Abstract
EMBO J (2013) 32: 629–644 doi:; DOI: 10.1038/emboj.2012.340; published online January 08 2013
The lymphatic system is indispensable for the collection and cycling of tissue-extravasated fluids, macromolecules and immune cells into the bloodstream. Different mechanisms, including sprouting, ballooning and budding of lymphatic endothelial cells from the cardinal vein, have been proposed for lymphatic vessel formation during mammalial embryogenesis. Hägerling et al (2013) now provide a cell-scale model of lymphoangiogenesis by applying selective plane illumination-based ultramicroscopy (Becker et al, 2008) to wholemount-immunostained mouse embryos. They describe VEGFR-3, VEGF-C and CCBE1 as key regulators of lymphatic endothelial cell budding and migration at the early emergence of lymphatics from venous endothelium.
Blood and lymphatic vessels are physically and functionally related throughout the life of vertebrates. However, despite very similar morphological features and overall organization, their development is finely tuned by different cues in different moments of embryonal development. The vascular system is the first functional organ system of the embryo. In mice, vasculogenesis starts at day E8.0 with de novo organization of blood vessels by in situ differentiation of hemangioblasts from the lateral plate mesoderm. At day E8.5, as the heart begins to beat, the deriving angioblastic blood islands coalesce to form the first vascular circuits, consisting of paired dorsal aortae, endocardial heart tube, anterior and posterior cardinal veins. Lymphatic endothelial cells differentiate from blood endothelial cells at day E9.0, when a polarized expression of the homeobox gene Sox18 induces the transcription factor Prox1, which becomes detectable by day E10.0 in cells of the dorsolateral cardinal vein. Besides Prox1, a main regulator and a specific marker of lymphatic commitment, several genes have been involved in the biogenesis of the lymphatic system (Schulte-Merker et al, 2011).
Yet, a complete understanding of the subtended mechanism(s) is lacking, mainly related to our inability to comprehensively image, in a dynamic fashion, the events that shape the basic architectures of developing embryos. Emerging technologies are providing new impulse for anatomical studies, by coupling the excellent resolutions of confocal microscopy with high penetration depths. Among these technologies, selective plane illumination microscopy (ultramicroscopy) is a fluorescence technique that uses a focused light sheet to illuminate the sample perpendicularly to the observation pathway. In this system, a micrometric optical sectioning is obtained by stepping the specimen chamber vertically through the laser light sheet. To image a thick specimen, for example, a whole embryo, light needs to penetrate for hundreds of microns, and for this purpose, the specimen has to be optically cleared. This is achieved by replacing the water in the specimen by a liquid with approximately the same refractive index as proteins: in this way, light scattering is strongly reduced, and the specimen becomes translucent (Becker et al, 2008).
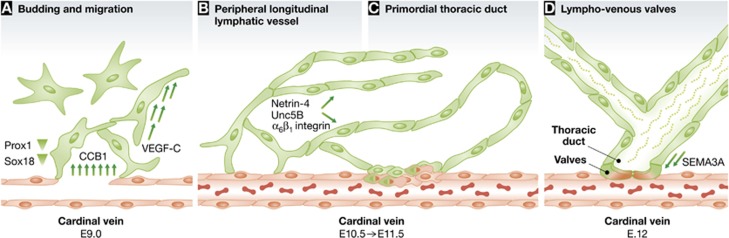
In an elegant morpho-functional study, Hägerling et al (2013) exploited the power of ultramicroscopy for a 3D reconstruction of the first steps in mouse lymphoangiogenesis at a single cell level. They imaged the vascular system of E9.5–E12.0 embryos by wholemount immunostaining for PECAM1 (arterial vessels) or VEGFR-3/Endomucin (venous vessels). At day E10.0, they observed the first Prox1-positive, that is, lymphatic cells emerging from the common cardinal vein as streams of spindle-shaped, migrating cells (Figure 1A). In this early phase, cells form a meshwork; differently from the arterial and venous vessels, these newly formed lymphatics have no lumen and no associated erythrocytes. In concomitance with the formation of such structures, by day E11.0, the authors report that VEGFR-3 expression is almost completely switched from blood endothelial to lymphatic endothelial cells, confirming a functional role for this receptor, and for its ligands VEGF-C and/or CCBE1, in lymphoangiogenesis (Bos et al, 2011). A massive and rapid expansion of the lymphatic meshwork is documented between days E10.0 and E10.25. Around day E10.5, lymphatic endothelial cells are shown to rapidly accumulate at the dorsal edge of the meshwork, forming luminized structures that coalesce to create the peripheral longitudinal lymphatic vessel (Figure 1B). A completely symmetrical structure, the primordial thoracic duct, is demonstrated to derive from cells similarly moving from the cardinal vein, and is fully developed by day E11.5 (Figure 1C). This is the first demonstration that the so-called lymphatic sac is actually constituted by two different entities, that is, the peripheral longitudinal lymphatic vessel and the precursor of the thoracic duct. At this same timepoint, imaging of longer lymphatic structures, extended dorsally from the peripheral longitudinal lymphatic vessel and formed by cells with a migratory phenotype, shows the organization of the dorsal superficial lymphatic plexus. By day E12.0, densely packed erythrocytes are evident in the lumen of the thoracic duct close to the cardinal vein, suggesting a continuity between these two structures and the development of valves for proper compartmentalization and prevention of venous backflow (Figure 1D). The interpretation of such ‘3D snapshots’ is further supported by comparative imaging of genetically engineered mice, lacking VEGFR-3, VEGF-C and/or CCBE1.
Figure 1.
Hägerling et al (2013) apply ultramicroscopy to whole mouse embryos concomitantly stained for different endothelial and lymphatic markers. Their single-cell level 3D imaging of the developing lymphatic structures allowed the definition of four hallmarks of lymphoangiogenesis: (A) Induced by a gradient of Sox18 in the epithelial cells, Prox1-positive, lymphatic endothelial cells emerge from the cardinal vein; CCB1 is responsible for the emersion of single cells, and VEGF-C induces directional migration. (B) These cells coalesce to form the peripheral longitudinal lymphatic vessel and (C) the primordial thoracic duct; the Netrin-4/Unc5B/α6β1 integrin axis is involved in the maintenance of their structural stabilization. (D) The first lymphovenous valves are formed in juxtaposed sites of the cardinal vein and of the primordial thoracic duct: Semaphorin 3A (SEMA3A) has been described in this process.
The work by Hägerling et al (2013) provides a significant contribution to the understanding of controversial models of lymphoangiogenesis. First, their data counteract the concepts that lymphatic endothelial cells emerge from the cardinal vein either (i) by sprouting, as recently suggested by the involvement of platelets in the separation of blood and lymph vessels (Uhrin et al, 2010), or (ii) by ballooning, giving raise to the lymph sacs in a single step (Francois et al, 2012). Their findings, instead, support a model of budding and migration (Yang et al, 2012). Second, they characterize differential roles for VEGFR-3 ligands, showing that (i) CCB1 is responsible for the emersion of single lymphatic endothelial cells from the venous district; (ii) VEGF-C acts as a directional migration cue. Third, they demonstrate that, despite a number of known homologies in the development of the nervous, vascular and lymphatic system, at least one common molecular pathway is exploited in vivo with a different readout. The axon guidance and sprouting angiogenesis ligand Netrin-4, its canonical receptor Unc5B, and its alternative receptor α6β1 integrin (Larrieu-Lahargue et al, 2011), indeed, are not expressed in the first emerging lymphatic endothelial cells, but only in formed structures starting from day E11.0–E11.5. This suggests a role in the structural maintenance of lymphatic vessels, rather than in the early lymphatic commitment. A similar, intriguing difference is provided by a recently identified role of Semaphorin 3A, another axon guidance cue involved in embryo-vascular remodelling (Serini et al, 2012) and in the formation of lymphatic valves (Jurisic et al, 2012).
Some questions remain: how is the separation of blood and lymphatic progenies firstly triggered by the spatial gradient of the transcription factors Sox18 and Prox1? Which are the molecules specifically involved in the shaping and lumening of lymphatic vessels? And, most intriguingly, why and how the pathways shared with the blood, lymphatic and nervous systems are exploited so differently? A number of molecular interactions and their precise appearance during embryo development still have to be clarified with particular emphasis on mixed regulatory circuits between transcriptional and post-transcriptional (microRNAs, long non-coding RNAs) mechanisms. This query will be greatly supported by the possibility of upgrading the reported ultramicroscopy technique toward a time-lapse imaging of living embryos, multiplex stained with differently-labelled specific antibodies. This goal seems not to be so far in the future.
Acknowledgments
The research activities of SM and FB are supported by grants from Italian Association for Cancer Research (contracts 10133, 12182 to FB and My First AIRC Grant, MFAG, to SM), Piedmont Foundation for Cancer Research (FPRC) Intramural Grant 5 × 1000 2008 and Rotaract 2030 (Rotaract Against Cancer 2011-2012) to SM; FP7 (Contract 318035); Ministry of the University and Research (MIUR) (Contract RBAP11BYNP) to FB.
Footnotes
The authors declare that they have no conflict of interest.
References
- Becker K, Jahrling N, Kramer ER, Schnorrer F, Dodt HU (2008) Ultramicroscopy: 3D reconstruction of large microscopical specimens. J Biophotonics 1: 36–42 [DOI] [PubMed] [Google Scholar]
- Bos FL, Caunt M, Peterson-Maduro J, Planas-Paz L, Kowalski J, Karpanen T, van Impel A, Tong R, Ernst JA, Korving J, van Es JH, Lammert E, Duckers HJ, Schulte-Merker S (2011) CCBE1 is essential for mammalian lymphatic vascular development and enhances the lymphangiogenic effect of vascular endothelial growth factor-C in vivo. Circ Res 109: 486–491 [DOI] [PubMed] [Google Scholar]
- Francois M, Short K, Secker GA, Combes A, Schwarz Q, Davidson TL, Smyth I, Hong YK, Harvey NL, Koopman P (2012) Segmental territories along the cardinal veins generate lymph sacs via a ballooning mechanism during embryonic lymphangiogenesis in mice. Dev Biol 364: 89–98 [DOI] [PubMed] [Google Scholar]
- Hägerling R, Pollmann C, Andreas M, Schmidt C, Nurmi H, Adams RH, Alitalo K, Andresen V, Schulte-Merker S, Kiefer F (2013) A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J 32: 629–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurisic G, Maby-El Hajjami H, Karaman S, Ochsenbein AM, Alitalo A, Siddiqui SS, Ochoa Pereira C, Petrova TV, Detmar M (2012) An unexpected role of semaphorin3a-neuropilin-1 signaling in lymphatic vessel maturation and valve formation. Circ Res 111: 426–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieu-Lahargue F, Welm AL, Thomas KR, Li DY (2011) Netrin-4 activates endothelial integrin {alpha}6{beta}1. Circ Res 109: 770–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Merker S, Sabine A, Petrova TV (2011) Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol 193: 607–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini G, Bussolino F, Maione F, Giraudo E (2012) Class 3 semaphorins: physiological vascular normalizing agents for anti-cancer therapy. J Intern Med doi:; DOI: 10.1111/joim.12017 [DOI] [PubMed] [Google Scholar]
- Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, Stockinger H, Fuertbauer E, Moser M, Haiko P, Fassler R, Alitalo K, Binder BR, Kerjaschki D (2010) Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood 115: 3997–4005 [DOI] [PubMed] [Google Scholar]
- Yang Y, Garcia-Verdugo JM, Soriano-Navarro M, Srinivasan RS, Scallan JP, Singh MK, Epstein JA, Oliver G (2012) Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood 120: 2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]