polymorphism by antagonistic pleiotropy is a beneficial reversal of dominance. In this case, when two alleles segregate at a locus, the allele that confers a trait value with higher fitness is dominant, with both alleles exhibiting dominance when it confers higher fitness for different traits. We found no evidence for a beneficial reversal of dominance or consistent heterozygote advantage across the traits. Of course, given the central importance of this gene in the production of neural transmitters and its influence on pigmentation (Hodgetts and O'Keefe 2006), a number of other traits could be affected by the polymorphism in Ddc. If so, the dominance relationships of these haplotypes on other affected phenotypes would need to be measured. This information, combined with measurements of the relative contribution of these traits to fitness, would provide a more thorough test of the role of antagonistic pleiotropy in maintaining variation at Ddc.
If antagonistic pleiotropy is not important for maintaining variation, what is? To recap, flies with the TCG genotype have the lowest dopamine levels and the lowest encapsulation rates but have the highest feeding rates, move more while foraging and have the best ability to avoid attack by the species of wasp (L. boulardi) that attacks at the highest rate and is the most lethal (summarized in Table 1). One potential explanation that offers a promising avenue for further work is that the community composition of parasitoids is known to differ geographically and temporally (Fleury et al. 2009). Geographic variation in the community composition of parasitoids selecting against alternative traits combined with gene flow among populations could be an important contributor to variation in these traits (Levene 1953, Dempster 1955). To the best of our knowledge there is no published information on geographic variation in parasitoid community composition along the eastern coast of the U.S. Such data would be useful in this context. We do have five years of field data from our two local sites in Maryland that suggest regular seasonal changes in parasitoid community composition. Aphaereta sp. are common early in the breeding season but not later in the summer, when parasitoids in the genus Leptopilina are dominant (Hodges, unpublished data). Therefore, regular temporal changes in selection by parasitoids may help preserve genetic variation in these traits (Ellner and Hairston 1994, Ellner and Sasaki 1996). Our field data also suggest that fly density increases as the summer breeding season progresses. This provides the potential for variation in the strength and direction of selection for density dependent competitive ability. Detailed information on the role of geographic and temporal variation of parasitoid community composition on larval trait evolution is needed. This, combined with data on geographic and temporal variation in density dependent selection on larval traits in Drosophila are needed to reveal the relative role of these ecological factors on the evolution and maintenance of variation in larval fitness traits.
Table 1.
Relative differences among Ddc genotypes for fitness traits assayed as well as life span measured by De Luca et al. 2003.
| Ddc Genotype | |||
|---|---|---|---|
| Trait | CAT/CAT | Heterozygote | TCG/TCG |
| Life span | Longer | Longer Not measured | Shorter |
| Immune response against Aphaereta sp. |
Higher | Higher | Lower |
| Immune response against L. boulardi |
NS | NS | NS |
| Ability to avoid parasitism by Aphaereta sp. |
NS | NS | NS |
| Ability to avoid parasitism by L. boulardi (L: Lab, F: Field) |
L: NS F: Lower |
L: Lower F: Lower |
L: Higher F: Higher |
| Distance move while foraging |
Shorter | Shorter | Longer |
| Feeding rate | Lower | Lower | Higher |
| Dopamine levels | NS | Higher | Lower |
Boxes with NS indicates no significant difference with the other Ddc genotypes
ACKNOWLEDGEMENTS
We thank Norman Wang, Macarena Busto, Marla Sokolowski, and Chere Petty for help and advice on the foraging assay. Alex Kraiijeveld and Michael Antolin provided useful suggestions for maintenance of the wasp colonies. Michael Antolin, Charlie Baer, Helen Rodd, Kim Hughes, Tami Mendelson, Cynthia O’Rourke, Tashuana Felix, Mary Durham, David Dunston, Brian Dalton, Frode Jacobsen, Rachel Sturge, and two anonymous reviewers provided valuable comments on the manuscript. We especially thank Joe Travis for a critical evaluation of this manuscript and for his insightful comments. We thank Boordy Vineyards and Boyer Farms for allowing us to use their sites to conduct field studies. We also thank Evan L. Floyd for help with the HPLC data analysis. This study was supported by grants from the National Science Foundation to JL, DEB-0349856, and a National Institutes of Health grant to MDL and JL, 5R01HL80812. TKH was supported in part by a Department of Education GAANN Grant P200A030235.
LITERATURE CITED
- Agrawal AA. Current trends in the evolutionary ecology of plant defence. Funct Ecol. 2011;25:420–432. [Google Scholar]
- Allemand R, Lemaitre C, Frey F, Bouletreau M, Vavre F, Nordlander G, van Alphen J, Carton Y. Phylogeny of six African Leptopilina species (Hymenoptera : Cynipoidea, Figitidae), parasitoids of Drosophila, with description of three new species. Annales De La Societe Entomologique De France. 2002;38:319–332. [Google Scholar]
- Anderson JT, Lee CR, Rushworth CA, Colautti RI, Mitchell-Olds T. Genetic trade-offs and conditional neutrality contribute to local adaptation. Mol Ecol. 2012 doi: 10.1111/j.1365-294X.2012.05522.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anholt RR, Mackay TF. Quantitative genetic analyses of complex behaviours in Drosophila. Nat Rev Genet. 2004;5:838–849. doi: 10.1038/nrg1472. [DOI] [PubMed] [Google Scholar]
- Armbruster WS. Multilevel analysis of morphometric data from natural plant populations: insights into ontogenetic, genetic, and selective correlations in Dalechampia scandens. Evolution. 1991;45:1229–1244. doi: 10.1111/j.1558-5646.1991.tb04389.x. [DOI] [PubMed] [Google Scholar]
- Arnold S. Constraints on phenotypic evolution. Amer Nat. 1992;140:S85–S107. doi: 10.1086/285398. [DOI] [PubMed] [Google Scholar]
- Baer CF, Lynch M. Correlated evolution of life-history with size at maturity in Daphnia pulicaria: patterns within and between populations. Genet Res. 2003;81:123–132. doi: 10.1017/s0016672303006098. [DOI] [PubMed] [Google Scholar]
- Baker RH, Wilkinson GS. Phylogenetic analysis of correlation structure in stalk-eyed flies (Diasemopsis, Diopsidae) Evolution. 2003;57:87–103. doi: 10.1111/j.0014-3820.2003.tb00218.x. [DOI] [PubMed] [Google Scholar]
- Bucan M, Abel T. The mouse: genetics meets behaviour. Nat Rev Genet. 2002;3:114–123. doi: 10.1038/nrg728. [DOI] [PubMed] [Google Scholar]
- Burnet B, Sewell D, Bos M. Genetic analysis of larval feeding behaviour in Drosophila melanogaster. II. Growth relations and competition among lines. Genet Res. 1977;30:149–161. doi: 10.1017/s0016672300015196. [DOI] [PubMed] [Google Scholar]
- Carbone MA, Jordan KW, Lyman RF, Harbison ST, Leips J, Morgan TJ, DeLuca M, Awadalla P, Mackay TF. Phenotypic variation and natural selection at catsup, a pleiotropic quantitative trait gene in Drosophila. Curr Biol. 2006;16:912–919. doi: 10.1016/j.cub.2006.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carton Y, Bouletreau M, Van Lenteren JC, Van Alphen JCM. The Drosophila parasitic wasps. In: Ashburner M, Thomson JN, Carson HL, editors. The genetics and biology of Drosophila. New York, N.Y: Academic Press; 1986. pp. 347–394. [Google Scholar]
- Carton Y, David JR. Reduction of fitness in Drosophila adults surviving parasitization by a cynipid wasp. Experientia. 1983;39:231–233. [Google Scholar]
- Carton Y, Sokolowski MB. Interactions between searching strategies of Drosophila parasitoids and the polymorphic behavior of their hosts. J Insect Beh. 1992;5:161–175. [Google Scholar]
- Christensen JG, Dairman W, Udenfriend S. On the identity of DOPA decarboxylase and 5-hydroxytryptophan decarboxylase. Proc Natl Acad Sci U S A. 1972;69:343–347. doi: 10.1073/pnas.69.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CM, Neckameyer WS. Serotonin synthesis by two distinct enzymes in Drosophila melanogaster. Archives of Insect Biochemistry and Physiology. 2005;59:12–31. doi: 10.1002/arch.20050. [DOI] [PubMed] [Google Scholar]
- Conner JK, Karoly K, Stewart C, Koelling VA, Sahli HF, Shaw FH. Rapid independent trait evolution despite a strong pleiotropic genetic correlation. Amer Nat. 2011;178:429–441. doi: 10.1086/661907. [DOI] [PubMed] [Google Scholar]
- Curtsinger JW, Service PM, Prout T. Antagonistic pleiotropy, reversal of dominance, and genetic polymorphism. Amer Nat. 1994;144:210–228. [Google Scholar]
- De Luca M, Roshina NV, Geiger-Thornsberry GL, Lyman RF, Pasyukova EG, Mackay TF. Dopa decarboxylase (Ddc) affects variation in Drosophila longevity. Nature Genet. 2003;34:429–433. doi: 10.1038/ng1218. [DOI] [PubMed] [Google Scholar]
- Dempster ER. Maintenance of genetic heterogeneity. Cold Spring Harbor Symposia on Quantitative Biology. 1955;20:25–31. doi: 10.1101/sqb.1955.020.01.005. [DOI] [PubMed] [Google Scholar]
- Ellner S, Hairston NG. Role of overlapping generations in maintaining genetic-variation in a fluctuating environment. Amer Nat. 1994;143:403–417. [Google Scholar]
- Ellner S, Sasaki A. Patterns of genetic polymorphism maintained by fluctuating selection with overlapping generations. Theoretical Population Biology. 1996;50:31–65. doi: 10.1006/tpbi.1996.0022. [DOI] [PubMed] [Google Scholar]
- Featherstone DE, Brodie K. Wrestling with pleiotropy: genomic and topological analysis of the yeast expression network. Bioessays. 2002;24:267–274. doi: 10.1002/bies.10054. [DOI] [PubMed] [Google Scholar]
- Fellowes MD, Kraaijeveld AR, Godfray HC. Trade-off associated with selection for increased ability to resist parasitoid attack in Drosophila melanogaster. Proc R Soc Lond [Biol] 1998;265:1553–1558. doi: 10.1098/rspb.1998.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellowes MD, Kraaijeveld AR, Godfray HC. Cross-resistance following artificial selection for increased defense against parasitoids in Drosophila melanogaster. Evolution. 1999a;53:966–972. doi: 10.1111/j.1558-5646.1999.tb05391.x. [DOI] [PubMed] [Google Scholar]
- Fellowes MDE, Kraaijeveld AR, Godfray HCJ. Association between feeding rate and parasitoid resistance in Drosophila melanogaster. Evolution. 1999b;53:1302–1305. doi: 10.1111/j.1558-5646.1999.tb04544.x. [DOI] [PubMed] [Google Scholar]
- Fleury F, Gilbert P, Ris N, Allemand R. Ecology and life history evolution of frugivorous Drosophila parasitoids. In: Prevost G, editor. Advances in Parasitology Parasitoids of Drosophila. London: Elsevier; 2009. pp. 3–44. [DOI] [PubMed] [Google Scholar]
- Fleury F, Ris N, Allemand R, Fouillet P, Carton Y, Bouletreau M. Ecological and genetic interactions in Drosophila-parasitoids communities: a case study with D. melanogaster D. simulans and their common Leptopilina parasitoids in south-eastern France. Genetica. 2004;120:181–194. doi: 10.1023/b:gene.0000017640.78087.9e. [DOI] [PubMed] [Google Scholar]
- Futuyma DJ, Mitter C. The evolution of component communities. Philos Trans R Soc London [Biol] 1996;351:1361–1366. [Google Scholar]
- Gandon S, Buckling A, Decaestecker E, Day T. Host-parasite coevolution and patterns of adaptation across time and space. J Evol Biol. 2008;21:1861–1866. doi: 10.1111/j.1420-9101.2008.01598.x. [DOI] [PubMed] [Google Scholar]
- Gandon S, Van Zandt PA. Local adaptation and host-parasite interactions. Trends Ecol Evolut. 1998;13:214–216. doi: 10.1016/s0169-5347(98)01358-5. [DOI] [PubMed] [Google Scholar]
- Geiger-Thornsberry GL, Mackay TF. Association of single-nucleotide polymorphisms at the Delta locus with genotype by environment interaction for sensory bristle number in drosophila Melanogaster. Genet Res. 2002;79:211–218. doi: 10.1017/s0016672302005621. [DOI] [PubMed] [Google Scholar]
- Gimeno C, Belshaw R, Quicke DLJ. Phylogenetic relationships of the Alysiinae/Opiinae (Hymenoptera: Braconidae) and the utility of cytochrome b, 16S and 28S D2 rRNA. Insect Mol Biol. 1997;6:273–284. doi: 10.1046/j.1365-2583.1997.00181.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D, Kelly AM, Rinaldi J, Klatt JD. Midbrain dopamine neurons reflect affiliation phenotypes in finches and are tightly coupled to courtship. Proc Natl Acad Sci U S A. 2009;106:8737–8742. doi: 10.1073/pnas.0811821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratten J, Wilson AJ, McRae AF, Beraldi D, Visscher PM, Pemberton JM, Slate J. A localized negative genetic correlation constrains microevolution of coat color in wild sheep. Science. 2008;319:318–320. doi: 10.1126/science.1151182. [DOI] [PubMed] [Google Scholar]
- Green DM, Kraaijeveld AR, Godfray HJC. Evolutionary interactions between Drosophila melanogaster and its parasitoid Asobara tabida. Heredity. 2000;85:450–458. doi: 10.1046/j.1365-2540.2000.00788.x. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The moulding of senescence by natural selection. Journal of Theoretical Biology. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- Harshman LG, Hoffmann AA. Laboratory selection experiments using Drosophila: what do they really tell us? Trends in Ecol Evol. 2000;15:32–36. doi: 10.1016/s0169-5347(99)01756-5. [DOI] [PubMed] [Google Scholar]
- Hedrick PW. Antagonistic pleiotropy and genetic polymorphism: a perspective. Heredity. 1999;82:126–133. [Google Scholar]
- Hita MT, Poirie M, Leblanc N, Lemeunier F, Lutcher F, Frey F, Periquet G, Carton Y. Genetic localization of a Drosophila melanogaster resistance gene to a parasitoid wasp and physical mapping of the region. Genome Res. 1999;9:471–481. [PMC free article] [PubMed] [Google Scholar]
- Hodgetts RB, O'Keefe SL. Dopa decarboxylase: a model gene-enzyme system for studying development, behavior, and systematics. Annu Rev Entomol. 2006;51:259–284. doi: 10.1146/annurev.ento.51.110104.151143. [DOI] [PubMed] [Google Scholar]
- Houle D. Characters as the units of evolutionary change. In: Wagner GP, editor. The Character Concept in Evolutionary Biology. San Diego: Academic Press; 2001. pp. 109–140. [Google Scholar]
- Hughes K, Sokolowski MB. Natural Selection in the laboratory for a change in resistance by Drosophila melanogaster to the parasitoid wasp Asobara tabida. J Insect Beh. 1996;9:477–491. [Google Scholar]
- Hughes KA, Reynolds RM. Evolutionary and mechanistic theories of aging. Annu Rev Entomol. 2005;50:421–445. doi: 10.1146/annurev.ento.50.071803.130409. [DOI] [PubMed] [Google Scholar]
- Janssen A, Driessen G, Haan MD, Roodbol N. The impact of parasitoids on natural populations of temperate woodland Drosophila. Neth J Zool. 1988;38:61–73. [Google Scholar]
- Jordan KW, Morgan TJ, Mackay TF. Quantitative trait loci for locomotor behavior in Drosophila melanogaster. Genetics. 2006;174:271–284. doi: 10.1534/genetics.106.058099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A, Mueller LD. Evolution of higher feeding rate in Drosophila due to density dependent natural selection. Evolution. 1988;42:1090–1093. doi: 10.1111/j.1558-5646.1988.tb02527.x. [DOI] [PubMed] [Google Scholar]
- Joshi A, Mueller LD. Density-dependent natural selection in Drosophila: trade-offs between larval food acquisition and utilization. Evol Ecol. 1996;10:463–474. [Google Scholar]
- Kenney-Hunt JP, Vaughn TT, Pletscher LS, Peripato A, Routman E, Cothran K, Durand D, Norgard E, Perel C, Cheverud JM. Quantitative trait loci for body size components in mice. Mamm Genome. 2006;17:526–537. doi: 10.1007/s00335-005-0160-6. [DOI] [PubMed] [Google Scholar]
- Kraaijeveld AR, Godfray HC. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature. 1997;389:278–280. doi: 10.1038/38483. [DOI] [PubMed] [Google Scholar]
- Kraaijeveld AR, Godfray HC. Evolution of host resistance and parasitoid counter-resistance. In: Prevost G, editor. Advances in Parasitology. London: Academic Press; 2009. [DOI] [PubMed] [Google Scholar]
- Kraaijeveld AR, van Alphen JJM. Foraging behavior and encapsulation ability of Drosophila melanogaster larvae: Correlated polymorphisms? (Diptera: Drosophilidae) J Insect Beh. 1995;8:305–312. [Google Scholar]
- Lande R. A quantitative genetic theory of life history evolution. Ecology. 1982;63:607–615. [Google Scholar]
- Lande R, Arnold S. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Lankau RA, Strauss SY. Community complexity drives patterns of natural selection on a chemical Defense of Brassica nigra. Amer Nat. 2008;171:150–161. doi: 10.1086/524959. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Kalamarz ME, Paddibhatla I, Small C, Rajwani R, Govind S. Virulence factors and strategies of Leptopilina spp.: selective responses in Drosophila hosts. In: Prevost G, editor. Parasitoids of Drosophila. Amsterdam: Academic Press; 2009. pp. 123–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroi AM, Bartke A, De Benedictis G, Franceschi C, Gartner A, Gonos ES, Fedei ME, Kivisild T, Lee S, Kartaf-Ozer N, Schumacher M, Sikora E, Slagboom E, Tatar M, Yashin AI, Vijg J, Zwaan B. What evidence is there for the existence of individual genes with antagonistic pleiotropic effects? Mech Ageing Dev. 2005;126:421–429. doi: 10.1016/j.mad.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Levene H. Genetic equilibrium when more than one ecological niche is available. Amer Nat. 1953;87:331–333. [Google Scholar]
- Lively CM, Dybdahl MF. Parasite adaptation to locally common host genotypes. Nature. 2000;405:679–681. doi: 10.1038/35015069. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Tempel BL. Genetic dissection of monoamine neurotransmitter synthesis in Drosophila. Nature. 1983;303:67–70. doi: 10.1038/303067a0. [DOI] [PubMed] [Google Scholar]
- Mitsui H, Van Achterberg K, Nordlander G, Kimura MT. Geographical distributions and host associations of larval parasitoids of frugivorous Drosophilidae in Japan. Journal of Natural History. 2007;41:1731–1738. [Google Scholar]
- Nappi AJ, Carton Y, Vass E. Reduced cellular immune competence of a temperature-sensitive dopa decarboxylase mutant strain of Drosophila melanogaster against the parasite Leptopilina boulardi. Comp Biochem Physiol B. 1992;101:453–460. doi: 10.1016/0305-0491(92)90027-o. [DOI] [PubMed] [Google Scholar]
- Nappi AJ, Christensen BM. Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochem Mol Biol. 2005;35:443–459. doi: 10.1016/j.ibmb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS. Dopamine and mushroom bodies in Drosophila: experience-dependent and -independent aspects of sexual behavior. Learn Memory. 1998;5:157–165. [PMC free article] [PubMed] [Google Scholar]
- Orr HA, Irving S. The genetics of adaptation: the genetic basis of resistance to wasp parasitism in Drosophila melanogaster. Evolution. 1997;51:1877–1885. doi: 10.1111/j.1558-5646.1997.tb05110.x. [DOI] [PubMed] [Google Scholar]
- Pendleton RG, Rasheed A, Paluru P, Joyner J, Jerome N, Meyers RD, Hillman R. A developmental role for catecholamines in Drosophila behavior. Pharmacol Biochem Behav. 2005;81:849–853. doi: 10.1016/j.pbb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Pendleton RG, Rasheed A, Sardina T, Tully T, Hillman R. Effects of tyrosine hydroxylase mutants on locomotor activity in Drosophila: a study in functional genomics. Behav Genet. 2002;32:89–94. doi: 10.1023/a:1015279221600. [DOI] [PubMed] [Google Scholar]
- Price T, Turelli M, Slatkin M. Peak shifts produced by correlated response to selection. Evolution. 1993;47:280–290. doi: 10.1111/j.1558-5646.1993.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Promislow DEL. New perspectives on comparative tests of antagonistic pleiotropy using Drosophila. Evolution. 1995;49:394–397. doi: 10.1111/j.1558-5646.1995.tb02255.x. [DOI] [PubMed] [Google Scholar]
- Roff DA, Fairbairn DJ. The evolution of trade-offs: where are we? J Evol Biol. 2007;20:433–447. doi: 10.1111/j.1420-9101.2006.01255.x. [DOI] [PubMed] [Google Scholar]
- Rose MR. Antagonistic pleiotropy, dominance, and genetic variation. Heredity. 1982;48:63–78. [Google Scholar]
- Rose MR. Life history evolution with antagonistic pleiotropy and overlapping generations. Theor Pop Biol. 1985;28:342–358. [Google Scholar]
- Rose MR, Charlesworth B. Genetics of life history in Drosophila melanogaster. II. Exploratory selection experiments. Genetics. 1981;97:187–196. doi: 10.1093/genetics/97.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarcelli N, Cheverud JM, Schaal BA, Kover PX. Antagonistic pleiotropic effects reduce the potential adaptive value of the FRIGIDA locus. Proc Natl Acad Sci U S A. 2007;104:16986–16991. doi: 10.1073/pnas.0708209104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenke TA, Morales J, Govind S, Clark AG. Contrasting infection strategies in generalist and specialist wasp parasitoids of Drosophila melanogaster. PLoS Pathogens. 2007;3:1486–1501. doi: 10.1371/journal.ppat.0030158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D. Adaptive radiation along genetic lines of least resistance. Evolution. 1996;50:1766–1774. doi: 10.1111/j.1558-5646.1996.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Schweitzer J, Driever W. Development of the dopamine systems in zebrafish. Adv Exp Med Biol. 2009;651:1–14. doi: 10.1007/978-1-4419-0322-8_1. [DOI] [PubMed] [Google Scholar]
- Sewell D, Burnet B, Connolly K. Genetic analysis of larval feeding behaviour in Drosophila melanogaster. Genet Res. 1975;24:163–173. doi: 10.1017/s0016672300015196. [DOI] [PubMed] [Google Scholar]
- Sokolowski MB. Foraging strategies of Drosophila melanogaster: a chromosomal analysis. Behav Genet. 1980;10:291–302. doi: 10.1007/BF01067774. [DOI] [PubMed] [Google Scholar]
- Sokolowski MB, Pereira HS, Hughes K. Evolution of foraging behavior in Drosophila by density-dependent selection. Proc Natl Acad Sci USA. 1997;94:7373–7377. doi: 10.1073/pnas.94.14.7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski MB, Turlings TCJ. Drosophila parasitoid-host interactions: vibrotaxis and ovipositor searching from the host's perspective. Can J Zool. 1987;65:461–464. [Google Scholar]
- Stathakis DG, Burton DY, McIvor WE, Krishnakumar S, Wright TR, O'Donnell JM. The catecholamines up (Catsup) protein of Drosophila melanogaster functions as a negative regulator of tyrosine hydroxylase activity. Genetics. 1999;153:361–382. doi: 10.1093/genetics/153.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathakis DG, Pentz ES, Freeman ME, Kullman J, Hankins GR, Pearlson NJ, Wright TR. The genetic and molecular organization of the Dopa decarboxylase gene cluster of Drosophila melanogaster. Genetics. 1995;141:629–655. doi: 10.1093/genetics/141.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppan SJ, Phillips PC, Houle D. Comparative quantitative genetics: evolution of the G matrix. Trends Ecol Evol. 2002;17:320–327. [Google Scholar]
- Strell C, Sievers A, Bastian P, Lang K, Niggemann B, Zanker KS, Entschladen F. Divergent effects of norepinephrine, dopamine and substance P on the activation, differentiation and effector functions of human cytotoxic T lymphocytes. BMC immunol. 2009;10:62. doi: 10.1186/1471-2172-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N. A phylogenic analysis of biogenic monoamines in the central nervous systems of various organisms and application to the fields of medical Biochemistry. In: Acworth IN, Naoi M, H P, Parvaez S, editors. Coulometric Electrode Array Detectors for HPLC, Progress in HPLC-HPCE VSP, Ultrecht. 1997. [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tellier A, Villareal LM, Giraud T. Antagonistic pleiotropy may help population-level selection in maintaining genetic polymorphism for transmission rate in a model phytopathogenic fungus. Heredity. 2007;98:45–52. doi: 10.1038/sj.hdy.6800902. [DOI] [PubMed] [Google Scholar]
- Turelli M, Barton NH. Polygenic variation maintained by balancing selection: pleiotropy, sex-dependent allelic effects and G x E interactions. Genetics. 2004;166:1053–1079. doi: 10.1093/genetics/166.2.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen JJM, Drijver RAB. Host selection by Asobara tabida Nees (Braconidae, Alysiinae), a larval parasitoid of fruit inhabiting Drosophila species. I. Host stage selection with Drosophila melanogaster as host species. Neth J Zool. 1982;32:215–231. [Google Scholar]
- Van Dooren TJ. Protected polymorphism and evolutionary stability in pleiotropic models with trait-specific dominance. Evolution. 2006;60:1991–2003. [PubMed] [Google Scholar]
- Vass E, Nappi AJ. Developmental and immunological aspects of Drosophila-parasitoid relationships. J Parasitol. 2000;86:1259–1270. doi: 10.1645/0022-3395(2000)086[1259:DAIAOD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Vermeulen CJ, Cremers TI, Westerink BH, Van De Zande L, Bijlsma R. Changes in dopamine levels and locomotor activity in response to selection on virgin lifespan in Drosophila melanogaster. Mech Ageing Dev. 2006;127:610–617. doi: 10.1016/j.mad.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Vet LEM, Sokolowski MB, MacDonald DE, Snellen H. Responses of a generalist and specialist parasitoid (Hymenoptera: Eucoilidae) to Drosophilid larval kairomones. J Insect Beh. 1993;6:615–624. [Google Scholar]
- Vet LEM, van Alphen JJM. A comparative functional approach to the host detection behavior of parasitic wasps. I. A qualitative study on Eucoilidiae and Alysiinae. Oikos. 1985;44:478–486. [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Winde I, Wittstock U. Insect herbivore counteradaptations to the plant glucosinolate-myrosinase system. Phytochemistry. 2011;72:1566–1575. doi: 10.1016/j.phytochem.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Wright TR. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv Genet. 1987;24:127–222. [PubMed] [Google Scholar]
- Wright TR. The Wilhelmine E Key 1992 Invitational lecture 1996 Phenotypic analysis of the Dopa decarboxylase gene cluster mutants in Drosophila melanogaster. J Hered. 87:175–190. doi: 10.1093/oxfordjournals.jhered.a022983. [DOI] [PubMed] [Google Scholar]


