Abstract
Background:
In CT scans of smokers with COPD, the subsegmental airway wall area percent (WA%) is greater and more strongly correlated with FEV1 % predicted than WA% obtained in the segmental airways. Because emphysema is linked to loss of airway tethering and may limit airway expansion, increases in WA% may be related to emphysema and not solely to remodeling. We aimed to first determine whether the stronger association of subsegmental vs segmental WA% with FEV1 % predicted is mitigated by emphysema and, second, to assess the relationships among emphysema, WA%, and total bronchial area (TBA).
Methods:
We analyzed CT scan segmental and subsegmental WA% (WA% = 100 × wall area/TBA) of six bronchial paths and corresponding lobar emphysema, lung function, and clinical data in 983 smokers with COPD.
Results:
Compared with segmental WA%, the subsegmental WA% had a greater effect on FEV1% predicted (−0.8% to −1.7% vs −1.9% to −2.6% per 1-unit increase in WA%, respectively; P < .05 for most bronchial paths). After adjusting for emphysema, the association between subsegmental WA% and FEV1 % predicted was weakened in two bronchial paths. Increases in WA% between bronchial segments correlated directly with emphysema in all bronchial paths (P < .05). In multivariate regression models, emphysema was directly related to subsegmental WA% in most bronchial paths and inversely related to subsegmental TBA in all bronchial paths.
Conclusion:
The greater effect of subsegmental WA% on airflow obstruction is mitigated by emphysema. Part of the emphysema effect might be due to loss of airway tethering, leading to a reduction in TBA and an increase in WA%.
Trial registry:
ClinicalTrials.gov; No.: NCT00608764; URL: www.clinicaltrials.gov
COPD is incompletely reversible expiratory airflow obstruction attributed to a combination of emphysema and airway disease.1 CT imaging is increasingly being used to characterize and quantify these processes for genetic, epidemiologic, and therapeutic investigations with the premise that their relative balance may define unique subsets of disease.2 Although the quantification of emphysema by CT imaging has been well established and now validated in multiple histopathologic investigations,3‐5 the quantification of airway disease has proven to be a greater challenge.6
It is believed that the site of expiratory airflow obstruction in COPD is the small airways that are < 2 mm in diameter.7,8 Although direct examination of these structures is beyond the resolution of clinical CT scanning techniques, work by Nakano and colleagues9 demonstrated that dimensions (ie, wall area percent [WA%]) of the central airways and their degree of remodeling on CT scan are correlated with the burden of distal small airways disease. It was subsequently discovered that the correlation between FEV1 % predicted and WA% is greater in subsegmental (and more peripheral) airways than in segmental airways.10,11 It has since become the standard in quantitative CT image analyses to study the most peripheral airways.12‐14
Previously, we and other groups demonstrated that with increasing disease severity and increasing burdens of emphysema, airways become less distensible.15,16 There may be several reasons for this observation, including mural fibrosis, which prevents dilation, and the disruption of the airway-parenchymal interdependence in emphysematous lungs,15 which may reduce radial traction on the airway.17 In either case, an incompletely expanded airway may appear to have an elevated WA% on CT scan that could be falsely attributed to a greater burden of airway disease. Because the increase in WA% beyond the segmental airways is the basis of the increased correlation between lung function and airway disease, it is important to uncover whether this measure is related to emphysema. Using CT imaging, epidemiologic, and functional data from a large cohort of smokers in the COPDGene Study, we aimed to (1) examine the effect of emphysema on the relationship between WA% and FEV1 % predicted and (2) assess the relationships among lobar emphysema, segmental and subsegmental WA%, and total bronchial area (TBA) along six bronchial paths.
Materials and Methods
The COPDGene Study has been previously described in detail.18 Briefly, the goal of the study was to determine the genetics and epidemiologic determinants of COPD in non-Hispanic white and African American smokers aged 45 to 80 years. Subjects with active lung disease other than asthma, emphysema, and COPD were excluded. An interim data analysis was planned following enrollment of the first 2,500 of 10,000 subjects. In this analysis, we included subjects from the first 2,500 enrolled who had Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage I to IV COPD19 and complete data on airway WA% measures. The COPDGene Study was approved by the institutional review boards of all participating centers. The current analysis was approved by the Partners Healthcare Research Committee (2007P-000554).
Demographic and clinical data with standardized questionnaires, including a modified Adult Respiratory Questionnaire, were collected.18 Spirometric measures of lung function were performed using the EasyOne spirometer (ndd Medical Technologies Inc) before and after the administration of a short-acting inhaled bronchodilator according to American Thoracic Society recommendations.20 Postbronchodilator FEV1 and FVC were expressed as % predicted values.21
CT Scans
Standardized volumetric CT scans of the chest were performed at both full inspiration and relaxed exhalation.18 The CT image acquisition protocol used was 120-kVp, 200-mAs, and 0.5-s rotation time for GE LightSpeed 16 and VCT 64 (General Electric), Siemens Sensation 16 and 64 (Siemens AG), and Philips 40-slice and 64-slice (Koninklijke Philips Electronics NV) scanners. Images were reconstructed using a standard algorithm at 0.625-mm slice thickness and 0.625-mm intervals for the GE scanners. Siemens CT images were reconstructed using a B46f algorithm at 0.75-mm slice thickness and 0.5-mm intervals. Reconstruction of Philips images was performed by using a B algorithm at 0.9-mm slice thickness and 0.45-mm intervals. Image analysis was performed on inspiratory CT scans using the dedicated CT image analysis software Pulmonary Workstation 2 and Plus (VIDA Diagnostics, Inc)22 at the core imaging center for the COPDGene Study. Whole-lung volume on the full inspiration CT scans was expressed as a % predicted total lung capacity.23 Emphysema was defined as the percent low-attenuation area <−950 Hounsfield units.3 To better understand the relationship between emphysema and airways at the lobar level, we used emphysema and volume measurements from the lobes where the airway measurements were obtained.
Details about airway measurements using VIDA Diagnostics software are described elsewhere.24 We used all available data for the segmental and subsegmental airways that were collected in the following six bronchial paths: (1) right upper lobe apical bronchus (RB1), (2) right middle lobe lateral bronchus (RB4), (3) right lower lobe posterior basal bronchus (RB10), (4) left upper lobe apicoposterior bronchus (LB1), (5) superior lingular bronchus (LB4), and (6) left lower lobe posterior basal bronchus (LB10). These bronchi were chosen based on the consensus of COPDGene Study investigators and prior studies.9,10,13 Additionally, this airway selection allowed for an airway assessment of all lung lobes. The average segmental and subsegmental measures of WA% (WA% = 100 × wall area/TBA) were calculated for each bronchial path. The relative difference in WA% between bronchial segments (hereafter, referred to as the increase in WA%) was calculated as follows: [(subsegmental WA% − segmental WA%)/segmental WA%] × 100.
Statistical Analysis
The difference between the effects of segmental and subsegmental WA% on FEV1 % predicted was assessed for each bronchial path by regression analysis. A Z test was used to compare β values between two different regression models.25 The effect of lobar emphysema on the relationship between segmental or subsegmental measures of WA% and FEV1 % predicted for each bronchial path was assessed with multivariate regression. We also analyzed the relationships among lobar emphysema, the increase in WA%, segmental or subsegmental WA%, and TBA by multivariate regression analysis. The multivariate models included variables with expected a priori relationships to the outcome, including lung volume. To account for differences in Hounsfield units among CT scanner brands/makes, we grouped the CT scanners into five groups according to a COPDGene physicist’s opinion of their performance in phantom studies. In this study, the Siemens Definition and Siemens Definition AS+ (Siemens AG) scanners were used as the reference. Because of the differences in the pathophysiology of airway disease in COPD and asthma, additional analyses incorporating asthma history were also reported. Prior asthma was defined as a self-reported physician diagnosis of the disease. A P < .05 was considered statistically significant. Analyses were performed using SAS, version 9.2 (SAS Institute Inc).
Results
Of the first 2,500 subjects, 1,272 had COPD in GOLD stages I to IV. Of these, 983 had complete segmental and subsegmental airway WA% data. Study population demographics as well as clinical, lung function, and CT scan emphysema data are shown in Table 1. Subjects with the most severe disease (GOLD stage IV) had lower BMI, were more likely to be former smokers, and had a higher % predicted total lung capacity and burden of emphysema as measured by CT scan. Among the 983 subjects, 202 reported a physician diagnosis of asthma. There were no significant differences in the characteristics listed in Table 1 between subjects with COPD alone and those with COPD and prior asthma, except that the latter subjects were more likely to be women and African American. Segmental and subsegmental airway WA% for each bronchial path and corresponding lobar emphysema and lobar volume data are provided in Table 2. Compared with segmental airway WA%, subsegmental airway WA% was higher in all bronchial paths (P < .0001 for each bronchial path). The relative increase in WA% ranged from 4.4% (RB1) to 8.1% (LB10). TBA was lower in subsegmental than segmental airways in all bronchial paths (P < .0001 for each bronchial path). Compared with subjects with complete data on both segmental and subsegmental WA%, those with missing data (n = 289) were more likely to be women (45% vs 54%, P = .004) and to have COPD GOLD stages III or IV (37% vs 58%, P < .0001). Subjects with missing data also had lower FEV1 % predicted (59% ± 23% vs 46% ± 22%, P < .0001) and more emphysema on CT scan (median [interquartile range], 9.3% [3.6%-19.7%] vs 12.1% [4.0%-26.2%]; P = .01).
Table 1.
—Characteristics of the 983 Subjects With COPD Included in the Analysis by GOLD Stage
| Characteristic | GOLD I (n = 189) | GOLD II (n = 434) | GOLD III (n = 252) | GOLD IV (n = 108) |
| Age, y | 64 ± 10 | 63 ± 8 | 65 ± 8 | 65 ± 8 |
| Female sex, % | 38 | 47 | 48 | 42 |
| White race, % | 87 | 81 | 86 | 81 |
| BMI, kg/m2 | 27 ± 5 | 29 ± 6 | 28 ± 6 | 26 ± 6 |
| Smoking history, pack-y | 45 ± 25 | 52 ± 25 | 57 ± 29 | 57 ± 29 |
| Smoking status, % | 47 | 41 | 30 | 12 |
| Asthma history, % | 12 | 22 | 25 | 21 |
| FEV1, % predicted | 92 ± 10 | 65 ± 9 | 40 ± 6 | 23 ± 5 |
| FVC, % predicted | 109 ± 14 | 87 ± 13 | 72 ± 12 | 56 ± 13 |
| FEV1/FVC ratio | 0.64 ± 0.05 | 0.57 ± 0.08 | 0.43 ± 0.08 | 0.31 ± 0.07 |
| TLCCT, % predicted | 101 ± 16 | 96 ± 16 | 103 ± 17 | 112 ± 14 |
| Whole-lung emphysema, % | 5.4 (2.5-8.9) | 6.1 (2.6-13.7) | 16.9 (7.7-26.9) | 30.0 (19.4-40.2) |
Data are presented as mean ± SD and median (interquartile range) for continuous variables and % for binary variables. GOLD = Global Initiative for Chronic Obstructive Lung Disease; TLCCT = CT scan-based total lung capacity.
Table 2.
—WA% and TBA by Airway Segment Along Six Bronchial Paths, Lobar Emphysema, and Lobar Volume in 983 Subjects With COPD
| WA%a |
TBA, mm2a |
||||||
| Bronchial Path | Segmental | Subsegmental | Segmental | Subsegmental | Lobe | Lobar Emphysema, % | Lobar Volume, mLb |
| RB1 | 61.86 ± 4.55 | 64.36 ± 3.27 | 55.8 ± 18.8 | 32.8 ± 10.1 | RUL | 9.2 (2.8-22.2) | 1,333 ± 380 |
| RB4 | 62.47 ± 3.96 | 65.10 ± 3.06 | 42.8 ± 15.1 | 24.1 ± 6.8 | RML | 9.2 (3.7-18.4) | 479 ± 169 |
| RB10 | 60.77 ± 4.16 | 64.55 ± 3.26 | 55.0 ± 15.3 | 32.9 ± 9.2 | RLL | 6.5 (2.2-17.4) | 1,430 ± 401 |
| LB1 | 64.11 ± 3.62 | 66.32 ± 3.15 | 41.9 ± 12.7 | 27.2 ± 7.9 | LUL | 9.8 (3.8-21.2) | 1,501 ± 399 |
| LB4 | 62.20 ± 3.86 | 64.86 ± 3.48 | 42.9 ± 15.8 | 24.6 ± 8.2 | LLL | 6.6 (2.6-16.3) | 1,361 ± 414 |
| LB10 | 59.73 ± 4.22 | 64.41 ± 3.37 | 61.0 ± 16.6 | 35.9 ± 10.3 | … | … | … |
Data are presented as mean ± SD or median (interquartile range). LB1 = left upper lobe apicoposterior bronchus; LB4 = superior lingular bronchus; LB10 = left lower lobe posterior basal bronchus; LLL = left lower lobe; LUL = left upper lobe; RB1 = right upper lobe apical bronchus; RB4 = right middle lobe lateral bronchus; RB10 = right lower lobe posterior basal bronchus; RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe; TBA = total bronchial area; WA% = wall area percent.
P < .0001 for segmental vs subsegmental WA% and segmental vs subsegmental TBA in each bronchial path. P values were calculated with paired t test.
Lobar volume data were missing in 33 subjects.
Influence of Emphysema on the Relationship Between FEV1 % Predicted and WA%
The effects of both segmental and subsegmental WA% on FEV1 % predicted were significant in all bronchial paths, although the effect of subsegmental WA% was stronger (the difference in β value was significant in all bronchial paths but LB10) (model 1 in Table 3). When lobar emphysema was included in these models, the effects of segmental and subsegmental WA% on FEV1 % predicted remained significant in all bronchial paths. Adjusting for emphysema strengthened the association between segmental WA% and FEV1 % predicted, whereas it weakened the relationship between subsegmental WA% and FEV1 % predicted in all bronchial paths but RB10. Change in the effect of subsegmental WA% on FEV1 % predicted was ≥ 10% and statistically significant (P < .05 for lobar emphysema) only in LB1 and LB4 (model 2 of Table 3).
Table 3.
—Effect Estimates (β Values) of Segmental and Subsegmental WA% of Two Models for FEV1 % Predicted by Bronchial Path
| Model 1: FEV1 % Predicted = Segmental or Subsegmental WA% |
Model 2: FEV1 % Predicted = Segmental or Subsegmental WA% + Lobar Emphysema |
|||||
| Airway Segment |
Airway Segment |
|||||
| Bronchial Path | Segmental | Subsegmental | β-Value Change | Segmental | Subsegmental | β-Value Change |
| RB1 | −0.78 | −2.22 | −1.44a | −1.13 | −2.05 | −0.92a |
| RB4 | −1.63 | −2.36 | −0.74a | −1.86 | −2.27 | −0.41 |
| RB10 | −1.47 | −2.07 | −0.60a | −1.70 | −2.14 | −0.43a |
| LB1 | −1.66 | −2.55 | −0.89a | −1.79 | −2.27 | −0.48a |
| LB4 | −1.49 | −2.25 | −0.76a | −1.52 | −2.00 | −0.48a |
| LB10 | −1.47 | −1.87 | −0.40 | −1.58 | −1.75 | −0.17 |
See Table 2 legend for expansion of abbreviations.
P < .05.
Relationship Between Lobar Emphysema and WA%
Lobar emphysema was directly related to the increase in WA% in all bronchial paths as follows: RB1, r = 0.17, P < .0001; RB4, r = 0.08, P = .02; RB10, r = 0.08, P = .01; LB1, r = 0.13, P < .0001; LB4, r = 0.08, P = .008; and LB10, r = 0.09, P = .003. In models adjusted for sex, age, height, pack-years of smoking, CT scanner brand/make, and lobar volume, lobar emphysema was directly related to subsegmental WA% in all bronchial paths but RB4. These associations were weak but statistically significant (P = .02 to < .0001). Lobar emphysema was inversely related to segmental WA% only in RB4 (P = .03) (Table 4). In all six bronchial paths, lobar volume was inversely related to segmental and subsegmental WA%. In multivariate analyses looking at subjects with COPD alone (ie, no history of asthma), the relationships between lobar emphysema and segmental or subsegmental WA% for most bronchial paths were similar (e-Table 1 (331.1KB, pdf) ) to those observed in the entire cohort. In the group of subjects with COPD and prior asthma, the relationship between lobar emphysema and segmental or subsegmental WA% was statistically significant only in LB4 (data not shown).
Table 4.
—Multivariate Regression Analysis for WA% in Six Bronchial Paths
| Lobar Emphysema |
||||
| Segmental Airway |
Subsegmental Airway |
|||
| Bronchial Path | Regression Coefficient | P Value | Regression Coefficient | P Value |
| RB1 | −0.02 | .13 | 0.03 | < .0001 |
| RB4 | −0.02 | .03 | 0.02 | .054 |
| RB10 | 0.001 | .92 | 0.02 | .02 |
| LB1 | 0.02 | .12 | 0.04 | < .0001 |
| LB4 | 0.009 | .43 | 0.04 | < .0001 |
| LB10 | 0.01 | .28 | 0.04 | < .0001 |
In these analyses, the sample size was 950; 33 subjects were excluded because their measurements of lobar lung volume were not available. In these models, the main predictor was lobar emphysema as measured by CT scan. Adjustment for sex (female = 1), age, height, pack-y smoking, CT scanner brand/make, and lobar volume was performed. See Table 2 legend for expansion of abbreviations.
Relationship Between Emphysema and TBA
To assess the impact of emphysema on TBA, we analyzed the relationship between lobar emphysema and TBA of subsegmental airways in models adjusted for sex, age, height, CT scanner brand/make, and lobar volume. In all bronchial paths but RB10, lobar emphysema had a significant inverse relationship with subsegmental TBA (Table 5). The relationship between lobar emphysema and subsegmental TBA for all bronchial paths was similar in the COPD group without asthma and the entire cohort (e-Table 2 (331.1KB, pdf) ). In the COPD group with asthma history, the relationship between lobar emphysema and subsegmental TBA reached statistical significance in only LB4 (data not shown).
Table 5.
—Multivariate Analysis for Subsegmental TBA in Six Bronchial Paths
| Lobar Emphysema |
||
| Bronchial Path | Regression Coefficient | P Value |
| RB1 | −0.12 | < .0001 |
| RB4 | −0.06 | .002 |
| RB10 | −0.04 | .10 |
| LB1 | −0.09 | < .0001 |
| LB4 | −0.10 | < .0001 |
| LB10 | −0.09 | .005 |
In these analyses, the sample size was 950; 33 subjects were excluded because their measurements of lobar lung volume were not available. In these models, the main predictor was lobar emphysema as measured by CT scan. Adjustment for sex (female = 1), age, height, pack-y smoking, CT scanner brand/make, and lobar volume was performed. See Table 2 legend for expansion of abbreviations.
Discussion
Measures of central airways by CT scan have been demonstrated to be related to the burden of distal small airway disease,9 and the more peripheral in the bronchial tree these CT image-based measures are made, the stronger the correlation to clinically significant measures of COPD severity.10‐13 Using data from the COPDGene Study, we observed that emphysema mitigates the increasing effect of WA% on FEV1 % predicted in subsegmental compared with segmental airways. CT scan measures of emphysema and lung volume appeared to have opposing effects on subsegmental WA%: An increasing amount of lobar emphysema was associated with an increase in WA% in all the bronchial paths. Additional analyses showed that a potential explanation for the effect of CT scan-measured emphysema on WA% is through its influence on TBA.
Consistent with prior investigations,10‐12 we observed that on univariate analysis, the effect of WA% on lung function was greater in the subsegmental than the segmental airways. After adjusting for emphysema, however, this effect was mitigated in two bronchial paths. The observed increase in correlation between subsegmental WA% and lung function is complex. The present analysis suggests that potential mechanisms for this correlation include both airway dilation with increasing lung volume and reductions in TBA because of the loss of airway tethering associated with emphysema. (An illustration of the conflicting effects of lung volume and emphysema on airway dimensions is shown in Fig 1.) Of note, the effect of segmental WA% on FEV1 % predicted was strengthened by lobar emphysema in three bronchial paths. This opposing effect of emphysema on the relationship between subsegmental and segmental WA% and FEV1 % predicted is difficult to explain. One potential explanation is that the degree to which emphysema disrupts airway-parenchymal interdependence is greater in subsegmental than in segmental airways, leading to a lesser effect of the former airways on lung function.
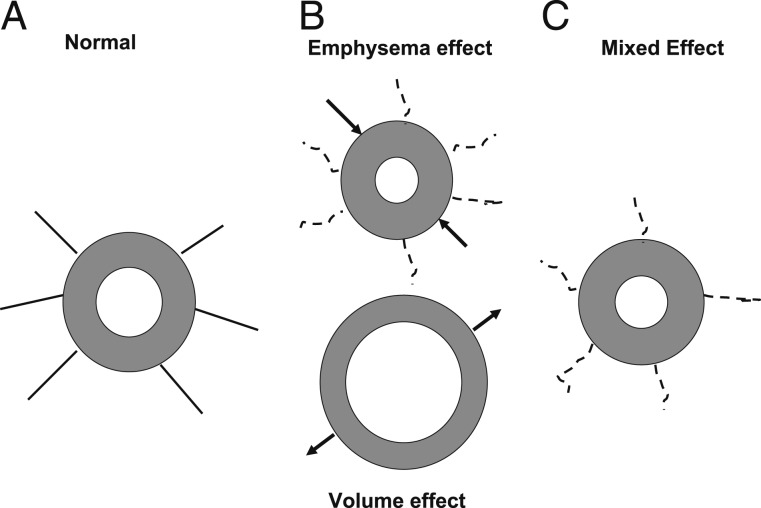
Figure 1.
Illustration of the conflicting effects of emphysema and lung volume on airway dimensions. A, Intact airway with attachments to the lung parenchyma. B, On the top, lung attachments are broken by emphysematous destruction, resulting in a diminished total bronchial area (TBA) (arrows) and increased wall area percent (WA%) (gray area). At the bottom, an increase in lung volume results in increased TBA (arrows) and lower WA%. C, The likely result of these two processes is an airway with lower TBA and an increased WA% compared with a normal airway.
Another possible explanation for the stronger relationship between FEV1 % predicted and subsegmental vs segmental WA% is a selection bias toward thicker airways resulting from inflammation and remodeling that are likely more identifiable on CT scan. Although it is true that there is limited resolution in clinical CT scanning, the range of airways we measured is broad and suggests that we have a representative sample of both larger and smaller airways (median [interquartile range] TBA, 59 [50-70] to 23 [19-28] mm2 in LB10 and RB4, respectively). Additionally, there is histopathologic evidence suggesting a loss of mural cartilage in COPD26,27; in extreme cases, there is frank airway loss as a result of parenchymal destruction.28 Therefore, we agree that the present data are biased to subjects who had airways to measure, but it is unlikely that there is additional selection bias because of remodeling of existing airways.
Greater burdens of CT scan measures of lobar emphysema were associated with the increase in WA% between airway segments and with lower TBA, suggesting that increases in subsegmental WA% are partly due to loss of lung elastic recoil with reductions in airway dilation. The present data support the notion of airway-parenchymal uncoupling suggested in prior investigations.15,16 We and others have observed that reduced airway distensibility (change in airway caliber) with lung inflation is associated with disease severity16 and an emphysema-predominant COPD phenotype.15 We extended these previous observations by demonstrating that airway dimensions are related to the burden of emphysema as measured by CT scan. A couple of limitations should be noticed. First, emphysema had opposing effects on subsegmental vs segmental airways dimensions. We believe that these opposing effects reflect the complex interaction among parenchyma, airways, and lung volume. One possibility is that airway tethering is more prominent in subsegmental than in segmental airways. Another explanation is that subsegmental airways are less affected by lung volume than are segmental airways. As a result, subsegmental airways dilate less, leading to greater WA%. Second, although the effect of emphysema on both subsegmental WA% and TBA was weak, it was consistent across the two subsets of subjects analyzed (the entire cohort and the subset of subjects with COPD and no history of asthma) (e-Tables 1, 2 (331.1KB, pdf) ). The weak effect of emphysema may be due to emphysema preferentially affecting airways smaller than those visualized on CT scan. A prior study in subjects with COPD found that destructive changes in alveolar walls reduce the number of alveolar attachments on airways, leading to parenchymal-airway uncoupling, which in turn diminishes airway dilation.17 This process may be less prominent in airways capable of being visualized on CT scan. Despite these limitations, we speculate that CT scan measures of WA% may not exclusively represent an airway remodeling process.
The observed direct relationship between subsegmental WA% and emphysema in multivariate analysis is in contrast to prior studies, which demonstrated an inverse association between wall thickness standardized at a 10-mm-perimeter airway and whole-lung emphysema.29,30 Differences in the airway measurements and regression models used among studies and the lack of adjustment for lung volume in prior investigations may partially explain this discrepancy.
There are several limitations to this investigation that must be noted. The first is that 23% of the subjects with COPD had missing data on either segmental or subsegmental airways, decreasing the statistical power of the analysis. Airway segmentation in subjects with a high burden of emphysema can be challenging because of distortion or even disappearance of airways as we previously observed.31 Cardiac and respiratory motion artifact may also make airway measurement difficult. Second, the effects of lobar emphysema on subsegmental WA% or TBA were weak, and more peripheral bronchial segments were not available for analysis, preventing a more thorough investigation of the effects of emphysema on distal airways. The present results, therefore, should be considered as initial steps in understanding the effect of lung parenchymal destruction on airway measurements. The finally limitation is the lack of physiologic data characterizing lung mechanics. In the absence of simultaneous measures of transpulmonary pressure, the analysis proceeded on the assumption that emphysematous destruction of the lung parenchyma is associated with loss of elastic recoil.
In summary, the results suggest an interplay among emphysema, lung volume, airway dimensions, and lung function. The standard CT imaging-based assessment of mural remodeling (ie, WA%) may reflect the combined influences of airway disease, lung volume, and the mechanical properties of the surrounding lung parenchyma. Furthermore, these interactions may partly account for the stronger correlations observed between subsegmental and segmental WA% and spirometric measures of lung function. Further studies are needed to elucidate the complex relationship between emphysema and airway disease in COPD.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Washko had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Diaz: contributed to the creation and final approval of the manuscript.
Dr Han: contributed to the creation and final approval of the manuscript.
Dr Come: contributed to the creation and final approval of the manuscript.
Dr San José Estépar: contributed to the collection of imaging data and creation and final approval of the manuscript and is a member of the Imaging Core for the COPDGene Study.
Mr Ross: contributed to the collection of imaging data and creation and final approval of the manuscript and is a member of the Imaging Core for the COPDGene Study.
Dr Kim: contributed to the creation and final approval of the manuscript.
Dr Dransfield: contributed to the creation and final approval of the manuscript.
Dr Curran-Everett: contributed to the creation and final approval of the manuscript.
Dr Schroeder: contributed to the collection of imaging data and creation and final approval of the manuscript and is a member of the Imaging Core for the COPDGene Study.
Dr Lynch: contributed to the collection of imaging data and creation and final approval of the manuscript and is a member of the Imaging Core for the COPDGene Study.
Dr Tschirren: contributed to the creation and final approval of the manuscript.
Dr Silverman: contributed to the data collection and the creation and final approval of the manuscript and is one of the two national principal investigators in the COPDGene Study.
Dr Washko: contributed to the collection of imaging data and creation and final approval of the manuscript and is a member of the Imaging Core for the COPDGene Study.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Han has been a speaker for Boehringer Ingelheim GmbH; Pfizer Inc; and GlaxoSmithKline plc and has consulted for Novartis AG; Genentech, Inc; GlaxoSmithKline plc; Pfizer Inc; Boehringer Ingelheim GmbH; and MedImmune, LLC. Dr Dransfield has received consultancy fees from GlaxoSmithKline plc, Boehringer Ingelheim GmbH, and Forest Laboratories, Inc. Dr Lynch was a paid consultant for Actelion Pharmaceuticals US, Inc; InterMune; Gilead; Perceptive Informatics, Inc; Novartis AG; and Janssen Biotech, Inc, and received grants or has grants pending with Siemens AG. Dr Tschirren is an employee of VIDA Diagnostics, Inc, the provider of the software used to perform the CT image measurements for the COPDGene Study. Dr Silverman received institutional grant support from the COPD Foundation and GlaxoSmithKline plc, received travel accommodations from the COPD Foundation, and was a consultant for and received lecture fees from GlaxoSmithKline plc and AstraZeneca. Drs Diaz, Come, San José Estépar, Kim, Curran-Everett, Schroeder, and Washko and Mr Ross have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of this study, collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: This work was performed at Brigham and Women’s Hospital.
Additional information: The e-Tables can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- LB1
left upper lobe apicoposterior bronchus
- LB4
superior lingular bronchus
- LB10
left lower lobe posterior basal bronchus
- RB1
right upper lobe apical bronchus
- RB4
right middle lobe lateral bronchus
- RB10
right lower lobe posterior basal bronchus
- TBA
total bronchial area
- WA%
wall area percent
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
Funding/Support: The COPDGene Study is funded by National Institutes of Health (NIH) [Grants U01 HL089897 and U01 HL089856]. This work is supported by the NIH [Grants K23 HL093351 (to Dr Han), T32 HL007633-26 (to Dr Come), K25 HL104085 (to Dr San José Estépar), K23 HL094696-01A2 (to Dr Kim), and K23 HL089353 (to Dr Washko)].
References
- 1.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364(9435):709-721 [DOI] [PubMed] [Google Scholar]
- 2.Coxson HO. Quantitative chest tomography in COPD research: chairman’s summary. Proc Am Thorac Soc. 2008;5(9):874-877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152(2):653-657 [DOI] [PubMed] [Google Scholar]
- 4.Kuwano K, Matsuba K, Ikeda T, et al. The diagnosis of mild emphysema. Correlation of computed tomography and pathology scores. Am Rev Respir Dis. 1990;141(1):169-178 [DOI] [PubMed] [Google Scholar]
- 5.Bergin C, Müller N, Nichols DM, et al. The diagnosis of emphysema. A computed tomographic-pathologic correlation. Am Rev Respir Dis. 1986;133(4):541-546 [DOI] [PubMed] [Google Scholar]
- 6.Coxson HO. Quantitative computed tomography assessment of airway wall dimensions: current status and potential applications for phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(9):940-945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278(25):1355-1360 [DOI] [PubMed] [Google Scholar]
- 8.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645-2653 [DOI] [PubMed] [Google Scholar]
- 9.Nakano Y, Wong JC, de Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171(2):142-146 [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa M, Nasuhara Y, Onodera Y, et al. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(12):1309-1315 [DOI] [PubMed] [Google Scholar]
- 11.Coxson HO, Quiney B, Sin DD, et al. Airway wall thickness assessed using computed tomography and optical coherence tomography. Am J Respir Crit Care Med. 2008;177(11):1201-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashiro T, Matsuoka S, Estépar RS, et al. Quantitative airway assessment on computed tomography in patients with alpha1-antitrypsin deficiency. COPD. 2009;6(6):468-477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasegawa M, Makita H, Nasuhara Y, et al. Relationship between improved airflow limitation and changes in airway calibre induced by inhaled anticholinergic agents in COPD. Thorax. 2009;64(4):332-338 [DOI] [PubMed] [Google Scholar]
- 14.Diaz AA, Bartholmai B, San José Estépar R, et al. Relationship of emphysema and airway disease assessed by CT to exercise capacity in COPD. Respir Med. 2010;104(8):1145-1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz AA, Come CE, Ross JC, et al. ; COPDGene Investigators Association between airway caliber changes with lung inflation and emphysema assessed by volumetric CT scan in subjects with COPD. Chest. 2012;141(3):736-744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scichilone N, La Sala A, Bellia M, et al. The airway response to deep inspirations decreases with COPD severity and is associated with airway distensibility assessed by computed tomography. J Appl Physiol. 2008;105(3):832-838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scichilone N, Bruno A, Marchese R, Vignola AM, Togias A, Bellia V. Association between reduced bronchodilatory effect of deep inspiration and loss of alveolar attachments. Respir Res. 2005;6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabe KF, Hurd S, Anzueto A, et al. ; Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532-555 [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152(3):1107-1136 [DOI] [PubMed] [Google Scholar]
- 21.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179-187 [DOI] [PubMed] [Google Scholar]
- 22.Hoffman EA, Simon BA, McLennan G. State of the art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(6):519-532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur Respir J. 1995;8(3):492-506 [DOI] [PubMed] [Google Scholar]
- 24.Kim YI, Schroeder J, Lynch D, et al. ; COPDGene Investigators Gender differences of airway dimensions in anatomically matched sites on CT in smokers. COPD. 2011;8(4):285-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clogg CC, Petkova E, Haritou A. Statistical methods for comparing regression coefficients between models. Am J Sociol. 1995;100(5):1261-1293 [Google Scholar]
- 26.Haraguchi M, Shimura S, Shirato K. Morphometric analysis of bronchial cartilage in chronic obstructive pulmonary disease and bronchial asthma. Am J Respir Crit Care Med. 1999;159(3):1005-1013 [DOI] [PubMed] [Google Scholar]
- 27.Wright RR. Bronchial atrophy and collapse in chronic obstructive pulmonary emphysema. Am J Pathol. 1960;37:63-77 [PMC free article] [PubMed] [Google Scholar]
- 28.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567-1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel BD, Coxson HO, Pillai SG, et al. ; International COPD Genetics Network Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178(5):500-505 [DOI] [PubMed] [Google Scholar]
- 30.Kim WJ, Silverman EK, Hoffman E, et al. ; NETT Research Group CT metrics of airway disease and emphysema in severe COPD. Chest. 2009;136(2):396-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diaz AA, Valim C, Yamashiro T, et al. Airway count and emphysema assessed by chest CT imaging predicts clinical outcome in smokers. Chest. 2010;138(4):880-887 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement