Abstract
A major role of respiratory mucus is to trap inhaled particles, including pathogens and environmental particulates, to limit body exposure. Despite the tremendous health implications, how particle size and surface chemistry affect mobility in respiratory mucus from humans without lung disease is not known. We prepared polymeric nanoparticles densely coated with low molecular weight polyethylene glycol (PEG) to minimize muco-adhesion, and compared their transport to that of uncoated particles in human respiratory mucus, which we collected from the endotracheal tubes of surgical patients with no respiratory comorbidities. We found that 100 and 200 nm diameter PEG-coated particles rapidly penetrated respiratory mucus, at rates exceeding their uncoated counterparts by approximately 15- and 35-fold, respectively. In contrast, PEG-coated particles ≥ 500 nm in diameter were sterically immobilized by the mucus mesh. Thus, even though respiratory mucus is a viscoelastic solid at the macroscopic level (as measured using a bulk rheometer), nanoparticles that are sufficiently small and muco-inert can penetrate the mucus as if it were primarily a viscous liquid. These findings help elucidate the barrier properties of respiratory mucus and provide design criteria for therapeutic nanoparticles capable of penetrating mucus to approach the underlying airway epithelium.
1. Introduction
Mucus lines the conducting airways, protecting the respiratory epithelium from the external environment. The respiratory mucus barrier comprises two layers: The gel layer, rich in secreted macromolecules, which is continuously cleared by ciliary beating, and the periciliary layer, consisting of macromolecules tethered to the airway surface, which facilitates ciliary activity [1]. A major role of the respiratory mucus gel is to trap inhaled particles so they can be swept from the airways by mucociliary clearance, thereby defending the lungs against pathogens and toxic materials [2]. However, it is not known what size particles are trapped, or how particle surface chemistry affects mobility, in the airway mucus gel layer of humans without respiratory disease [3]. Airway mucus is difficult to collect and analyze because individuals with healthy respiratory systems generally cannot spontaneously expectorate it, and the mucus gel layer is only a few to a few tens of micrometers thick [2, 4]. Yet measuring the permeability of this mucus gel is essential for understanding how effectively it protects the lungs from continuously inhaled pathogens and environmental particulates. Studying the interaction between respiratory mucus and nanoparticles may also elucidate design criteria for inhaled therapeutic nanoparticles to treat diseases such as lung cancer; these particles must penetrate the mucosal barrier to avoid rapid clearance and achieve the pharmacokinetic profile requisite for effective therapeutic outcomes [5]. Finally, characterizing respiratory mucus from humans without pulmonary disease may provide a benchmark against which to compare respiratory secretions from patients with diseases that affect the airways, including asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis (CF).
Nanoparticle diffusion has previously been measured in a variety of human mucus secretions, including cervicovaginal (CV) mucus [6], CF sputum [7, 8], and chronic rhinosinusitis mucus [9]. Conventional, uncoated polymeric particles have hydrophobic surfaces that adhere to hydrophobic domains on mucin fibers – the long, entangled glycoproteins responsible for the mucus gel structure [10]. We discovered that coating polymeric nanoparticles with a dense layer of low molecular weight polyethylene glycol (PEG) minimizes particle adhesion to mucus [6]. PEG minimizes protein adhesion at biomaterial surfaces because the hydrated PEG layer enthalpically resists release of water molecules and entropically resists compression [11]. Using PEG-coated particles, we were able to probe the mucus microstructure [12]. Surprisingly, we found that PEG-coated particles as large as 200 nm in diameter can penetrate sputum freshly expectorated by CF patients [7], and coated particles as large as 500 nm can penetrate CV mucus collected from healthy women [6], whereas comparably sized uncoated particles are immobilized by the mucus mesh. Tracking the motion of individual PEG-coated and uncoated particles in fresh, minimally diluted mucus samples offers several advantages compared to traditional approaches for studying mucus gel structure and permeability. Electron microscopy, for instance, requires fixation methods that can change the mucus structure [10]. Transport studies using only conventional, uncoated polymeric particles cannot distinguish between adhesive and steric contributions to particle trapping. Finally, bulk diffusion measurements do not reveal transport rates at the single-particle level, and thus cannot identify particle subpopulations of interest, such as fast-moving outliers.
Mucus secretions from various wet epithelia are similar in overall properties – they are viscoelastic gels comprised of water and mucins, as well as salts, non-mucin proteins, and lipids – but their exact biochemical compositions, clearance rates, and microbiota differ with anatomical site and disease state [2]. These factors alter the mucus microstructure and permeability, and motivate the analysis of mucus from various tissues and conditions. Here, our aim was to study normal human airway mucus, which we define as airway mucus from individuals without respiratory disease. As our model for normal airway mucus, we collected the mucus from endotracheal tubes of patients without respiratory comorbidities who underwent elective, non-cardiothoracic surgery, as previously described [13]. This collection method is noteworthy because it imposes no additional burden on the patient, and in contrast to bronchiolar lavage or to sputum induction using nebulized hypertonic saline, it minimizes sample dilution and salivary contamination [13, 14]. We performed scanning electron microscopy to visualize the mucus samples and conducted biochemical assays to analyze their basic composition. To elucidate the physicochemical properties of nanoparticles that govern their transport rates in normal airway mucus, we measured the mobility of PEG-coated and uncoated 100, 200, and 500 nm polymeric particles in fresh mucus samples using high-resolution fluorescence microscopy and multiple-particle tracking analysis. We further investigated the mucus gel structure and the barrier it poses to nanoparticle diffusion by comparing the bulk rheological properties of respiratory mucus, as measured using a cone-and-plate rheometer, with the microrheological properties, as probed by PEG-coated nanoparticles.
2. Methods
2.1 Mucus sample collection
Human airway mucus samples were collected in accordance with a protocol approved by the Johns Hopkins Medicine Institutional Review Board (study number NA_00038606). Samples were collected by the endotracheal (ET) tube method, as previously described [13, 15]. Patients who required intubation as part of general anesthesia for elective, non-cardiothoracic surgery at the Johns Hopkins Hospital were identified. Only patients with no cardiopulmonary or respiratory comorbidities and no smoking history were included in this study. At the end of surgery, the ET tube was removed from the patient, and the distal 10 cm portion, including the balloon cuff, was cut and placed in a 50 mL centrifuge tube. The specimens were then spun at 1000 rpm (220 × g) for 30 s, yielding an average mucus volume of 0.5 mL. Mucus with visible blood contamination was not included in the analysis. Mucus samples were stored at 4 °C and analyzed within 24 hours of collection, excluding portions for the mucin and DNA assays, which were frozen at −20 °C until use. The data presented here is from male and female patients; the mean patient age was 56 years, with standard deviation 17 years.
2.2 Rheology of respiratory mucus
Bulk rheological properties of airway mucus were measured using a strain-controlled rheometer (RFS3; TA Instruments) with cone-and-plate geometry (cone diameter 25 mm and angle 0.1 rad). All measurements were conducted at room temperature in a humidified chamber. Oscillatory tests, performed at small strain amplitudes to minimize shear damage to the mucus samples, were used to measure the frequency-dependent elastic modulus, G′(ω), and viscous modulus, G″(ω), of the mucus [16]. (The elastic and viscous moduli, respectively, are the in-phase and out-of-phase components of stress induced in the material, divided by the magnitude of the applied strain.) After the mucus sample was loaded onto the rheometer, it was allowed to equilibrate for five minutes. Then, the linear viscoelastic region, for which the viscous and elastic moduli are independent of strain amplitude [17], was determined by conducting strain amplitude sweeps from 0.2 to 10% strain at frequencies of 1, 6.28, and 100 rad/s. Based on the strain sweep tests, 1% strain was determined to be within the linear viscoelastic region, and was thus used for the frequency sweep test (from 0.1 to 100 rad/s).
2.3 Determination of mucin, DNA, and total solids content
Mucin concentration was determined based on the reaction of 2-cyanoacetamide (Sigma-Aldrich) with O-linked glycoproteins, as previously described [8, 18]. Mucus aliquots were diluted 20-fold and homogenized by vortexing for at least 15 min. Then, 50 μL of this suspension was mixed with 60 μL of an alkaline solution of 2-cyanoacetamide (200 μL of 0.6 M 2-cyanoacetmaide added to 1 mL of 0.15 M NaOH). The mixture was incubated at 100 °C for 30 min, after which 0.5 mL of 0.6 M borate buffer, pH 8.0, was added to it. Fluorescence intensity was measured at excitation and emission wavelengths of 340 and 420 nm, respectively. Mucin concentrations were calculated by comparing the fluorescence intensity readings to a standard curve generated using known concentrations of mucin from bovine submaxillary gland (Sigma-Aldrich).
A fluorimetric assay was also used to measure DNA concentration, based on the reaction of 3,5-diaminobenzoic acid dihydrochloride (DABA; Sigma-Aldrich) with DNA [8]. Mucus aliquots were diluted 5-fold and homogenized by vortexing for at least 15 min. Then, 30 μL of this suspension was reacted with 30 μL of 20% wt/vol DABA solution. After incubating for 1 h at 60 °C, 1 mL of 1.75 M HCl was then added to stop the reaction. The fluorescence was measured at excitation and emission wavelengths of 400 and 520 nm, respectively. DNA concentrations were calculated with reference to a standard curve generated using known concentrations of DNA from salmon testes (Sigma-Aldrich).
The total solids content of mucus was determined by freeze-drying. Mucus samples were frozen in liquid N2 and placed in a lyophilizer (FreeZone 4.5 Plus; Labconco) for at least 12 h to extract water from the samples. The ratio of mucus mass before versus after lyophilization is the total solids content.
2.4 Scanning electron microscopy
Respiratory mucus samples were prepared for scanning electron microscopy (SEM) based on a protocol previously used for CV mucus and CF sputum [8, 19]. Mucus samples were fixed for 1 h in 2% glutaraldehyde in 100 mM sodium cacodylate buffer, pH 7.2, containing 3 mM CaCl2. The samples were then rinsed in buffer and postfixed in 1% OsO4 in 100 mM sodium cacodylate buffer for 1 h on ice in the dark. Following a brief rinse with distilled water, samples were stained with 2% uranyl acetate for 1 h and then dehydrated through a graded series of ethanol solutions. Upon complete dehydration, samples were soaked in a 50:50 mixture of ethanol and hexamethyldisilazane (HMDS), followed by pure HMDS. Mucus samples were then dessicated under vacuum overnight. The samples were attached to aluminum stub mounts via carbon adhesive tabs (Ted Pella), and then coated with 20 nm of AuPd with a sputter coater (Desk III; Denton Vacuum). The samples were imaged with a field-emission scanning electron microscope (LEO 1530 FESEM; Zeiss) operating at 1 kV.
2.5 Nanoparticle preparation and characterization
Fluorescent, carboxylate-modified polystyrene spheres (PS-COOH) sized 100, 200, and 500 nm in diameter were purchased from Molecular Probes. The surface density of carboxyl groups on the particles, calculated using data provided by the manufacturer, ranged from 3 to 8 COOH/nm2 depending on the lot. PEG-coated particles were prepared by covalently modifying the PS-COOH particles with 5 kDa methoxy-PEG-amine (Creative PEGWorks) using carbodiimide coupling chemistry [20]. Briefly, 100 μL of PS-COOH particle suspension, supplied by the manufacturer as 2% solids, were washed and resuspended to 4-fold dilution in ultrapure water in a 1.5 mL microcentrifuge tube. PEG was added to the particle suspension in excess (2-fold excess for the 100 nm particles and 5-fold excess for the 200 and 500 nm particles), based on the number of carboxyl groups on the particles. After mixing to dissolve the PEG, N-hydroxysulfosuccinimide sodium salt (sulfo-NHS; Sigma-Aldrich) was added to the tube, followed by 600 uL of 200 mM borate buffer, pH 8.2, and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC; Invitrogen). NHS and EDC concentrations were 10 mM and 6 mM, respectively. Particles suspensions were placed on a tube rotator for 4 hours, then centrifuged and washed with ultrapure water. Particles were resuspended in ultrapure water to the original concentration. Aliquots for particle transport experiments were diluted with ultrapure water to obtain concentrations appropriate for tracking individual particles. Particle suspensions were stored at 4 °C until use.
The surface density of PEG on PS-PEG particles prepared according to this protocol was recently measured to be 0.09 PEG/nm2, using a nuclear magnetic resonance (NMR) method, as reported by our lab [20]. Particle size and zeta-potential were measured by dynamic light scattering and laser Doppler anemometry, respectively, using a Zetasizer Nano ZS90 (Malvern Instruments), which has a 90° scattering angle. Both size and zeta-potential were measured at 25 °C, with the particles suspended in phosphate buffered 10 mM NaCl solution, pH 7.4.
2.6 Multiple particle tracking in respiratory mucus
Nanoparticle transport in airway mucus was studied by multiple particle tracking (MPT). The particle suspensions were added to mucus samples at a final dilution of 4% vol/vol in custom-made microscopy chambers. After gently stirring the samples, the chambers were sealed to prevent sample dehydration, then incubated for 1.5 h. Particle motion in the mucus samples was observed at room temperature using an inverted epifluorescence microscope (Axio Observer; Zeiss) with a 100×/1.46 NA oil-immersion objective. Movies were collected for 20 s at a temporal resolution of 67 ms using an EMCCD camera (Evolve 512; Photometrics). Movies were analyzed with MetaMorph software (Molecular Devices) to extract the x and y positions of particle centroids over time. At least 100 PS-COOH and PS-PEG particles of each size were tracked for at least 50 frames in each of five mucus samples. For each trajectory, the time-averaged mean squared displacement (MSD) was calculated as a function of time scale, τ, as 〈Δr2(τ)〉 = 〈[x(t + τ) − x(t)]2〉 + 〈[y(t + τ) − y(t)]2〉. The tracking resolution, determined by tracking particles immobilized to glass with a strong adhesive [21], was determined to be 10 nm. Based on the SEM images, airway mucus may be assumed to be isotropic (though not homogeneous), so the 2-dimensional MSD measured here by MPT equals two-thirds of the 3-dimensional MSD. The ensemble-averaged MSD (〈MSD〉) for all particles of a given type in a given mucus sample was calculated by taking the geometric mean of the individual particles’ time-averaged MSDs. We fit the 〈MSD〉 data to the equation 〈MSD〉 = kτα to obtain α, which is a measure of the extent of impediment to particle diffusion (α = 1 for pure Brownian motion and α < 1 for subdiffusion). This was accomplished by calculating the slope of log(〈MSD〉) vs. log(τ) using the least-squares method. Additional information on MPT measurements is available in review articles [21, 22].
2.7 Particle tracking microrheology analysis
Particle tracking microrheology analysis was performed by applying the generalized Stokes-Einstein relation (GSER) to the PS-PEG particle MSD data. The GSER relates the thermal motion of particles to the viscoelastic moduli of the material environment they probe. The unilateral Laplace transform of the MSD, , is related to the viscoelastic spectrum of the material, G̃(s), according to the GSER by . Here, kB is Boltzmann’s constant, T is the absolute temperature, a is the particle radius, and s is the complex Laplace frequency. This can be used to find the complex modulus, G* (ω) = G′(ω) + iG″(ω), as detailed elsewhere [23, 24]. In this way, the particles’ displacements can be used to calculate the microscopic viscoelasticity of the environment they probe. A material’s viscoelastic response can alternatively [25] be interpreted as a complex viscosity, η*(ω), whose magnitude is defined as |η*(ω)| = |G*(ω)|/ω. Complex viscosity thus incorporates both viscous and elastic contributions. In the limiting case, for a purely viscous Newtonian liquid such as water, the complex viscosity equals the standard steady shear viscosity.
2.8 Viscosity of mucus interstitial fluid
The viscosity of mucus interstitial fluid was determined by centrifuging mucus samples for 1 h at 21,000 × g, removing the supernatant, and then measuring the supernatant viscosity by particle tracking microrheology (we confirmed that these supernatant viscosity measurements did not depend on probe particle size).
2.9 Statistical analysis
Statistical analysis (for Fig. 6) was performed by one-way analysis of variance (ANOVA) using logarithmically transformed data. Subsequent multiple comparison tests were performed using Tukey’s HSD procedure. Analysis was conducted using the Statistics Toolbox in MATLAB R2012a (MathWorks). Differences were considered to be statistically significant at a level of P < 0.01.
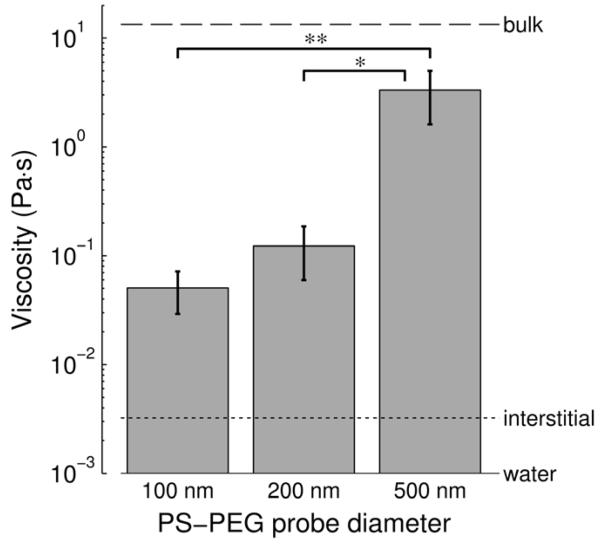
Figure 6.
Micro- vs. macroscopic viscosity of human respiratory mucus. Viscosity reported is |η(ω)*|, the magnitude of the complex viscosity, at ω = 1 rad/s. Complex viscosity incorporates both the viscous and elastic contributions to a material’s rheological behavior. Microscopic viscosity experienced by muco-inert 100, 200, and 500 nm PS-PEG probes in respiratory mucus was measured by particle tracking microrheology (PTM). Macroscopic viscosity, also called bulk viscosity (dashed horizontal line), was measured using a cone-and-plate rheometer. Mucus interstitial fluid (dotted line) was characterized by centrifuging mucus samples and measuring the supernatant’s viscosity by PTM. For reference, the viscosity of water at 20 °C is a lso shown (solid horizontal line). Viscosity values represent n = 5 mucus samples, and error bars indicate standard error of the mean. Brackets with asterisks denote statistically significant differences (*, P < 0.01; **, P < 0.005)
3. Results
3.1 Bulk rheology
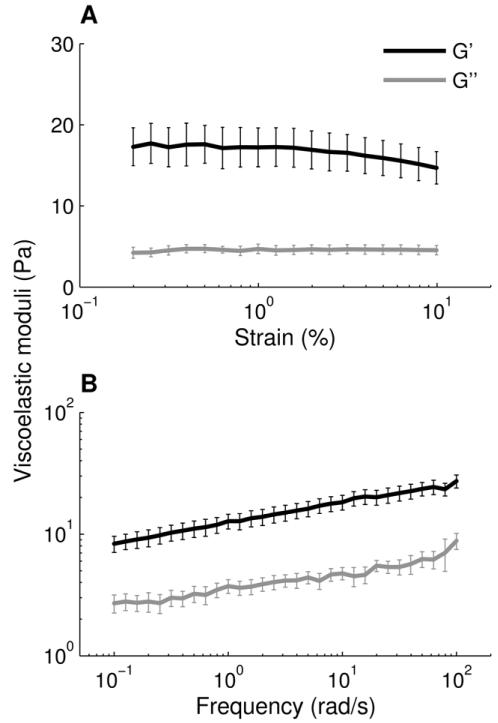
To characterize the bulk material properties of respiratory mucus, we measured the viscoelastic moduli of mucus samples (n = 5) using a cone-and-plate rheometer. We determined from strain sweep tests that 1% strain is within the linear viscoelastic region (Fig. 1A). We then measured the frequency-dependent viscoelastic moduli at 1% strain and found that the elastic modulus, G′(ω) exceeded the viscous modulus, G″(ω), over the 3 decades of frequency tested (Fig. 1B). A frequency of ω = 1 rad/s is traditionally used in mucus rheology studies to approximate the low velocities of mucociliary clearance [26, 27]. The ratio of the viscous to elastic modulus, tan(δ) = G″(ω)/G′(ω), was 0.30 at ω = 1 rad/s; this corresponds to a phase angle of δ = 17°. Materials with 0 < G″/G′ < 1, or equivalently 0° < δ < 45°, are categorized as viscoelastic solids. The viscous and elastic moduli exhibit a weak frequency dependence, with log(G′) and log(G″) both having slopes around 0.15 when plotted against log(ω). Overall, this rheological signature is characteristic of a crosslinked gel, as expected [27].
Figure 1.
Bulk rheology of airway mucus. (A) Strain-dependent viscous and elastic moduli from 0.2 to 10% strain at a frequency of 1 Hz (6.28 rad/s). (B) Frequency-dependent viscous and elastic moduli from 0.1 to 100 rad/s at 1% strain. Error bars represent standard error of the mean for n = 5 mucus samples.
3.2 Biochemical analysis
To assess mucus sample quality and composition, we conducted basic biochemical analysis. The total solids content of airway mucus was 6.9 ± 2.8% (n = 13). The measured mucin content ranged from 8 to 22% of the total solids, while DNA comprised less than 1% of the total solids on average. The pH of the mucus samples ranged from 7 to 8.5.
3.3 Microstructure of respiratory mucus
We imaged airway mucus using scanning electron microscopy, which visually confirmed that the mucus samples have a meshwork architecture (Fig. 2). In the SEM images, pores in the mucus mesh ranged from tens to hundreds of nm in diameter, with many pores smaller than 100 nm; we note, however, that SEM sample preparation likely contracts the mucus microstructure [10] . The fibers appeared to be randomly oriented, which suggests that the mucus samples are isotropic. The SEM images of normal airway mucus were similar in overall appearance to previously published images of CF sputum [8, 28].
Figure 2.
Scanning electron micrograph shows network microstructure of airway mucus. Scale bar represents 500 nm.
3.4 Transport of particles in respiratory mucus
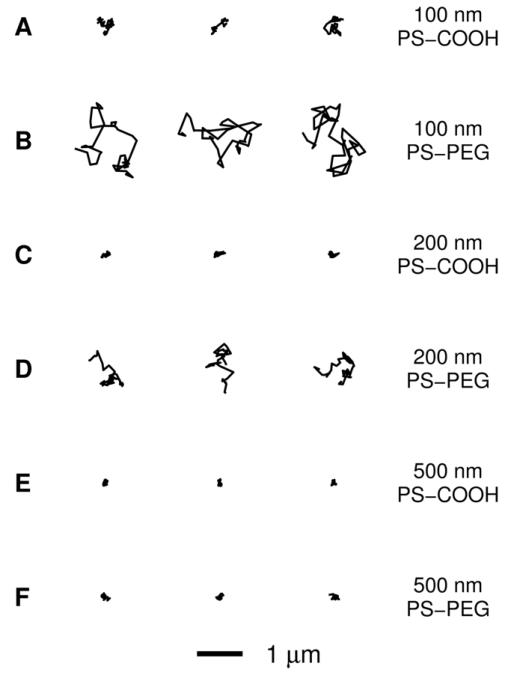
We compared the thermally-driven motion of PEG-coated polystyrene nanoparticles (PS-PEG) to that of conventional carboxylate-modified PS nanoparticles (PS-COOH) in fresh human respiratory mucus using multiple-particle tracking (MPT). The dense PEG layer on the PS-PEG particles was confirmed by their near-neutral surface charge, compared to the highly negative surface charge of the PS-COOH particles (Table 1). We have also recently quantified the PEG density using an NMR-based method [20]. The 100 nm diameter PS-COOH particles exhibited hindered trajectories in airway mucus, and the 200 and 500 nm PS-COOH particles were essentially immobilized (Fig. 3A, C and E). In contrast, the 100 and 200 nm PS-PEG particle trajectories were more diffusive (Fig. 3B and D). The 500 nm PS-PEG particles exhibited constrained traces (Fig. 3F) indistinguishable from those of the 500 nm PS-COOH particles.
Table 1.
Particle characterization and transport summary
| Particle sizea, nm | Material | Diameterb, nm | ζ-potentialc, mV |
Dw/〈Deff〉d |
α e |
|---|---|---|---|---|---|
| 100 | PS-COOH | 92 ± 3 | −50 ± 3 | 390 | 0.68 |
| 100 | PS-PEG | 99 ± 1 | −1 ± 1 | 26 | 0.88 |
| 200 | PS-COOH | 188 ± 1 | −54 ± 4 | 1600 | 0.54 |
| 200 | PS-PEG | 219 ± 3 | −5 ± 1 | 41 | 0.82 |
| 500 | PS-COOH | 508 ± 2 | −73 ± 3 | 980 | 0.54 |
| 500 | PS-PEG | 549 ± 6 | −3 ± 1 | 590 | 0.59 |
Provided by the manufacturer.
Measured by dynamic light scattering. Error values represent standard deviation of 3 measurements.
Measured in 10 mM NaCl at pH 7.4. Error values represent standard deviation of 3 measurements.
Dw is the diffusivity of particles in water, as calculated from the Stokes–Einstein equation. 〈Deff〉 is the effective diffusivity of particles in mucus measured at a time scale of 1 s, and it is calculated as 〈Deff〉 = 〈MSD(τ)〉/4τ with τ = 1 s. The ratio Dw/〈Deff〉 indicates the average factor by which the transport of particles in mucus is slowed compared to in water.
Calculated by fitting 〈MSD(τ)〉 to 〈MSD(τ)〉 = kτα for τ between 0.2 and 3.2 s. For anomalous diffusion, 0 < α < 1. Smaller values of α indicate more hindered particle motion.
Figure 3.
Representative trajectories of nanoparticles in airway mucus. Trajectories show 3 s of motion. The trajectories presented are within one standard error of the mean MSD for each particle type. (A) 100 nm PS-COOH particles. (B) 100 nm PS-PEG particles. (C) 200 nm PS-COOH particles. (D) 200 nm PS-PEG particles. (E) 500 nm PS-COOH particles. (F) 500 nm PS-PEG particles.
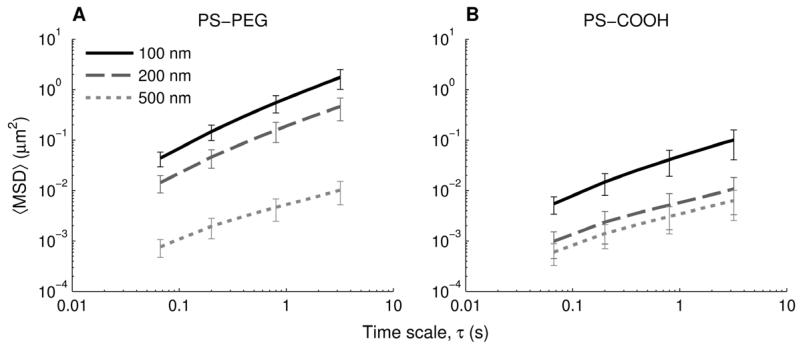
To quantify these differences, we calculated the mean squared displacements (MSDs) of at least 100 individual particles of each particle type in each mucus sample (n = 5). At a time scale (τ) of 1 s, the geometric ensemble-averaged MSD (〈MSD〉) of 100 and 200 nm PS-PEG particles was 14-fold and 33-fold greater, respectively, compared to the 100 nm and 200 nm PS-COOH particles (Fig. 4). However, the 〈MSD〉 of the 500 nm PS-PEG particles was similar to that of the 500 nm PS-COOH particles for all time scales. At a time scale of 1 s, the 100 and 200 nm PS-PEG particles were slowed only 26- and 41-fold, respectively, compared to their theoretical diffusivities in water, as calculated with the Stokes-Einstein equation. In contrast, the 100 and 200 nm uncoated particles were slowed 390- and 1600-fold, respectively, compared to their theoretical diffusivities in water. The 500 nm particles, both PS-COOH and PS-PEG, were slowed more than 500-fold compared to their theoretical diffusivities in water (Table 1).
Figure 4.
Ensemble-averaged geometric mean squared displacement (〈MSD〉) as a function of timescale for nanoparticles in airway mucus. (A) 100, 200, and 500 nm PS-PEG particles. (B) 100, 200, and 500 nm PS-COOH particles. Data represents 5 mucus samples, with at least 100 particles of each particle type tracked per sample. Error bars are presented as standard error of the mean.
To further assess the extent to which normal airway mucus hinders particle motion, we fit the ensemble-averaged MSDs to 〈MSD〉 = kτα and extracted α, the anomalous diffusion exponent. For pure Brownian motion in a viscous liquid, α = 1; for anomalous subdiffusion in complex environments over short time scales, μ < 1, with smaller μ indicating more hindered particle motion [22]. The value of μ for 100 and 200 nm PS-PEG particles was 0.88 and 0.82, respectively, while for the 100 and 200 nm PS-COOH particles, it was 0.68 and 0.54, respectively (Table 1). The α values were only 0.59 and 0.54 for 500 nm PS-PEG and PS-COOH particles, respectively.
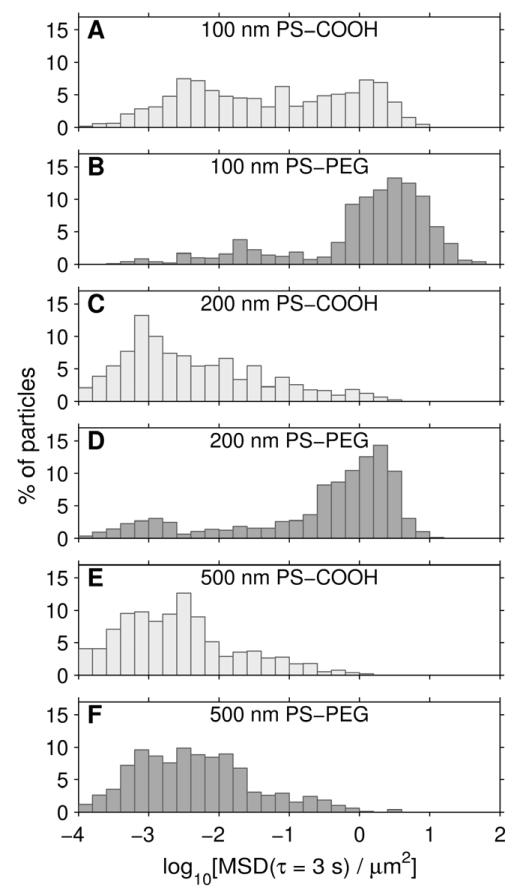
Fast-moving particles are of particular interest, as they are more likely to penetrate the heterogeneous mucus layer quickly enough to reduce their removal by mucociliary clearance and approach the underlying epithelium. In addition to ensemble-averaged transport rates, MPT also provides individual particle data, which can be represented in a histogram of individual particle MSDs (Fig. 5; this data is presented at τ = 3 s to facilitate comparison with the 3 s long trajectories in Fig. 3). Particles with MSDs less than 0.1 μm2 at a time scale of 3 s appeared immmobile in our time-lapse videos. The MSD value of 0.1 μm2 at τ = 3 s is approximately 500-fold lower than that of a 100 nm particle diffusing in water. If we define particles below this cutoff as immobile and above it as mobile, we find 84%, 75%, and 8% of 100, 200, and 500 nm PS-PEG particles, respectively, were mobile. The 100 nm PS-COOH particles did have a sizable mobile fraction by the above definition, over 42%, whereas only 11% of 200 nm and 5% of 500 nm PS-COOH particles were mobile.
Figure 5.
Distribution of individual particle mean squared displacements at a time scale of 3 s. Data represents 5 mucus samples, with at least 100 particles of each type tracked per sample. (A) 100 nm PS-COOH particles. (B) 100 nm PS-PEG particles. (C) 200 nm PS-COOH particles. (D) 200 nm PS-PEG particles. (E) 500 nm PS-COOH particles. (F) 500 nm PS-PEG particles.
3.5 Micro- vs. macroscopic rheology
To deepen our understanding of the mucus gel structure and the barrier it poses to different sizes of muco-inert particles, we compared the macroscopic (bulk) rheological properties of respiratory mucus, as measured using a cone-and-plate rheometer, with the microscopic rheological properties, as measured by particle tracking microrheology (PTM). To facilitate this comparison, the values we report here are |η*(ω)|, the magnitude of the complex viscosity, at ω = 1 rad/s. At the bulk level, respiratory mucus had a complex viscosity magnitude more than 13,000 times that of water. In contrast, the measured viscosity of mucus interstitial fluid was approximately 3 times that of water (Fig. 6), in good agreement with a previous study on CF sputum [29]. The 100 and 200 nm PS-PEG particles experienced viscosities only 16- and 38-fold higher than that of the mucus interstitial fluid, respectively, and more than 250- and 100-fold lower than the bulk viscosity (Fig. 6). In contrast, the 500 nm PS-PEG particles experienced an average viscosity more than 1000 times greater than that of the mucus interstitial fluid, and only 4-fold lower than the bulk viscosity of mucus.
4. Discussion
In this study, we explored the barrier properties of human respiratory mucus collected from individuals without lung disease. We found that polymeric nanoparticles as large as 200 nm, if densely coated with low molecular weight PEG, can rapidly diffuse through respiratory mucus. This finding will help guide the design of inhaled therapeutic nanoparticles – including drug, gene, and vaccine carriers – which offer the potential for increased efficacy and reduced side effects compared to traditional approaches for treating and preventing respiratory disease. To be maximally effective, inhaled chemotherapy-loaded nanoparticles for lung cancer [30] and nanoparticle-based vaccines for tuberculosis and influenza [31, 32] may need to achieve broad coverage throughout the airways and avoid rapid clearance by the mucociliary escalator [5]. We recently reported that mucus-penetrating nanoparticle formulations can improve the distribution, retention, and efficacy of vaginally administered drugs [33]. Likewise, particles that can rapidly penetrate airway mucus may achieve reduced clearance and improved airway distribution, retention, and pharmacokinetic profile. The present work also enhances understanding of the barrier properties of airway mucus to inhaled environmental particles, such as viruses and particulate matter pollution, both of which are major global health risks [34].
The findings in this study suggest that airway mucus is a selectively permeable barrier, whose barrier properties depend upon both particle size and adhesiveness. Small, PEG-coated particles exhibit enhanced transport in airway mucus compared to similarly sized uncoated particles because the dense PEG coating reduces particle adhesion to the mucus network, permitting the particles to diffuse more freely [6, 7, 12]. The low viscosities experienced by 100 and 200 nm PEG-coated particles indicate that these particles resist muco-adhesion and furthermore are small enough to diffuse through fluid-filled openings in the mucus network, so they are less sensitive to the bulk rheological properties of the mucus. To these particles, on average, the mucus barrier is a low-viscosity liquid. In contrast, the 500 nm particles, even with PEG coatings, experience significantly larger viscosities, approaching the mucus bulk viscosity. This is most likely because their large size relative to the mucus mesh size precludes the particles from readily percolating through the mucus gel. Particles that are large relative to the mucus mesh spacing, similarly to particles that firmly adhere to mucus, are sensitive to the elasticity of the biopolymer network and its solid-like bulk rheology.
Our finding that PEG-coated particles as large as 200 nm in diameter can diffuse in respiratory mucus appears to conflict with our visual analysis of SEM images, in which the majority of pores appeared to be smaller than 100 nm. As previously documented, this discrepancy is likely caused by alteration of the mucus network during SEM sample preparation, which entails fixation and dehydration of the mucus [10]. Furthermore, polymers in solutions and gels like mucus are dynamic, which a static SEM image cannot show [10, 35]. Because of this dynamic nature, a non-adhesive particle whose diameter is comparable to the average mesh size may be able to percolate through the mucus [35, 36], which might not be evident from SEM image analysis. Particle tracking allowed us to overcome these challenges and probe the microstructure of fresh, minimally perturbed airway mucus samples.
To effectively shield against adhesive hydrophobic and electrostatic interactions and minimize protein binding, PEG must be densely grafted to the particle surface. PEG tends to adopt an extended “brush” conformation if the surface density is sufficiently high that the distance between grafted PEG chains is smaller than their Flory radius (the unconstrained polymer size in a good solvent) [37, 38]. In contrast, the PEG adopts a “mushroom” conformation when PEG density is lower, such that adjacent chains are far enough apart to generally avoid overlap. We recently reported that our 100 nm PS-PEG particles with near-neutral zeta-potential had approximately 0.09 PEG chains per nm2 of particle surface, or one chain per ~11 nm2, which is within the brush regime for 5 kDa PEG [20]. Dense PEG brush coatings have been shown to better shield nanoparticles against interactions with biomolecules [20, 37].
While we found that PEG-coated particles as large as 200 nm in diameter can diffuse in respiratory mucus gel, we note that in vivo, the mucus gel layer sits atop the periciliary layer (PCL), which is expected to serve as a further barrier to particles. Recent evidence suggests that the PCL is not a watery liquid, as previously thought, but rather consists of a dense brush of macromolecules grafted to cilia and the epithelial surface [1]. In primary human bronchial epithelial (HBE) cell cultures, the periciliary brush has a maximum mesh size of approximately 40 nm [1]. This may provide an extra layer of protection to the airway epithelia, beyond that of the mucus gel studied here, in airway regions with an intact PCL. There may be gaps in the PCL above secretory cells [39], so particles might be able to more readily breach the lung’s protective barrier at those locations. We must also emphasize that in healthy individuals, ciliary beating constantly propels mucus out of the lungs, while continuous mucin secretion simultaneously replenishes the mucus gel layer [2]. To reach the PCL, a nanoparticle must therefore diffuse through the mucus gel layer faster than it is swept away by mucociliary activity.
We recognize that mucus collected by the endotracheal tube method may be altered compared to the mucus gel layer in vivo. When the patients were in the operating room, their endotracheal tubes were connected to a passive heat and moisture exchanger (Humid-Vent) to humidify the ventilated air. Still, the ventilated air may be drier than air humidified by passing through the upper respiratory tract during normal respiration. Endotracheal tube mucus could thus be dehydrated compared to mucus in vivo [13]. If so, the work here would set a conservative bound on the nanoparticle size capable of penetrating respiratory mucus. However, our rheological and biochemical data suggest that the endotracheal tube mucus samples were of good quality. First, in terms of rheology, we measured tan(δ) = 0.30 at ω = 1 rad/s; this is in close agreement with previously published values for respiratory mucus collected by the endotracheal tube method (0.33) and by a bronchoscopy brush method (0.28) from individuals with no respiratory disease [13, 15]. Second, in terms of total solids content, we measured an average of 6.9% solids. This is in reasonable agreement with the 5.2% solids reported by Matthews et al. for “normal” mucus, which they collected from laryngectomized patients [40]. Other papers report that normal mucus is 2 to 3% solids, but those values are based on mucus collected from HBE cell cultures [1, 4]. Third, with regards to biochemical composition, we measured mucin contents ranging from 8 to 22% of the total solids, which is consistent with reports that mucins account for less than 30% of the mucus solids [2]. We found that DNA was less than 1% of the total solids on average, as expected for healthy respiratory mucus, and in contrast to the elevated (approximately10-fold higher) DNA concentration in CF sputum, for instance [2, 8, 40].
Previously, we characterized sputum expectorated by adult CF patients [7]. Comparing that work with the present study, PEG-coated 200 nm particles were slowed 65-fold in CF sputum and 41-fold in normal airway mucus at a timescale of 1 s, compared to their theoretical diffusivities in water. This difference may be due to the increased concentrations of DNA, actin, and other debris released by dead inflammatory cells in CF sputum [2]. In both normal mucus and CF sputum, 500 nm particles were immobilized. Although the collection methods are different, making direct comparison challenging, this finding implies that both normal and CF mucus are significant barriers to nanoparticle transport. We note that in our previous work with CF sputum, 100 nm PS-PEG particles were immobilized, which we attributed to difficulty coating these smaller, more highly curved particles with a dense PEG layer [7]. However, we have since engineered improved 100 nm particles with denser PEG coatings, as discussed [6, 12, 20]. We show here that these improved 100 nm PS-PEG particles can penetrate normal airway mucus, and they are also capable of penetrating CF sputum (unpublished data). Thus, the difference in transport of 100 nm PS-PEG particles between our previous CF paper and the present work with normal airway mucus reflects the quality of the PEG coating, not the disease state.
We found that there are profound differences between respiratory mucus and mucus collected from the female reproductive tract [6, 12]. Whereas 500 nm PS-PEG particles are immobilized in airway mucus, they can effectively penetrate cervicovaginal mucus (CVM) from healthy women. This indicates that the airway mucus collected here has a significantly tighter mesh than does CVM. This may reflect the greater need for particle trapping in the airways, which are continuously exposed to the external environment. Despite its larger mesh spacing, CVM’s bulk viscous and elastic moduli are roughly one order of magnitude greater than those of airway mucus [41]. This may be explained as follows: Mucins in native CVM are bundled by hydrophobic interactions [41], and may be more bundled than are mucins in airway mucus. Increased mucin bundling in CVM would produce thicker mucin fibers, resulting in larger viscoelastic moduli and a more porous microstructure, of CVM compared to airway mucus. This finding agrees with experiments using a model actin gel, where it was found that for a fixed actin concentration, increased actin fiber bundling caused both increased mesh size and increased elastic modulus [42]. To further understand how mucus differs between different anatomical locations, the techniques used here could also be applied to characterize the rheology and permeability of mucus from other mucosal tissues, such as the GI tract.
Another difference we found in this study was that approximately 40% of 100 nm PS-COOH particles are mobile in respiratory mucus, whereas 100 nm PS-COOH particles are almost entirely immobilized in CVM [6]. This suggests that particles with hydrophobic and anionic surface regions, such as PS-COOH particles, may be less adhesive to respiratory mucus than they are to CVM. It is possible that abundant endogenous surfactants in airway mucus [43, 44] may coat hydrophobic regions on mucins or on the particles, making the PS-COOH particles less adhesive to respiratory mucus. The consequences of this phenomenon on particle transport are more noticeable for smaller particles, most likely because smaller particles are less likely to form multiple contacts with the mucus gel network, and thus are less avidly adhered. One finding supporting this explanation is that, compared to untreated CF sputum, 200 nm PS-COOH particles and adhesive rod-shaped gene vectors exhibit enhanced transport in sputum treated with N-acetyl cysteine, which increases the pore size by cleaving disulfide crosslinks in the mucin mesh [28, 45]. The 100 nm PS-COOH particle transport data suggests that viruses and very small environmental pollution particles that deposit in the airways may be capable of penetrating the airway mucus gel layer to some extent, increasing the threat they pose to the body. For instance, our results suggest that the influenza virus (d ≈ 100 nm, smaller than the mucus mesh size) should be able to penetrate respiratory mucus, unless it is strongly immobilized by adhesive interactions with mucus constituents.
5. Conclusion
Using multiple particle tracking of muco-inert probes, we determined the microstructure of respiratory mucus collected from endotracheal tubes of humans without lung disease. We found that PEG-coated particles as large as 200 nm are capable of penetrating normal human airway mucus. Whereas respiratory mucus is a viscoelastic solid at the bulk level, small PS-PEG particles ≤ 200 nm can penetrate the mucus as if it were primarily a viscous liquid. We also established principles and methods that can be applied to design and test novel therapeutic mucus-penetrating nanoparticles for airway delivery, as well as to study the transport of respiratory viruses and environmental nanoparticles in physiologically relevant mucus samples.
Acknowledgements
The authors gratefully acknowledge Elizabeth Nance for assistance with particle PEGylation, Shimon Unterman and Rangaramanujam Kannan for assistance with rheology, and George Lin and Conan So for help with particle tracking. We thank Michael Delanoy and Barbara Smith from the Johns Hopkins School of Medicine Microscope Facility for assistance with scanning electron microscopy. We also thank Ying-Ying Wang, Tao Yu, Anthony Kim, Craig Schneider, Jane Chisholm, and Richard Cone for insightful discussions. We are indebted to the many faculty, staff, and fellows in the Johns Hopkins Department of Neurosurgery and the Department of Anesthesiology and Critical Care Medicine who assisted with sample collection.
This work was supported by the NIH (grants P01HL51811, P50HL107190, and R01EB003558) and the Cystic Fibrosis Foundation (grant HANES07XX0). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The mucus-penetrating particle technology described in this publication is being developed by Kala Pharmaceuticals. Dr. Hanes is co-founder of Kala and serves on its Board of Directors. Dr. Hanes owns company stock, which is subject to certain restrictions under Johns Hopkins University policy. The terms of this arrangement are being managed by Johns Hopkins University in accordance with its conflict of interest policies.
References
- [1].Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–41. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–47. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sanders NN, De Smedt SC, Demeester J. The physical properties of biogels and their permeability for macromolecular drugs and colloidal drug carriers. J Pharm Sci. 2000;89:835–49. doi: 10.1002/1520-6017(200007)89:7<835::AID-JPS1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- [4].Tarran R, Grubb BR, Gatzy JT, Davis CW, Boucher RC. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J Gen Physiol. 2001;118:223–36. doi: 10.1085/jgp.118.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–71. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, et al. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci U S A. 2007;104:1482–7. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Suk JS, Lai SK, Wang YY, Ensign LM, Zeitlin PL, Boyle MP, et al. The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials. 2009;30:2591–7. doi: 10.1016/j.biomaterials.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sanders NN, De Smedt SC, Van Rompaey E, Simoens P, De Baets F, Demeester J. Cystic fibrosis sputum: a barrier to the transport of nanospheres. Am J Respir Crit Care Med. 2000;162:1905–11. doi: 10.1164/ajrccm.162.5.9909009. [DOI] [PubMed] [Google Scholar]
- [9].Lai SK, Suk JS, Pace A, Wang YY, Yang M, Mert O, et al. Drug carrier nanoparticles that penetrate human chronic rhinosinusitis mucus. Biomaterials. 2011;32:6285–90. doi: 10.1016/j.biomaterials.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cone RA. Barrier properties of mucus. Adv Drug Deliv Rev. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- [11].Valentine MT, Perlman ZE, Gardel ML, Shin JH, Matsudaira P, Mitchison TJ, et al. Colloid surface chemistry critically affects multiple particle tracking measurements of biomaterials. Biophys J. 2004;86:4004–14. doi: 10.1529/biophysj.103.037812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lai SK, Wang YY, Hida K, Cone R, Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc Natl Acad Sci U S A. 2010;107:598–603. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rubin BK, Ramirez O, Zayas JG, Finegan B, King M. Collection and analysis of respiratory mucus from subjects without lung disease. Am Rev Respir Dis. 1990;141:1040–3. doi: 10.1164/ajrccm/141.4_Pt_1.1040. [DOI] [PubMed] [Google Scholar]
- [14].Fahy JV, Wong H, Liu J, Boushey HA. Comparison of samples collected by sputum induction and bronchoscopy from asthmatic and healthy subjects. Am J Respir Crit Care Med. 1995;152:53–8. doi: 10.1164/ajrccm.152.1.7599862. [DOI] [PubMed] [Google Scholar]
- [15].Rubin BK, Finegan B, Ramirez O, King M. General anesthesia does not alter the viscoelastic or transport properties of human respiratory mucus. Chest. 1990;98:101–4. doi: 10.1378/chest.98.1.101. [DOI] [PubMed] [Google Scholar]
- [16].Nielsen H, Hvidt S, Sheils CA, Janmey PA. Elastic contributions dominate the viscoelastic properties of sputum from cystic fibrosis patients. Biophys Chem. 2004;112:193–200. doi: 10.1016/j.bpc.2004.07.019. [DOI] [PubMed] [Google Scholar]
- [17].Janmey PA, Georges PC, Hvidt S. Basic rheology for biologists. Methods Cell Biol. 2007;83:3–27. doi: 10.1016/S0091-679X(07)83001-9. [DOI] [PubMed] [Google Scholar]
- [18].Crowther RS, Wetmore RF. Fluorometric assay of O-linked glycoproteins by reaction with 2-cyanoacetamide. Anal Biochem. 1987;163:170–4. doi: 10.1016/0003-2697(87)90108-4. [DOI] [PubMed] [Google Scholar]
- [19].Saltzman WM, Radomsky ML, Whaley KJ, Cone RA. Antibody diffusion in human cervical mucus. Biophys J. 1994;66:508–15. doi: 10.1016/s0006-3495(94)80802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu Q, Swaminathan G, et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012;4:149ra19. doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Suh J, Dawson M, Hanes J. Real-time multiple-particle tracking: applications to drug and gene delivery. Adv Drug Deliv Rev. 2005;57:63–78. doi: 10.1016/j.addr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- [22].Saxton MJ, Jacobson K. Single-particle tracking: applications to membrane dynamics. Annu Rev Biophys Biomol Struct. 1997;26:373–99. doi: 10.1146/annurev.biophys.26.1.373. [DOI] [PubMed] [Google Scholar]
- [23].Mason TG, Weitz DA. Optical measurements of frequency-dependent linear viscoelastic moduli of complex fluids. Phys Rev Lett. 1995;74:1250–3. doi: 10.1103/PhysRevLett.74.1250. [DOI] [PubMed] [Google Scholar]
- [24].Mason TG, Ganesan K, vanZanten JH, Wirtz D, Kuo SC. Particle tracking microrheology of complex fluids. Phys Rev Lett. 1997;79:3282–5. [Google Scholar]
- [25].Macosko CW. Rheology: principles, measurements, and applications. VCH; New York: 1994. [Google Scholar]
- [26].App EM, Kieselmann R, Reinhardt D, Lindemann H, Dasgupta B, King M, et al. Sputum rheology changes in cystic fibrosis lung disease following two different types of physiotherapy: flutter vs autogenic drainage. Chest. 1998;114:171–7. doi: 10.1378/chest.114.1.171. [DOI] [PubMed] [Google Scholar]
- [27].Innes AL, Carrington SD, Thornton DJ, Kirkham S, Rousseau K, Dougherty RH, et al. Ex vivo sputum analysis reveals impairment of protease-dependent mucus degradation by plasma proteins in acute asthma. Am J Respir Crit Care Med. 2009;180:203–10. doi: 10.1164/rccm.200807-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Suk JS, Lai SK, Boylan NJ, Dawson MR, Boyle MP, Hanes J. Rapid transport of muco-inert nanoparticles in cystic fibrosis sputum treated with N-acetyl cysteine. Nanomedicine (Lond) 2011;6:365–75. doi: 10.2217/nnm.10.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Braeckmans K, Peeters L, Sanders NN, De Smedt SC, Demeester J. Three-dimensional fluorescence recovery after photobleaching with the confocal scanning laser microscope. Biophys J. 2003;85:2240–52. doi: 10.1016/s0006-3495(03)74649-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Garbuzenko OB, Saad M, Pozharov VP, Reuhl KR, Mainelis G, Minko T. Inhibition of lung tumor growth by complex pulmonary delivery of drugs with oligonucleotides as suppressors of cellular resistance. Proc Natl Acad Sci U S A. 2010;107:10737–42. doi: 10.1073/pnas.1004604107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ballester M, Nembrini C, Dhar N, de Titta A, de Piano C, Pasquier M, et al. Nanoparticle conjugation and pulmonary delivery enhance the protective efficacy of Ag85B and CpG against tuberculosis. Vaccine. 2011;29:6959–66. doi: 10.1016/j.vaccine.2011.07.039. [DOI] [PubMed] [Google Scholar]
- [32].Nembrini C, Stano A, Dane KY, Ballester M, van der Vlies AJ, Marsland BJ, et al. Nanoparticle conjugation of antigen enhances cytotoxic T-cell responses in pulmonary vaccination. Proc Natl Acad Sci U S A. 2011;108:E989–97. doi: 10.1073/pnas.1104264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ensign LM, Tang BC, Wang Y-Y, Tse TA, Hoen T, Cone R, et al. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci Transl Med. 2012;4:138ra79. doi: 10.1126/scitranslmed.3003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].World Health Organization . Global health risks: mortality and burden of disease attributable to selected major risks. World Health Organization; Geneva, Switzerland: 2009. [Google Scholar]
- [35].Cai LH, Panyukov S, Rubinstein M. Mobility of nonsticky nanoparticles in polymer liquids. Macromolecules. 2011;44:7853–63. doi: 10.1021/ma201583q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wong IY, Gardel ML, Reichman DR, Weeks ER, Valentine MT, Bausch AR, et al. Anomalous diffusion probes microstructure dynamics of entangled F-actin networks. Phys Rev Lett. 2004;92:178101. doi: 10.1103/PhysRevLett.92.178101. [DOI] [PubMed] [Google Scholar]
- [37].Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond) 2011;6:715–28. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].de Gennes PG. Conformations of polymers attached to an interface. Macromolecules. 1980;13:1069–75. [Google Scholar]
- [39].Dickey BF. Biochemistry. Walking on solid ground. Science. 2012;337:924–5. doi: 10.1126/science.1227091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Matthews LW, Spector S, Lemm J, Potter JL. Studies on pulmonary secretions. I. The over-all chemical composition of pulmonary secretions from patients with cystic fibrosis, bronchiectasis, and laryngectomy. Am Rev Respir Dis. 1963;88:199–204. doi: 10.1164/arrd.1963.88.2.199. [DOI] [PubMed] [Google Scholar]
- [41].Lai SK, Wang YY, Cone R, Wirtz D, Hanes J. Altering mucus rheology to “solidify” human mucus at the nanoscale. PLoS One. 2009;4:e4294. doi: 10.1371/journal.pone.0004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shin JH, Gardel ML, Mahadevan L, Matsudaira P, Weitz DA. Relating microstructure to rheology of a bundled and cross-linked F-actin network in vitro. Proc Natl Acad Sci U S A. 2004;101:9636–41. doi: 10.1073/pnas.0308733101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bernhard W, Haagsman HP, Tschernig T, Poets CF, Postle AD, van Eijk ME, et al. Conductive airway surfactant: surface-tension function, biochemical composition, and possible alveolar origin. Am J Respir Cell Mol Biol. 1997;17:41–50. doi: 10.1165/ajrcmb.17.1.2594. [DOI] [PubMed] [Google Scholar]
- [44].Gehr P, Green FH, Geiser M, Im Hof V, Lee MM, Schurch S. Airway surfactant, a primary defense barrier: mechanical and immunological aspects. J Aerosol Med. 1996;9:163–81. doi: 10.1089/jam.1996.9.163. [DOI] [PubMed] [Google Scholar]
- [45].Suk JS, Boylan NJ, Trehan K, Tang BC, Schneider CS, Lin JM, et al. N-acetylcysteine enhances cystic fibrosis sputum penetration and airway gene transfer by highly compacted DNA nanoparticles. Mol Ther. 2011;19:1981–9. doi: 10.1038/mt.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]