Abstract
Double umbilical cord blood transplantation (dUCBT), developed as a strategy to treat larger patients with hematologic malignancies, frequently leads to the long-term establishment of a new hematopoietic system maintained by cells derived from a single UCB unit. However, predicting which unit will predominate has remained elusive. This retrospective study examined risk factor associated with unit predominance in 262 patients with hematologic malignancies who underwent dUCBT with subsequent hematopoietic recovery and complete chimerism between 2001–2009. Dual chimerism was detected at day 21–28, with subsequent single chimerism in 97% of cases by day +100 and beyond. Risk factors included nucleated cell dose, CD34+ and CD3+ cell dose, colony forming units-granulocyte macrophage dose, donor-recipient human leukocyte antigen (HLA) match, sex and ABO match, order of infusion, and cell viability. In the myeloablative setting, CD3+ cell dose was the only factor associated with unit predominance (OR 4.4, 95%CI, 1.8–10.6; p<0.01), but in the non-myeloablative setting, CD3+ cell dose (OR 2.1, 95%CI, 1.0–4.2; p=0.05) and HLA match (OR 3.4, 95%CI, 1.0–11.4; p=0.05) were independent factor associated with unit predominance. Taken together, these findings suggest that immune reactivity plays a role in unit predominance and should be considered during graft selection and graft manipulation.
Introduction
Double umbilical cord blood transplantation (dUCBT) was developed as a strategy to overcome the cell-dose limitation of a single UCB unit and allow adults and larger adolescents to proceed to transplantation (1). In addition, dUCBT has served as a model to evaluate novel graft manipulations that not only add a measure of safety (with an unmanipulated unit) but also permit tracking the contribution of the manipulated unit to short- and long-term hematopoietic recovery (2–4). Early after dUCBT (day +21) both UCB units contribute to hematopoiesis in 40–50% of patients, but by day +100 one unit predominates in the vast majority of patients (5, 6). We previously reported that the UCB unit with a higher CD3+ cell dose was more likely to predominate (1). However, the differences in CD3+ cell dose were minimal, and subsequent studies with additional patients at our institution failed to confirm these initial observations.
This current lack of evidence is a major limitation to dUCBT. Knowing which UCB unit characteristics make a unit more likely to predominate would optimize UCB graft selection algorithms by allowing for the selection of two UCB units with a high probability of long-term engraftment. In addition, this knowledge would be important to studies of graft manipulations, both as a safety measure in selecting for manipulation the unit less likely to predominate and as a control measure in assessing which graft-related variables must be taken into account when establishing whether the graft manipulation was effective. For these reasons we elected to reevaluate the risk factors associated with UCB unit predominance in a larger number of patients.
Patients and methods
Patients
This study was a retrospective analysis based on data from the University of Minnesota Blood and Marrow Transplantation Database. Eligibility criteria for inclusion in the study were hematologic malignancy, transplantation with two partially human leukocyte antigen (HLA)-matched unmanipulated UCB units, and hematopoietic recovery with complete chimerism by day +100. For the purposes of this study, cases were defined as the UCB unit achieving predominance, and controls were the non-predominant unit. Patients who received a prior allograft were excluded, though prior autologous transplantation was allowed. Patients treated in protocols that involved graft manipulations or who experienced persistent dual chimerism were excluded. Patient demographics, laboratory data, and clinical outcomes were prospectively collected. Graft selection criteria, conditioning regimen, immunosuppressive regimens, and supportive care were previously reported (1, 6, 7). As per institutional routine, UCB units were infused randomly with a < 30-min interval between the end of the first infusion and start of the second. UCB unit handling and processing followed established institutional practice as previously reported (1, 6). All patients were treated in transplantation protocols approved by the University of Minnesota Institutional Review Board and provided written informed consent according to the principles of the Declaration of Helsinki.
Chimerism Assessment
Chimerism was determined on bone marrow (BM) samples obtained at days +21, +100, +180, +360, and +730 after transplantation, with additional time points as clinically indicated by analysis using quantitative PCR of informative polymorphic variable number tandem repeat (VNTR) or short tandem repeat (STR) regions that distinguished the recipient and donor (8, 9). Chimerism was performed in unseparated BM. Fluorescent PCR products were separated using an Applied Biosystems 373 Sequencer or an Applied Biosystems 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA), and GeneScan software (Applied Biosystems) was used to correlate allele peak areas to the percentage of donor or recipient DNA (accuracy ±5%) (1). Complete chimerism was defined as < 5% of marrow cells derived from the host; dual chimerism was defined as both units contributing to host hematopoiesis; mixed chimerism was defined as > 5% of marrow cells derived from the host and >5% of marrow cells derived from one or both units. In order to determine the whole bone marrow chimerism level at day +100 above which an UCB would always be the long-term predominant, we studied levels above 60% at 5% intervals. Due to the chimerism assay’s accuracy of ±5%, 55% chimerism of unit 1 and 45% chimerism of unit 2, could potentially represent a chimerism of 50%/50% between the 2 units, we started at 60%. With a cutoff chimerism of 65%, there were 2 units (in 2 different patients) that did not predominate beyond day +100. In contrast, for any UCB units with 70% chimerism by day +100, it was always the predominant unit beyond that time point. Thus, unit predominance was defined as a UCB unit that represented ≥ 70% of whole marrow chimerism by day +100 post-transplantation.
Statistical methods
Logistic regression was performed to determine associations between the “winning” unit of a dUCB transplant and various factors at day +100 after UCB transplantation (10). Univariate and multivariate analysis was performed by assessing the odds of the one unit (which was chosen at random) becoming the dominant donor unit in a logistic regression model. Factors considered were infused nucleated cell (NC) dose and viability, CD34+ and CD3+ cell doses, infused colony forming units-granulocyte macrophage (CFU-GM) dose, unit-recipient HLA matching, sex and ABO matching, and order of UCB unit infusion. In this study we only included patients who engrafted after dUCB transplantation. Thus, by study design patient related factors (e.g. age, disease, prior autologous transplant) could not included in the analysis as they equally apply to both units with every patient having a predominant and a non-predominant UCB unit.
Results
Patient characteristics
A database search identified 306 patients with hematologic malignancies who achieved complete chimerism following transplantation with two partially HLA-matched UCB units between 2001–2009. Patients were excluded due to graft failure (n=29), graft manipulation (n=7), persistent dual chimerism (n=6), or unavailable chimerism data (n=2). A total of 262 patients were included in the analysis. The study population was notable for a median age of 44 years, median weight of 78 kg, lower proportion (39%) of myeloablative (MA) conditioning, higher proportion (58%) of acute leukemia, and median follow-up of 2.7 years (range, 0.5–7.2). All patients received cyclosporine and mycophenolate mofetil (MMF) as graft-vs.-host disease prophylaxis. Patient and transplant characteristics are summarized in Table 1.
Table 1.
Patient, graft, and transplant characteristics
| Factors | Frequency |
|---|---|
| Total number of patients | 262 |
| Age | |
| Median (range) | 44 (2–69) |
| Weight (kg) | |
| Median (range) | 78 (15–149) |
| Male | 164 (63%) |
| Disease | |
| Acute lymphoblastic leukemia | 53 (20%) |
| Acute myeloid leukemia | 99 (38%) |
| Chronic myeloid leukemia | 12 (5%) |
| Myelodysplasia | 21 (8%) |
| Chronic lymphocytic leukemia | 9 (3%) |
| Non-Hodgkin lymphoma | 38 (15%) |
| Hodgkin Lymphoma | 19 (7%) |
| Juvenile myelomonocytic leukemia | 5 (2%) |
| Multiple myeloma | 6 (2%) |
| Disease risk | |
| Standard | 158 (60%) |
| High | 104 (40%) |
| Prior autologous transplant | 36 (14%) |
| HLA Match | |
| 4/6+4/6 | 94 (36%) |
| 4/6+5/6 | 61 (23%) |
| 4/6+6/6 | 4 (2%) |
| 5/6+5/6 | 79 (30%) |
| 5/6+6/6 | 11 (4%) |
| 6/6+6/6 | 13 (5%) |
| ABO histocompatibility | |
| Match, Match | 52 (20%) |
| Match, Minor Mismatch | 61 (23%) |
| Match, Major Mismatch | 37 (14%) |
| Minor Mismatch, Minor Mismatch | 40 (15%) |
| Minor Mismatch, Major Mismatch | 39 (15%) |
| Major mismatch, Major Mismatch | 30 (11%) |
| Missing | 3 (1%) |
| Sex Match | |
| Match, Match | 52 (20%) |
| Match, Mismatch | 145 (55%) |
| Mismatch, Mismatch | 62 (24%) |
| Missing | 3 (%) |
| Conditioning | |
| Myeloablative: Cy/Flu/TBI1320 cGY | 102 (39%) |
| Nonmyeloablative: Cy/Flu/TBI200 cGy | 109 (42%) |
| Nonmyeloablative: Cy/Flu/TBI200 cGY/ATG | 51 (19%) |
| Years to Last Contact (among survivors) | |
| Median (range) | 2.7 (0.5–7.2) |
Cy: cyclophosphamide; Flu: fludarabine; TBI: total body irradiation; ATG: anti-thymocyte globuline.
Graft characteristics
As summarized in Table 1, the majority of patients received at least one unit with only 4/6 HLA matches (61%, n=159). In 52 patients (20%), both units were sex and ABO matched with the recipient. The median NC viability and dose, CD34+ and CD3+ cell doses, and CFU-GM dose were similar for the predominant and non-predominant units (Table 2).
Table 2.
Characteristics of predominant and non-predominant UCB units.
| Factors | Predominant | Non-predominant |
|---|---|---|
|
Nucleated cell dose (×107/kg) Median (range) |
1.9 (0.5–10.7) | 1.8 (0.6–8.1) |
|
CD34+ cell dose (×105/kg) Median (range) |
2.0 (0.1–14.0) | 2.0 (0.3–14.0) |
|
CD3+ cell dose (×106/kg) Median (range) |
7.0 (1.0–52.0) | 6.0 (1.0–21.0) |
|
CD3- nucleated cell dose (×107/kg) Median (range) |
1.2 (0.02–5.5) | 1.1 (0.01–6.0) |
|
CFU-GM cell dose (×105/kg) Median (range) |
0.2 (0.01–1.3) | 0.2 (0.01–8.7) |
|
VAOPI* % (range) |
68 (34–94) | 69 (40–88) |
Post-thaw viability was determined by acridine orange and propidium iodide fluorescent dyes (VAOPI). CFU-GM: colony forming unit granulocyte-macrophage.
Engraftment Chimerism Kinetics
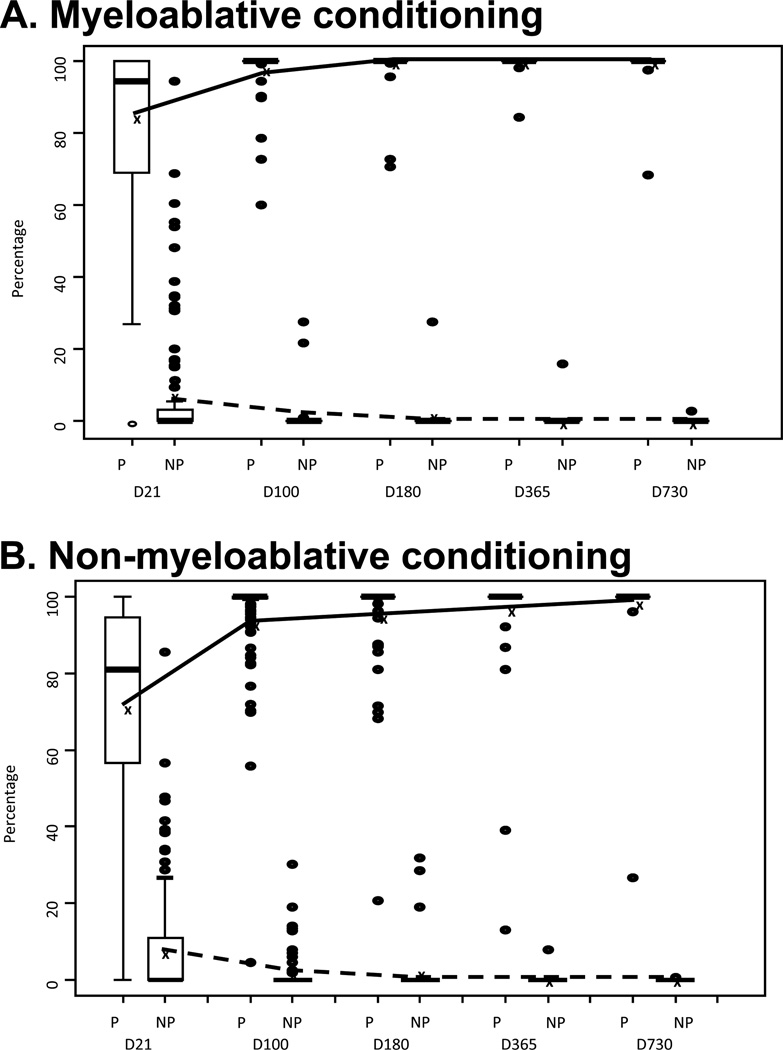
Engraftment studies showed that by day +21 after transplantation a predominant unit (as defined by ≥70% chimerism) was observed in 73 out of 90 (81%) patients after MA conditioning (Figure 1A), but in only 88 out of 145 (61%) patients after non-myeloablative (NMA) conditioning (Figure 1B) (p<0.01). However, this difference was no longer present by day +100 and beyond (Figure 1), at which point a predominant unit was evident in the majority of patients (97% and 94% for MA and NMA, respectively; p=0.35).
Figure 1.
Chimerism kinetics of predominant and non-predominant UCB units in patients receiving myeloablative (A) and non-myeloablative (B) conditioning. The box represents the interquartile range and median, and the dots represent the extreme values in the data set. The lines represent the mean percentage of chimerisms of the predominant (solid) and non-predominant (dashed) units for each time point after transplant. The box representing the interquartile range is not visible for Day 100 and later time points due to minimal data distribution.
Factors influencing predominance
In univariate analysis, the predominant UCB unit was more likely to have higher NC (60%, 95%CI, 57–63, p<0.01) and CD3+ cell doses (68%, 95%CI, 65–71, p<0.01) (Figure 2A). However, NC and CD3+ cell doses were strongly correlated (R=0.68, p<0.01). In order to determine whether the effect of the NC dose was due to the CD3+ cell content or whether it also involved other cells that we were not accounted for, we examined whether the CD3-negative fraction of the NC dose influenced engraftment. The unit with the larger CD3-negative fraction of the NC dose predominated 53% (95%CI 47–59, p=0.38) of the time. Thus, the CD3+ cell dose rather than NC dose was studied in multivariable models. Notably, the difference in CD3+ cell dose between UCB units was small (Figure 2B), with the median difference being only 0.1 × 107/kg (inter-quartile range: −0.1 to +0.3) and the largest difference being 2.0 × 107/kg.
Figure 2.
Effect of CD3+ cell dose (× 107/kg) on umbilical cord blood unit predominance. (A) Correlation between the CD3+ cell dose of the predominant and non-predominant units. Each dot represents the CD3+ cell dose of the predominant and non-predominant units in a single patient. (B) Absolute difference in the CD3+ cell dose between the predominant and non-predominant units derived from data shown in (A). A positive value, above the x-axis, means that the predominant unit had a larger CD3+ cell dose. A negative value, below the x-axis, means that the predominant unit had a lower CD3+ cell dose.
In multivariable analysis, after adjusting for HLA matching, the UCB unit with the higher CD3+ cell dose had higher odds of becoming predominant (OR 2.0, 95%CI, 1.6–4.9, p=<0.01). As the kinetics of chimerism were slightly different between MA and NMA conditioning, we also analyzed these two groups separately. Among MA dUCBT recipients, CD3+ cell dose remained the sole independent predictor of the predominant unit (OR 4.4, 95%CI, 1.8–10.6, p<0.01) with a larger dose more likely predominating. After NMA dUCBT, higher CD3+ cell dose (OR 2.1, 95%CI, 1.0–4.2, p=0.05) and better HLA-matching (OR 3.4, 95%CI 1.0–11.4, p=0.05) were independent predictors of the predominant unit.
Discussion
Previous reports by us (1, 6) and others (11–14) demonstrate that long-term hematopoiesis in dUCBT recipients is maintained by cells derived from a single UCB unit. The present study investigated the kinetics of single unit engraftment after dUCBT and the factors associated with UCB predominance. Unique to our study is the large number of patients that allowed us to determine different predictors leading to UCB predominance following MA (CD3+ cell dose) and NMA (CD3+ cell dose and HLA-matching) conditioning. Neutrophil recovery after MA conditioning typically takes longer and, by the time it occurs, is associated with full chimerism (1). In contrast, neutrophil recovery after NMA conditioning takes less time and is often associated with residual donor hematopoiesis and a period of mixed chimerism, in which both residual recipient and non-predominant unit cells persist (6). It is possible that the influence of HLA-matching in the setting of NMA conditioning could be due to residual host T cells interacting with T cells contained in the two UCB grafts, while these host cells have been eradicated or rendered non-functional following MA conditioning. Consistent with this possibility, we only observed differences in the kinetics of single UCB unit dominance in MA and NMA recipients very early (day +21) after transplant.
Others have shown that unit predominance may also be influenced by post-thaw viability (15), length of time between the infusion of the 2 UCB units (13), and ex vivo expansion (3, 4) . Our data suggest that, following dUCBT with two unmanipulated units of similar quality (as measured by viability) that are infused within a short interval (≤30min), the main determinants of UCB predominance are CD3+ cell dose and, in the setting of NMA conditioning, HLA-matching. While the importance of T cells to establish chimerism and ensure stem cell engraftment is widely accepted (16, 17), the concept that T cells also mediate predominance after dUCBT has been described by us and others in smaller numbers of patients (1, 12, 14, 18). Gutman et al. showed that after dUCBT a subset of CD8+ memory T cells derived from the engrafting unit rapidly emerged after transplant and produced interferon-γ in response to the non-predominant UCB unit (12). It has also been shown that when a T-cell depleted ex vivo expanded unit is combined with an unmanipulated unit, the latter always predominates, further suggesting the importance of T cells(3, 4).
Multiple factors related to the UCB unit and environment play a role in long-term unit predominance after dUCBT. Despite the evidence suggesting an immunologic-mediated mechanism underlying unit predominance, there are several unanswered questions. How is it possible that the typically small difference in T-cell dose between two UCB units determines long-term predominance? While the interferon-γ response is an attractive explanation, it is not clear why unit “A” rather than unit “B” develops it and why only one of the two units develops an interferon-γ response. Although a better understanding of the mechanism involved in long-term predominance is needed, our findings have immediate implications for UCB unit selection and interpretation of results of clinical trials that involve graft manipulation. For example, in studies of ex vivo expansion that involve T-cell depletion, the smaller UCB unit should be used for the manipulation as it would have a lower chance of long-term predominance but could still shorten the period of neutropenia (3, 4). In contrast, when studying agents that may facilitate homing and engraftment, both the CD3+ cell dose and HLA-matching may have to be taken into account to determine if the agent is active or not (19, 20).
Acknowledgments
We thank Michael J. Franklin (University of Minnesota) for editing the manuscript.
This work was supported in part by grants from the National Cancer Institute CA65493 (C.G.B, T.E.D, B.R.B, J.S.M, P.B.M, and J.E.W.) and CA77598 (T.E.D), Children’s Cancer Research Fund (J.E.W., T.E.D and M.R.V.), Universidad Católica de Chile (P.R.), American Cancer Society Audrey Meyer Mars International Fellowship in Clinical Oncology (P.R.), American Society of Blood and Marrow Transplantation Robert A. Good New Investigator Award (C.G.B.), and Leukemia and Lymphoma Society Scholar in Clinical Research Award (C.G.B.).
Footnotes
Disclosure: There are no conflicts of interest to disclose.
Authors Contributions
Pablo Ramirez was involved in the study conception, data collection and analysis, draft and final approval of the version to be published.
John E. Wagner was involved in the study conception, design, data analysis, review and final approval of the version to be published.
Todd E. DeFor was involved in the data analysis and review and final approval of the version to be published.
Bruce R. Blazar was involved in the study conception and in the review and final approval of the version to be published.
Michael R. Verneris was involved in the review and final approval of the version to be published.
Jeffrey S. Miller was involved in the study conception and in the review and final approval of the version to be published.
David H. McKenna was involved in the review and final approval of the version to be published.
Daniel J. Weisdorf was involved in the review and final approval of the version to be published.
Philip B McGlave was involved in the study conception and in the review and final approval of the version to be published.
Claudio G. Brunstein was involved in the study conception, design, data analysis, review and final approval of the version to be published.
References
- 1.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 2.Brunstein CG, Barker JN, Weisdorf DJ, et al. Intra-BM injection to enhance engraftment after myeloablative umbilical cord blood transplantation with two partially HLA-matched units. Bone Marrow Transplant. 2009;43(12):935–940. doi: 10.1038/bmt.2008.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delaney C, Heimfeld S, Brashem-Stein C, et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16(2):232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Lima M, Robinson S, McMannis J, et al. Mesenchymal Stem Cell Based Cord Blood Expansion Leads to Rapid Engraftment of Platelets and Neutrophils. Blood. 2010;116:164. [Google Scholar]
- 5.Ballen KK, Spitzer TR, Yeap BY, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13(1):82–89. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker JN, Weisdorf DJ, DeFor TE, et al. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102(5):1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 8.Schichman SA, Suess P, Vertino AM, Gray PS. Comparison of short tandem repeat and variable number tandem repeat genetic markers for quantitative determination of allogeneic bone marrow transplant engraftment. Bone Marrow Transplant. 2002;29(3):243–248. doi: 10.1038/sj.bmt.1703360. [DOI] [PubMed] [Google Scholar]
- 9.Kristt D, Stein J, Yaniv I, Klein T. Assessing quantitative chimerism longitudinally: technical considerations, clinical applications and routine feasibility. Bone Marrow Transplant. 2007;39(5):255–268. doi: 10.1038/sj.bmt.1705576. [DOI] [PubMed] [Google Scholar]
- 10.Snedecor G, Cochran W. Statistical Methods. 8th ed. Iowa State University Press; 1989. p^pp. [Google Scholar]
- 11.Avery S, Shi W, Lubin M, et al. Influence of infused cell dose and HLA-match on engraftment after double-unit cord blood allografts. Blood. 2010 doi: 10.1182/blood-2010-08-300491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutman JA, Turtle CJ, Manley TJ, et al. Single-unit dominance after double-unit umbilical cord blood transplantation coincides with a specific CD8+ T-cell response against the nonengrafted unit. Blood. 2010;115(4):757–765. doi: 10.1182/blood-2009-07-228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haspel RL, Kao G, Yeap BY, et al. Preinfusion variables predict the predominant unit in the setting of reduced-intensity double cord blood transplantation. Bone Marrow Transplant. 2008;41(6):523–529. doi: 10.1038/sj.bmt.1705933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanda J, Rizzieri DA, Gasparetto C, et al. Adult Dual Umbilical Cord Blood Transplantation Using Myeloablative Total Body Irradiation (1350cGy) and Fludarabine Conditioning. Biol Blood Marrow Transplant. 2010 doi: 10.1016/j.bbmt.2010.09.009. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scaradavou A, Smith KM, Hawke R, et al. Cord blood units with low CD34+ cell viability have a low probability of engraftment after double unit transplantation. Biol Blood Marrow Transplant. 2010;16(4):500–508. doi: 10.1016/j.bbmt.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98(12):3192–3204. doi: 10.1182/blood.v98.12.3192. [DOI] [PubMed] [Google Scholar]
- 17.Marmont AM, Horowitz MM, Gale RP, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78(8):2120–2130. [PubMed] [Google Scholar]
- 18.Eldjerou LK, Chaudhury S, Baisre-de Leon A, et al. An in vivo model of double-unit cord blood transplantation that correlates with clinical engraftment. Blood. 2010;116(19):3999–4006. doi: 10.1182/blood-2010-03-276212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ratajczak MZ, Reca R, Wysoczynski M, et al. Modulation of the SDF-1-CXCR4 axis by the third complement component (C3)--implications for trafficking of CXCR4+ stem cells. Exp Hematol. 2006;34(8):986–995. doi: 10.1016/j.exphem.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113(22):5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]