Abstract
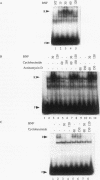
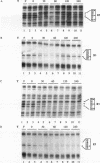
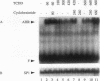
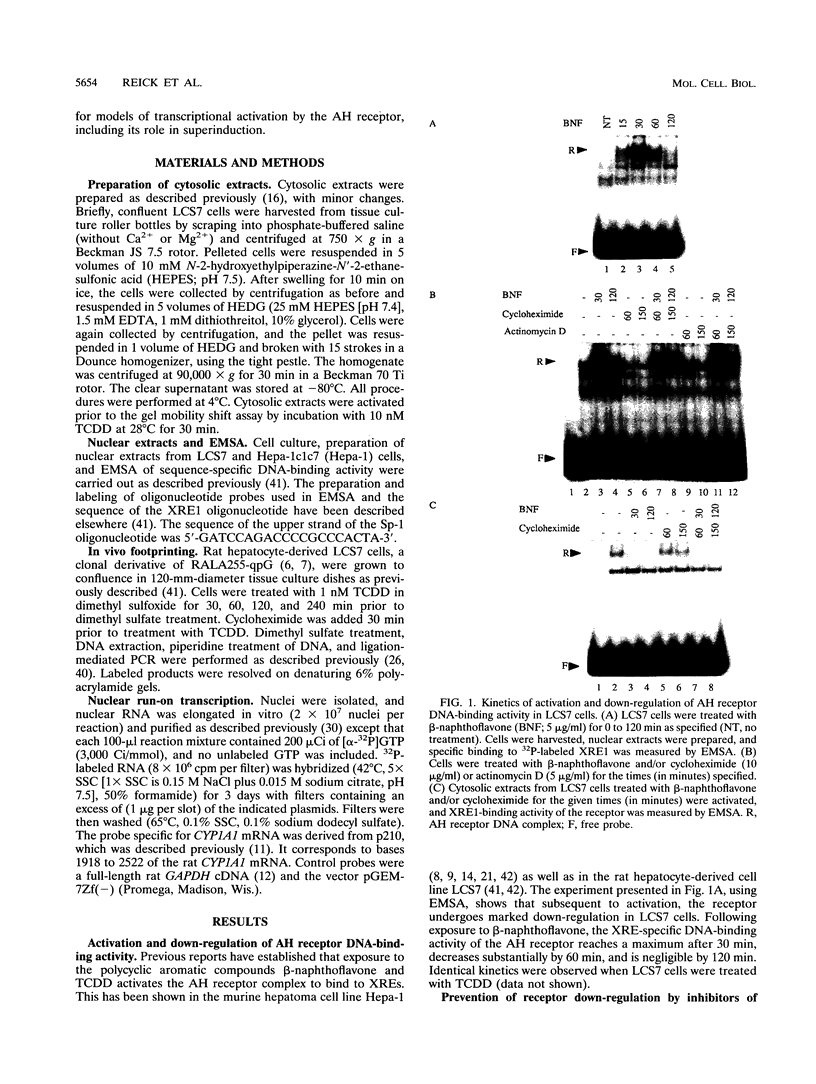
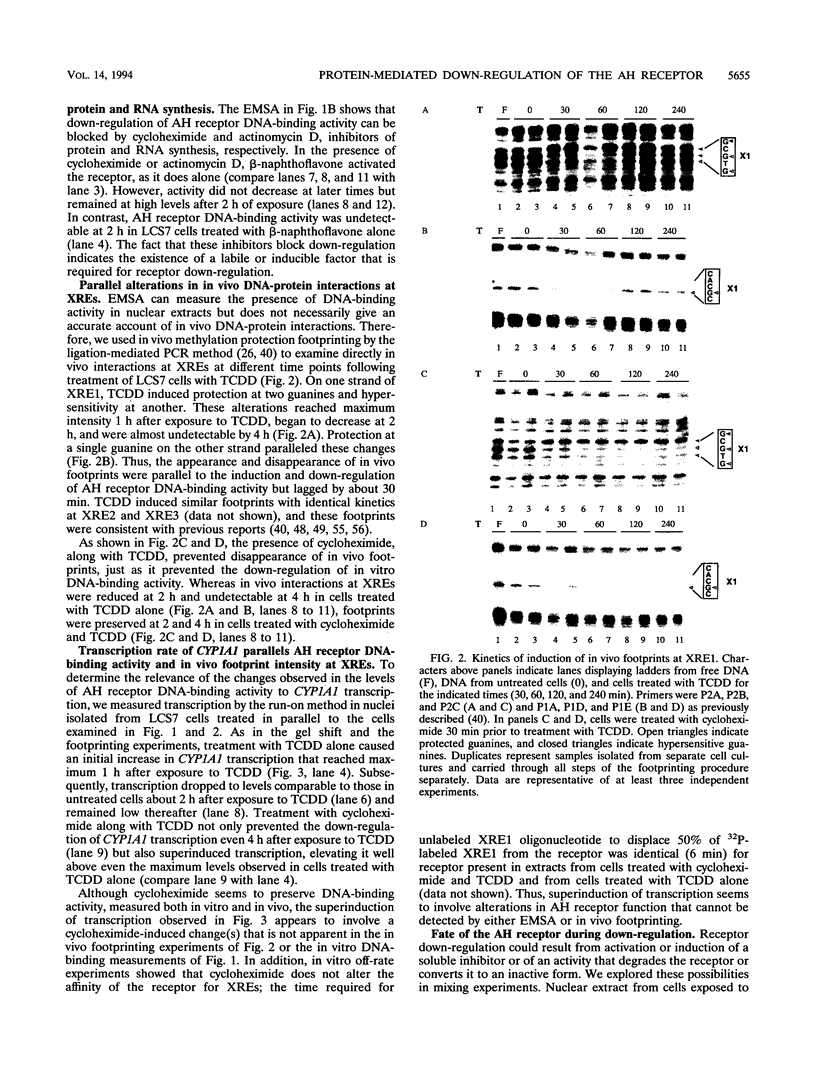
Aryl hydrocarbons (AHs) such as 2,3,7,8-tetrachlorodibenzo-p-dioxin and benzo[a]pyrene activate the sequence-specific DNA-binding activity of the AH receptor. In the rat hepatocyte-derived cell line LCS7, DNA-binding activity peaked after 30 min and was then down-regulated, reaching negligible levels by 2 h. Down-regulation could be blocked, and DNA-binding activity maintained at maximum for many hours by inhibiting protein or RNA synthesis, implying that down-regulation is a mediated process requiring a labile or inducible protein. CYP1A1 transcription and in vivo DNA-protein interactions at xenobiotic response elements were down-regulated in parallel with DNA-binding activity in nuclear extracts, and these changes could also be blocked by inhibitors of protein synthesis. The correlation between AH receptor DNA-binding activity, intensity of in vivo footprints at xenobiotic response elements, and CYP1A1 transcription rate implies that down-regulation of AH receptor DNA-binding activity is important in regulating CYP1A1 transcription and that receptor is required continuously to maintain transcription. This correlation extends to the murine hepatoma cell line Hepa-1c1c7, in which slower kinetics of activation and down-regulation of CYP1A1 transcription paralleled slower activation and down-regulation of AH receptor DNA-binding activity. The difference in kinetics between cell lines also implies that AH receptor DNA-binding activity is modulated by a mechanism that may be influenced by cell-specific regulatory pathways. The above observations in conjunction with mixing experiments and comparisons of cytoplasmic and nuclear extracts indicate that down-regulation of AH receptor DNA-binding activity is probably due either to degradation or to conversion of the receptor to form that is inactive in both DNA binding and transactivation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer T. K., Lefebvre P., Wolford R. G., Hager G. L. Transcription factor loading on the MMTV promoter: a bimodal mechanism for promoter activation. Science. 1992 Mar 20;255(5051):1573–1576. doi: 10.1126/science.1347958. [DOI] [PubMed] [Google Scholar]
- Berghard A., Gradin K., Pongratz I., Whitelaw M., Poellinger L. Cross-coupling of signal transduction pathways: the dioxin receptor mediates induction of cytochrome P-450IA1 expression via a protein kinase C-dependent mechanism. Mol Cell Biol. 1993 Jan;13(1):677–689. doi: 10.1128/mcb.13.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood E. M., Eisenman R. N. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991 Mar 8;251(4998):1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- Burbach K. M., Poland A., Bradfield C. A. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier F., Owens R. A., Nebert D. W., Puga A. Dioxin-dependent activation of murine Cyp1a-1 gene transcription requires protein kinase C-dependent phosphorylation. Mol Cell Biol. 1992 Apr;12(4):1856–1863. doi: 10.1128/mcb.12.4.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Schlegel-Haueter S. E. Study of liver differentiation in vitro. J Cell Biol. 1981 May;89(2):216–222. doi: 10.1083/jcb.89.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y. Temperature-sensitive adult liver cell line dependent on glucocorticoid for differentiation. Mol Cell Biol. 1983 Jun;3(6):1013–1020. doi: 10.1128/mcb.3.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison M. S., Fisher J. M., Whitlock J. P., Jr Inducible, receptor-dependent protein-DNA interactions at a dioxin-responsive transcriptional enhancer. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2528–2532. doi: 10.1073/pnas.85.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison M. S., Fisher J. M., Whitlock J. P., Jr The DNA recognition site for the dioxin-Ah receptor complex. Nucleotide sequence and functional analysis. J Biol Chem. 1988 Nov 25;263(33):17221–17224. [PubMed] [Google Scholar]
- Dolwick K. M., Swanson H. I., Bradfield C. A. In vitro analysis of Ah receptor domains involved in ligand-activated DNA recognition. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8566–8570. doi: 10.1073/pnas.90.18.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J. B., Pastewka J. V., Chalberg S. C., Gozukara E., Guengerich F. P., Gelboin H. V. Noncoordinate regulation of the mRNAs encoding cytochromes P-450BNF/MC-B and P-450ISF/BNF-G. Arch Biochem Biophys. 1986 Jan;244(1):261–272. doi: 10.1016/0003-9861(86)90116-5. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Sogawa K., Yamane M., Fujii-Kuriyama Y. Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: a similarity to glucocorticoid regulatory elements. Nucleic Acids Res. 1987 May 26;15(10):4179–4191. doi: 10.1093/nar/15.10.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Yamane M., Fujii-Kuriyama Y. A DNA-binding factor specific for xenobiotic responsive elements of P-450c gene exists as a cryptic form in cytoplasm: its possible translocation to nucleus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5859–5863. doi: 10.1073/pnas.85.16.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiewicz T. A., Bauman P. A. Heterogeneity of the rat hepatic Ah receptor and evidence for transformation in vitro and in vivo. J Biol Chem. 1987 Feb 15;262(5):2116–2120. [PubMed] [Google Scholar]
- Gasiewicz T. A., Neal R. A. The examination and quantitation of tissue cytosolic receptors for 2,3,7,8-tetrachlorodibenzo-p-dioxin using hydroxylapatite. Anal Biochem. 1982 Jul 15;124(1):1–11. doi: 10.1016/0003-2697(82)90212-3. [DOI] [PubMed] [Google Scholar]
- Gelboin H. V. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev. 1980 Oct;60(4):1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J. Molecular genetics of the P-450 superfamily. Pharmacol Ther. 1990;45(1):1–38. doi: 10.1016/0163-7258(90)90006-n. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Nebert D. W. Autoregulation plus upstream positive and negative control regions associated with transcriptional activation of the mouse P1(450) gene. Nucleic Acids Res. 1985 Oct 25;13(20):7269–7288. doi: 10.1093/nar/13.20.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradin K., Wilhelmsson A., Poellinger L., Berghard A. Nonresponsiveness of normal human fibroblasts to dioxin correlates with the presence of a constitutive xenobiotic response element-binding factor. J Biol Chem. 1993 Feb 25;268(6):4061–4068. [PubMed] [Google Scholar]
- Hapgood J., Cuthill S., Denis M., Poellinger L., Gustafsson J. A. Specific protein-DNA interactions at a xenobiotic-responsive element: copurification of dioxin receptor and DNA-binding activity. Proc Natl Acad Sci U S A. 1989 Jan;86(1):60–64. doi: 10.1073/pnas.86.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry E. C., Rucci G., Gasiewicz T. A. Characterization of multiple forms of the Ah receptor: comparison of species and tissues. Biochemistry. 1989 Jul 25;28(15):6430–6440. doi: 10.1021/bi00441a041. [DOI] [PubMed] [Google Scholar]
- Hoffman E. C., Reyes H., Chu F. F., Sander F., Conley L. H., Brooks B. A., Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991 May 17;252(5008):954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- Lusska A., Wu L., Whitlock J. P., Jr Superinduction of CYP1A1 transcription by cycloheximide. Role of the DNA binding site for the liganded Ah receptor. J Biol Chem. 1992 Jul 25;267(21):15146–15151. [PubMed] [Google Scholar]
- Mueller P. R., Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989 Nov 10;246(4931):780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- Nambu J. R., Lewis J. O., Wharton K. A., Jr, Crews S. T. The Drosophila single-minded gene encodes a helix-loop-helix protein that acts as a master regulator of CNS midline development. Cell. 1991 Dec 20;67(6):1157–1167. doi: 10.1016/0092-8674(91)90292-7. [DOI] [PubMed] [Google Scholar]
- Nebert D. W. The Ah locus: genetic differences in toxicity, cancer, mutation, and birth defects. Crit Rev Toxicol. 1989;20(3):153–174. doi: 10.3109/10408448909017908. [DOI] [PubMed] [Google Scholar]
- Neuhold L. A., Shirayoshi Y., Ozato K., Jones J. E., Nebert D. W. Regulation of mouse CYP1A1 gene expression by dioxin: requirement of two cis-acting elements during induction. Mol Cell Biol. 1989 Jun;9(6):2378–2386. doi: 10.1128/mcb.9.6.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco D. S., Boyum K. W., Merchant S. N., Chalberg S. C., Fagan J. B. Transcriptional and post-transcriptional regulation of the genes encoding cytochromes P-450c and P-450d in vivo and in primary hepatocyte cultures. J Biol Chem. 1988 Jun 25;263(18):8671–8676. [PubMed] [Google Scholar]
- Pelkonen O., Nebert D. W. Metabolism of polycyclic aromatic hydrocarbons: etiologic role in carcinogenesis. Pharmacol Rev. 1982 Jun;34(2):189–222. [PubMed] [Google Scholar]
- Perdew G. H. Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem. 1988 Sep 25;263(27):13802–13805. [PubMed] [Google Scholar]
- Pimental R. A., Liang B., Yee G. K., Wilhelmsson A., Poellinger L., Paulson K. E. Dioxin receptor and C/EBP regulate the function of the glutathione S-transferase Ya gene xenobiotic response element. Mol Cell Biol. 1993 Jul;13(7):4365–4373. doi: 10.1128/mcb.13.7.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Pongratz I., Mason G. G., Poellinger L. Dual roles of the 90-kDa heat shock protein hsp90 in modulating functional activities of the dioxin receptor. Evidence that the dioxin receptor functionally belongs to a subclass of nuclear receptors which require hsp90 both for ligand binding activity and repression of intrinsic DNA binding activity. J Biol Chem. 1992 Jul 5;267(19):13728–13734. [PubMed] [Google Scholar]
- Prokipcak R. D., Okey A. B. Downregulation of the Ah receptor in mouse hepatoma cells treated in culture with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Can J Physiol Pharmacol. 1991 Aug;69(8):1204–1210. doi: 10.1139/y91-176. [DOI] [PubMed] [Google Scholar]
- Reyes H., Reisz-Porszasz S., Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992 May 22;256(5060):1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- Rigaud G., Roux J., Pictet R., Grange T. In vivo footprinting of rat TAT gene: dynamic interplay between the glucocorticoid receptor and a liver-specific factor. Cell. 1991 Nov 29;67(5):977–986. doi: 10.1016/0092-8674(91)90370-e. [DOI] [PubMed] [Google Scholar]
- Robertson R. W., Zhang L., Pasco D. S., Fagan J. B. Aryl hydrocarbon-induced interactions at multiple DNA elements of diverse sequence--a multicomponent mechanism for activation of cytochrome P4501A1 (CYP1A1) gene transcription. Nucleic Acids Res. 1994 May 11;22(9):1741–1749. doi: 10.1093/nar/22.9.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatcioglu F., Perry D. J., Pasco D. S., Fagan J. B. Aryl hydrocarbon (Ah) receptor DNA-binding activity. Sequence specificity and Zn2+ requirement. J Biol Chem. 1990 Jun 5;265(16):9251–9258. [PubMed] [Google Scholar]
- Saatcioglu F., Perry D. J., Pasco D. S., Fagan J. B. Multiple DNA-binding factors interact with overlapping specificities at the aryl hydrocarbon response element of the cytochrome P450IA1 gene. Mol Cell Biol. 1990 Dec;10(12):6408–6416. doi: 10.1128/mcb.10.12.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S. H. Comparative toxicology and mechanism of action of polychlorinated dibenzo-p-dioxins and dibenzofurans. Annu Rev Pharmacol Toxicol. 1986;26:371–399. doi: 10.1146/annurev.pa.26.040186.002103. [DOI] [PubMed] [Google Scholar]
- Shen E. S., Whitlock J. P., Jr Protein-DNA interactions at a dioxin-responsive enhancer. Mutational analysis of the DNA-binding site for the liganded Ah receptor. J Biol Chem. 1992 Apr 5;267(10):6815–6819. [PubMed] [Google Scholar]
- Swanson H. I., Perdew G. H. Half-life of aryl hydrocarbon receptor in Hepa 1 cells: evidence for ligand-dependent alterations in cytosolic receptor levels. Arch Biochem Biophys. 1993 Apr;302(1):167–174. doi: 10.1006/abbi.1993.1195. [DOI] [PubMed] [Google Scholar]
- Teifeld R. M., Fagan J. B., Pasco D. S. Transient superinducibility of cytochrome P450c (CYP1A1) mRNA and transcription. DNA. 1989 Jun;8(5):329–338. doi: 10.1089/dna.1.1989.8.329. [DOI] [PubMed] [Google Scholar]
- Watson A. J., Hankinson O. Dioxin- and Ah receptor-dependent protein binding to xenobiotic responsive elements and G-rich DNA studied by in vivo footprinting. J Biol Chem. 1992 Apr 5;267(10):6874–6878. [PubMed] [Google Scholar]
- Watson A. J., Weir-Brown K. I., Bannister R. M., Chu F. F., Reisz-Porszasz S., Fujii-Kuriyama Y., Sogawa K., Hankinson O. Mechanism of action of a repressor of dioxin-dependent induction of Cyp1a1 gene transcription. Mol Cell Biol. 1992 May;12(5):2115–2123. doi: 10.1128/mcb.12.5.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Wen L. P., Koeiman N., Whitlock J. P., Jr Dioxin-inducible, Ah receptor-dependent transcription in vitro. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8545–8549. doi: 10.1073/pnas.87.21.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw M., Pongratz I., Wilhelmsson A., Gustafsson J. A., Poellinger L. Ligand-dependent recruitment of the Arnt coregulator determines DNA recognition by the dioxin receptor. Mol Cell Biol. 1993 Apr;13(4):2504–2514. doi: 10.1128/mcb.13.4.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J. P., Jr Genetic and molecular aspects of 2,3,7,8-tetrachlorodibenzo-p-dioxin action. Annu Rev Pharmacol Toxicol. 1990;30:251–277. doi: 10.1146/annurev.pa.30.040190.001343. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson A., Cuthill S., Denis M., Wikström A. C., Gustafsson J. A., Poellinger L. The specific DNA binding activity of the dioxin receptor is modulated by the 90 kd heat shock protein. EMBO J. 1990 Jan;9(1):69–76. doi: 10.1002/j.1460-2075.1990.tb08081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Whitlock J. P., Jr Mechanism of dioxin action: Ah receptor-mediated increase in promoter accessibility in vivo. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4811–4815. doi: 10.1073/pnas.89.11.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Whitlock J. P., Jr Mechanism of dioxin action: receptor-enhancer interactions in intact cells. Nucleic Acids Res. 1993 Jan 11;21(1):119–125. doi: 10.1093/nar/21.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]