Abstract
The elucidation of epigenetic alterations in the autism brain has potential to provide new insights into the molecular mechanisms underlying abnormal gene expression in this disorder. Given strong evidence that engrailed-2 (EN-2) is a developmentally expressed gene relevant to cerebellar abnormalities and autism, the epigenetic evaluation of this candidate gene was undertaken in 26 case and control post-mortem cerebellar samples. Assessments included global DNA methylation, EN-2 promoter methylation, EN-2 gene expression and EN-2 protein levels. Chromatin immunoprecipitation was used to evaluate trimethylation status of histone H3 lysine 27 (H3K27) associated with gene downregulation and histone H3 lysine 4 (H3K4) associated with gene activation. The results revealed an unusual pattern of global and EN-2 promoter region DNA hypermethylation accompanied by significant increases in EN-2 gene expression and protein levels. Consistent with EN-2 overexpression, histone H3K27 trimethylation mark in the EN-2 promoter was significantly decreased in the autism samples relative to matched controls. Supporting a link between reduced histone H3K27 trimethylation and increased EN-2 gene expression, the mean level of histone H3K4 trimethylation was elevated in the autism cerebellar samples. Together, these results suggest that the normal EN-2 downregulation that signals Purkinje cell maturation during late prenatal and early-postnatal development may not have occurred in some individuals with autism and that the postnatal persistence of EN-2 overexpression may contribute to autism cerebellar abnormalities.
Keywords: Autism, cerebellum, DNA, EN-2, Epigenetics, histone methylation
Introduction
Engrailed-2 (EN-2) is considered to be an autism susceptibility gene based on neuroanatomical parallels between autism and cerebellar developmental abnormalities in rodent models, and also on family linkage studies indicating an overtransmission of EN-2 polymorphic variants from parents to affected children.1 In mice, EN-2 is highly expressed in Purkinje cells during fetal and early-postnatal development acting primarily as a transcriptional repressor until it is downregulated during the perinatal period.2 EN-2 is also expressed in hindbrain nuclei involved in the development of serotonin (raphe nucleus) and norepinephrine (locus coeruleus) neurotransmitter systems that have been implicated in autism.3 Importantly, normal timing of Purkinje cell maturation and cerebellar patterning is critically dependent on perinatal EN-2 downregulation,2 which is disrupted with EN-2 overexpression.4 Although human studies are limited, expression analysis of 18- to -21-week-old fetuses indicated widespread EN-2 gene expression throughout the mid-/hindbrain regions including the cerebellar cortex and deep nuclei at 40-week gestation.5, 6 The human EN-2 gene is composed of two exons separated by a single intron and spans only 8 kb of DNA mapping to 7q36.3, just distal to a region that has been reproducibly associated with autism in linkage studies.7, 8
Anomalies in the cerebellum are arguably the most reproducible neuroanatomical alterations in the autism brain. Most frequently reported is a reduction in Purkinje cell number that appears to occur in the late prenatal period coinciding with the downregulation of EN-2, although other studies suggest this may not occur in all patients with autism.2, 9, 10, 11, 12, 13 Imaging studies have reported vermis hypoplasia and reduction in cerebellar volume in some patients with autism,14 and deficits in fine and gross motor function are common.15 Evidence from neuroimaging of acquired cerebellar lesions suggests that disruption of reciprocal connections to the cortex during critical periods of development may contribute to impaired higher cognitive functions associated with autism symptomatology.16, 17 Several cerebellar abnormalities have been observed in mouse models that either overexpress or under-express EN-2. Ectopic overexpression of EN-2 during fetal development results in selective Purkinje cell loss, reduced cerebellar volume and delayed maturation and migration of the germinal layer.2, 18 Further, EN-2 overexpression in late fetal and early-postnatal development in mice is associated with deficits in dentritogenesis and changes in the pattern of afferent innervation.4, 19, 20 However, for comparative purposes, it must be noted that rodent perinatal neurodevelopment (birth to postnatal days 2–7) approximately corresponds to the human third trimester.21 Interestingly, En-2−/− knockout mice exhibit similar abnormal cerebellar patterning, reduced Purkinje cell numbers and abnormal dendritic foliation underscoring the importance of normal EN-2 regulation during neurodevelopment.22 In addition, the EN-2 null mice display abnormal behaviors relevant to autism including deficits in social behaviors, spatial and memory tasks, and a cerebellar-specific increase in serotonin.23 Together, available evidence suggests that the failure to downregulate EN-2 expression late in development or decreased expression during embryogenesis results in altered cerebellar development that resembles postnatal cerebellar abnormalities in individuals with autism.
Materials and methods
Post-mortem Cerebellum
DNA and nuclei were isolated from frozen blocks of cerebellar cortex from 13 autism and 13 unaffected control individuals obtained from the National Institute of Child Health and Development Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD and from the Autism Tissue Program at the Harvard Brain Tissue Resource Center, Belmont, MA. All donors had a confirmed diagnosis of autism based on Diagnostic and Statistical Manual of Mental Disorders and Autistic Diagnostic Interview Revised. Autism and control groups were matched, as closely as possible for post-mortem interval (PMI), age, gender, race and cause of death.
Global DNA methylation
DNA was extracted from frozen cerebellum blocks using the Puregene DNA Purification kit (Qiagen, Valencia, CA, USA). Purified DNA was digested into component nucleotides as previously described in detail.24 DNA base separation and quantification of 5-methylcytosine (5-mC) and cytosine was performed with a Dionex HPLC-UV system (Sunnyvale, CA, USA) coupled to an electrospray ionization tandem mass spectrometer (Thermo-Finnigan LCQ, Waltham, MA, USA) using a Phenomenex Gemini column (C18, 150 × 2.0 mm, 3 μm, Torrance, CA, USA) and expressed as percent 5-mC/total cytosine in DNA.
EN-2 promoter methylation by McrBC-PCR assay
DNA was extracted and purified with QIAamp DNA Micro Kit (Qiagen) according to the manufacturer's instructions. DNA concentration was determined by NanoDrop 2000c/2000 UV-Vis spectrophotomer (Thermo Fisher Scientific, Middletown, VA, USA) and quality was confirmed by agarose electrophoresis. Genomic DNA (400 ng) was digested with 10 U of McrBC endonuclease (New England BioLabs, St Louis, MO, USA) overnight at 37 °C. McrBC is a methylation-specific endonuclease, which, as opposed to methylation-sensitive restriction (MSR) enzymes, cleaves DNA containing 5-mC on one or both strands, but does not act on unmethylated DNA.25 Strand breaks induced by cleavage of methylated DNA by McrBC abrogates PCR amplification, whereas unmethylated cytosines remain intact and can be detected by quantitative PCR product recovery. Following the McrBC treatment, quantitative PCR with primers within the EN-2 promoter (Figure 1) was used to analyze methylation status of EN-2 promoter. Undigested DNA served as control. Methylated genomic DNA from human male Jurkat cells (New England BioLabs) was used as DNA standard and given value of 100% methylation. Percent CpG methylation in the EN-2 promoter was calculated as ratio of PCR product recovery after DNA digestion with McrBC relative to undigested DNA, normalized to methylated Jurkat genomic DNA: (ΔCtsample/ΔCtCpG methylated jurkat genomic DNA × 100%) where ΔCt=Ctdigested DNA−Ctundigested DNA.
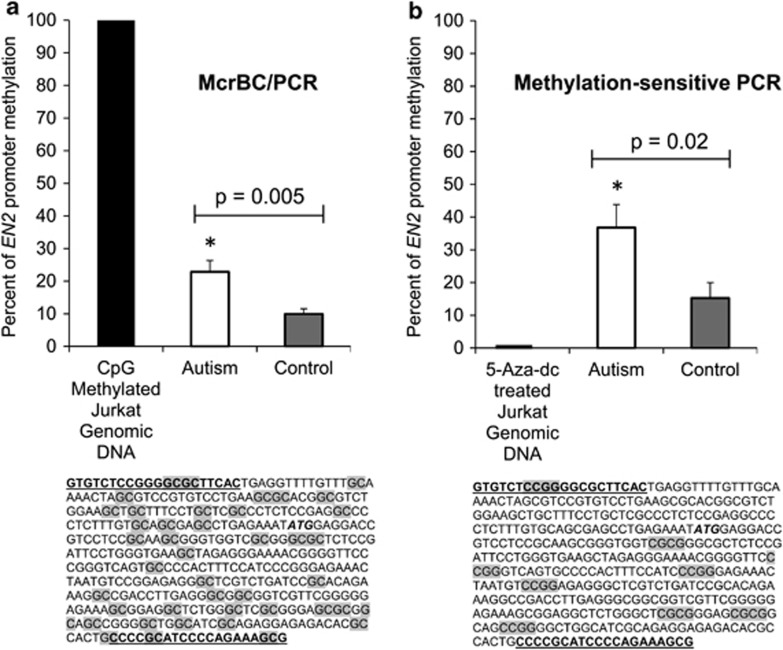
Figure 1.
(a) Engrailed-2 (EN-2) promoter methylation status in 13 case and 13 control cerebellum samples using the McrBC-PCR restriction assay; target CpG sites for restriction within the 5′-promoter sequence are highlighted. (b) EN-2 promoter methylation status using methylation-sensitive restriction PCR with HpaII/BstUI restriction enzymes; target CpG sites within the 5′-promoter sequence are highlighted.
EN-2 promoter methylation by MSR-PCR
Genomic DNA (400 ng) was digested overnight with 5 U of MSR endonuclease HpaII at 37 °C following with 5 U of MSR endonuclease BstUI at 60 °C according to the manufacturer's instructions (New England BioLabs). Double digestion of DNA by HpaII/BstUI induces DNA strand breaks at unmethylated CCGG and CGCG sequences, and abrogates PCR amplification.26 Conversely, methylated cytosines prevent enzyme cleavage and can be detected by PCR product recovery. Undigested DNA served as control. Unmethylated genomic DNA from human male Jurkat cells was used as a standard for EN-2 gene promoter methylation and given value of 0%. The results are presented as ratio of PCR product recovery after the digestion of DNA with HpaII/BstUI relative to undigested DNA, normalized to 5-Aza-dc-treated Jurkat Genomic DNA:
 |
 |
EN-2 gene expression by quantitative reverse transcription PCR
Total RNA was extracted from cerebellum brain tissues using TRI Reagent (Ambion, Life Technologies, Grand Island, NY, USA) and purified with RNeasy Mini Kit (Qiagen). RNA quality (RNA integrity number) was assessed with Agilent RNA 6000 Nano Kit and Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Total RNA (2 μg) was reverse transcribed using random primers and a High Capacity complementary DNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). The levels of EN-2 gene transcripts were determined in triplicate by quantitative reverse transcription PCR using TaqMan Gene Expression Assays (Hs00171321_m1*, Applied Biosystems). Relative quantification of gene expression was performed by using the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene as an internal control. The EN-2 gene expression was presented as mean 2−(CT EN-2 – CT GAPDH) as described previously.27
Western blot analysis of EN-2 protein level
Brain tissue lysates were prepared by homogenization of 30 mg of tissue in lysis buffer supplemented with protease and phosphatase inhibitors. Extracts (50 μg) containing equal quantities of proteins were separated by SDS-polyacrylamide gel electrophoresis on 15% polyacrylamide gels and transferred to polyvinylidene difluoride membranes. Human fetal normal cerebellum (ab30070, Abcam) was used as positive control. Membranes were probed with primary antibodies against EN-2 protein (Rabbit polyclonal antibody to EN-2, ab28731, 1:1000, Abcam). Horseradish peroxidase-coupled donkey anti-rabbit secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used for visualization. Chemiluminescence detection was performed with the Immobilon Western Chemiluminescent horseradish peroxidase Substrate (Millipore Corporation, Billerica, MA, USA) and measured directly by a UVP BioSpectrum Imaging System (Upland, CA, USA). Equal protein loading was confirmed by immunostaining against β-actin (1:5000; Sigma-Aldrich, St Louis, MO, USA).
Chromatin immunoprecipitation assay of H3K4me3 and H3K27me3
Chromatin immunoprecipitation (ChIP) assays were performed using the ChIP Assay Kit (Millipore) and primary antibodies: ChIPAb+ Trimethyl-Histone H3 (Lys27) set and ChIPAb+ Trimethyl-Histone H3 (Lys4) set. Positive and negative control antibodies were included according to the manufacturer's instructions. Purified immunoprecipitated DNA and input DNA were subsequently coamplified by quantitative PCR. The ChIP primers within the EN-2 promoter were designed with Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) tools based on the EN-2 promoter sequences shown in Figure 1. ChIP Primer Pair 1 covered the EN-2 ATG start site and included forward primer: 5′-TCCTGCTCGCCCTCTCCGAG-3′ and reverse primer: 5′-ACTGACCCGGGAACCCCGTT-3′ (product length—139 bp). All assays were run in triplicate and data expressed as the mean (±s.e.) percent input DNA after adjusting for total input DNA: 100 × 2(adjusted input Ct−IP Ct). Histone integrity was confirmed by western blot and gel electrophoresis.
Statistical analysis
Statistical analysis was performed using Graphpad Prism software (La Jolla, CA, USA). Normal distribution of the data was determined using the Kolmogorov–Smirnov test. The paired t-test was used for matched case–control data that were normally distributed and the nonparametric Wilcoxin matched-pairs signed-rank test was used for data that were not normally distributed. Correlations were determined using linear regression analysis within Graphpad software. Results are expressed as means±s.e.m. with statistical significance set at 0.05.
Results
Demographics of tissue donors
The case–control tissue demographics are presented in Table 1. There were no mean differences in age, gender, race or PMI between the autism and control groups (P>0.05). In both groups, 69% (9/13) of individuals were male. The mean age was 15.5 year±9.5 in the autism group (eight children under 18 years and five adults) and 15.8 year±8.6 (nine children under 18 years and four adults) in the control group. The mean PMI was 18.4±5.7 h for the autism samples and 15.7±6.2 h in the control samples. Age, gender, race and PMI were the primary variables matched between groups; however case–control matching for cause of death is more difficult given limited sample availability but these were matched as closely as possible. For example, asphyxia, smoke inhalation or aspiration in cases were matched to asthma and drowning in controls. Head trauma was matched with brain bruising and GI bleeding matched with multiple injuries.
Table 1. Case–control tissue distribution.
| Case no. | Gendera | Ageb | PMI (h)c | Race | Cause of death |
|---|---|---|---|---|---|
| Autism cases | |||||
| UMB 1182 | F | 9 | 24 | African American | Smoke inhalation |
| AN16115 | F | 11 | 13 | White | Drowning |
| AN00764 | M | 20 | 24 | White | Bruising of brain |
| AN08792 | M | 30 | 20 | White | GI Bleeding |
| AN11989 | M | 30 | 16 | White | Heart failure |
| UMB 4671 | F | 4 | 13 | African American | Multiple injuries |
| UMB 4721 | M | 8 | 16 | African American | Drowning |
| UMB 4231 | M | 8 | 12 | African American | Drowning |
| AN19511 | M | 8 | 22 | White | Sarcoma |
| AN16641 | M | 9 | 27 | White | Seizure disorder |
| UMB 4899 | M | 14 | 9 | White | Drowning |
| AN09730 | M | 22 | 25 | White | Aspiration |
| AN12457 | F | 29 | 18 | White | Seizure disorder |
| Control cases | |||||
| UMB 1407 | F | 9 | 20 | African American | Asthma |
| UMB 0856 | F | 29 | 7 | White | Asthma |
| UMB 1322 | M | 16 | 25 | White | Head trauma |
| AN10833 | M | 22 | 21 | Unknown | Unknown |
| AN15622 | M | 30 | 15 | White | Asphyxia |
| UMB 1708 | F | 8 | 20 | African American | Multiple injuries |
| UMB 1793 | M | 11 | 19 | African American | Drowning |
| UMB 4787 | M | 12 | 15 | African American | Asthma |
| UMB 4543 | M | 28 | 13 | White | Multiple injuries |
| UMB 616 | M | 12 | 25 | White | Multiple injuries |
| UMB 1670 | M | 13 | 5 | White | Asphyxia |
| UMB 1185 | M | 4 | 17 | White | Drowning |
| UMB 754 | F | 11 | 12 | Unknown | Asthma |
Abbreviations: F, female; M, male; PMI, post-mortem interval.
N (% male) for autism group: 11 (69.3%); N (% male) for control group: 11 (69.3%).
Mean (s.d.) age for autism group: 15.5 year (9.5); mean (s.d.) age for control group: 15.8 (8.6).
Mean (s.d.) PMI for autism group: 18.4 (5.7); mean (s.d.) PMI for control group: 15.7 ( 6.2).
EN-2 promoter region methylation
The EN-2 promoter region was found to be significantly hypermethylated in the 13 cerebellar samples compared with 13 control samples as determined by the two independent methylation assays with different CpG recognition sites in the EN-2 promoter. Figure 1a presents the percent methylation in case and control samples across the EN-2 promoter as determined by McrBC-PCR assay. The percent methylated cytosines in the autism cerebellum was 23±4% compared with 10±2% in the control samples (P=0.005). The second assay, MSR-PCR confirmed the McrBC results and demonstrated similar EN-2 promoter region hypermethylation (Figure 1b) with 38±7% of the CCGG and CGCG sites methylated compared with 15±5% in controls (P=0.02). The results from both methylation assays were highly correlated (P=0.037; data not shown). Together, these results confirm that the EN-2 promoter region in the autism cerebellar cortex is significantly hypermethylated relative to that of unaffected controls.
Global DNA methylation
Global DNA methylation was quantified in using tandem mass spectrometry and expressed as the percent 5-mC/total cytosine content in DNA. In contrast to global DNA hypomethylation previously reported in immune cells from children with autism,28 DNA extracted from autism cerebellum samples was significantly hypermethylated relative to control samples. The mean percent 5-mC±s.d. was 5.9±1.2 in 13 case cerebellum compared with 4.7±1.1 in 13 controls (P= 0.002) and is presented in Figure 2a. The positive correlation between EN-2 promoter methylation and global DNA methylation (% 5-mC) is shown in Figure 2b (P=0.016).
Figure 2.
(a) Global DNA methylation (percent 5-methylcytosine (5-mC)/total cytosine) in 13 case and 13 control cerebellar samples. (b) Positive correlation between percent 5-mC in DNA and engrailed-2 (EN-2) promoter methylation.
EN-2 gene expression
To assure RNA integrity and valid gene expression data from post-mortem samples, RNA integrity number was determined using the Agilent RNA 6000 Nano Kit and Agilent 2100 Bioanalyzer (Agilent Technologies). The mean RNA integrity number value for cerebellar and control samples was 6.7±1.5 (range 5.9–8.3), which is considered to be excellent quality for the quantitative reverse transcription PCR gene expression analysis. GADPH was used as reference control and has been previously validated as a stable reference gene in post-mortem cerebellar tissues and found not to be correlated with age, PMI or cause of death.29 Unexpectedly, the mean gene expression level of EN-2 was significantly higher in the autism samples despite evidence of promoter region hypermethylation. As shown in Figure 3a, the mean±s.e. amount of target RNA in the autism cerebellum was 0.09±0.02 and 0.04÷0.003 in control samples (P=0.04). In Figure 3b, the positive correlation between EN-2 promoter methylation in case and control samples, and EN-2 gene expression is shown (P=0.007).
Figure 3.
(a) Engrailed-2 (EN-2) gene expression in 13 case and 13 control tissue samples. The expression of EN-2 gene was determined by quantitative reverse transcription PCR and presented as mean 2−(CT EN-2 – CT GAPDH) as described previously.27 (b) Positive correlation between EN-2 promoter methylation and EN-2 gene expression. (c) Level of EN-2 protein in 13 case and 13 control cerebellar samples.
EN-2 protein level
Given the unexpected increase in EN-2 gene expression in the presence of promoter region hypermethylation, levels of EN-2 protein were then evaluated by western blot and expressed as fluorescence units (DLU) normalized to β-actin. As shown in Figure 3c, EN-2 protein levels in the cerebellum were significantly increased in the autism cerebellar samples (9.2±0.86 DLU) compared with the control samples (6.8±0.55 DLU) with a P-value of 0.02. The increased level of EN-2 protein is consistent with the increase in EN-2 gene expression (Figure 3a).
EN-2 promoter region trimethylation of histones H3K27 and H3K4
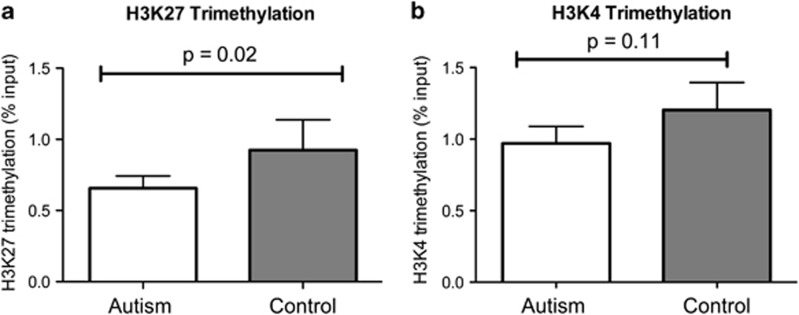
The methylation status of histones H3K27 (associated with gene silencing) and histone H3 lysine 4 (H3K4; associated with gene activation) was investigated as a possible explanation for the increased EN-2 gene expression in the presence of promoter region DNA hypermethylation. The content and integrity of histone protein was confirmed by western blot and gel electrophoresis. Two samples from both case and control groups were omitted from the ChIP analysis due to histone degradation resulting in 11 case and 11 control samples. There was no difference in histone content between the remaining analyzed samples in case and control samples (115 767±12 786 and 124,578±11 541 fluorescent units, respectively; P=0.6). Figure 4 presents ChIP results as the calculated percent input of immunoprecipitated DNA for histone H3K4 trimethylation and histone H3K27 trimethylation after normalization to total input DNA. The mean level of H3K27 trimethylation (associated with gene repression) was significantly decreased in the autism compared with control samples (P= 0.02), and the mean level of histone H3K4 trimethylation (associated with gene activation) in the EN-2 promoter was increased in autism relative to the matched control samples with marginal significance (P=0.11).
Figure 4.
(a) Histone H3 lysine 27 (H3K27) trimethylation within the engrailed-2 (EN-2) promoter region in 11 case and 11 control cerebellar samples. (b) Histone H3 lysine 4 (H3K4) trimethylation within the EN-2 promoter region in 11 case and 11 control cerebellar samples.
Discussion
The elucidation of epigenetic alterations in the autism brain has the potential to provide new insights into the molecular mechanisms underlying abnormal gene expression associated with this disorder. The epigenetic evaluation of EN-2 in the autism cerebellum herein indicates a persistent upregulation of this developmentally expressed homobox gene that normally undergoes perinatal downregulation to insure normal timing and onset of Purkinje cell differentiation. The results of the present investigation are consistent with the possibility that the sustained EN-2 overexpression may be due to epigenetic abnormalities in histone methylation patterns that may contribute to Purkinje cell loss in some individuals with autism.
Recent studies in humans and rodent models have discovered an unexpected connection between gene promoter DNA/histone methylation and memory formation, learning and behavior. For example, epigenetic alterations in brain-derived neurotrophic factor gene expression in the hippocampus were associated with changes in brain-derived neurotrophic factor promoter methylation and memory consolidation after contextual fear conditioning or after early-life maltreatment in mouse models.30, 31 In addition to DNA methylation changes, histone modification and chromatin remodeling have been similarly implicated in synaptic plasticity and learning behavior.32, 33, 34 These results suggest that DNA methylation and histone modifications are dynamically regulated in brain and may contribute to deficits in attention, learning, heritable memory and altered behavioral phenotype in autism.35 Evidence of the cerebellar contribution to autism core symptoms and behaviors has been detailed recently in two excellent reviews.16, 17 Deficits in attention, cognitive function, affective behavior, visual-spatial organization and expressive language have been documented in multiple neuroimaging studies and reports of localized cerebellar lesions.36, 37 Fine and gross motor impairments are common among individuals with autism and have been associated with symptom severity.15 Interestingly, enhanced motor skills in 2-year-old children with autism was a predictor of the subsequent loss of diagnosis at 4 years of age and implicates cerebellar involvement in autism symptom severity and prognosis.38
The increase in EN-2 gene expression and protein levels in the presence of global and promoter region DNA hypermethylation was an unexpected finding for two reasons. In a previous report, we documented genome-wide DNA hypomethylation in immune cells from children with autism relative to age-matched controls. The discordant results in brain and immune cells suggest that alterations in DNA methylation density occur in a tissue-specific manner in autism and that peripheral cell DNA methylation patterns may not be a valid surrogate for alterations in the brain. Secondly, we had expected that EN-2 promoter region hypermethylation would be associated with a decrease in gene expression as previously reported for the MeCP2 promoter in autism cerebral cortex39 and for many cancer-related tumor suppressor genes40 and in schizophrenia.41 However, recent reports of gene activation despite the presence of promoter hypermethylation have emerged to underscore the complexity and multiple layers of molecular control involved in the regulation of gene expression.42 It is possible that promoter DNA hypermethylation of the EN-2 gene in the autism cerebellum preceded the histone modifications, and was initially intended to support downregulation of EN-2 during perinatal development. However, the observed increase in EN-2 gene and protein expression would argue that transcriptional upregulation by other epigenetic mechanisms predominated over the repressive tendencies of DNA cytosine methylation. This suggestion is supported by previous reports showing either a high expression of genes, for example, hTERT, despite their promoter DNA hypermethylation, or reactivation of silenced genes, for example, SFRP1, E-cadherin, without loss of promoter DNA hypermethylation.43, 44, 45
The increase in EN-2 gene and protein level in the autism cerebellum may be partly explained by alterations in promoter histone H3 methylation status. The significant decrease in H3K27 trimethylation (gene repression) was accompanied by a marginally significant increase in H3K4 trimethylation (gene activation) (Figure 4). Both of these alterations, independently or combined, have been associated with gene upregulation during early development. Consistent with our results, several recent studies have attributed expression/reactivation of DNA hypermethylated genes to the increased levels of upregulating histone marks, for example, acetyl-H3K9, methyl-H3K4 or reduced levels of repressive chromatin marks, for example, trimethyl-H3K9 and trimethyl-H3K27.41, 42, 43
The patterns of histone H3K27 and H3K4 trimethylation are dynamically regulated during early development by specific methyltransferases and demethylases, and are thought to underlie the establishment of lineage-specific gene expression programs in the brain. For example, a recently identified H3K27 trimethylation-specific demethylase, JMJD3, has been shown to mediate the upregulation of key genes involved in neurogenesis and the commitment to the neural lineage, including homeobox genes such as EN-2.46, 47 Interestingly, JMJD3 demethylase expression was found to be acutely upregulated in endothelial cells under conditions of oxidative stress following spinal cord injury. The associated decrease in H3K27 trimethylation in the IL-6 gene promoter suggests a regulatory role for this histone modification in the induction of the neuroinflammatory response. Consistent with these observations, our group recently reported evidence of oxidative stress associated with protein and DNA damage in the identical cerebellar samples evaluated in this report.48 Finally, the tissue-specific distribution of MeCP2 in the brain has been shown to be due to its association with chromatin regions containing trimethylated H3K27. The significant decrease in H3K27 trimethylation observed in the present study could offer an alternative mechanism for the inability of MeCP2 to bind to DNA. Mutations in MeCP2 have been associated with comorbid autism in Rett syndrome and similarly prevent MeCP2 DNA binding resulting in gene overexpression. In future studies, it will be important to determine whether the binding of MeCP2 to the EN-2 promoter is decreased and correlated with EN-2 overexpression as a converging genetic/epigenetic mechanism.
Similar to H3K27, reversible methylation and demethylation of H3K4 dynamically alters gene expression during early stages of development in mouse models.49 In humans, a patient with autism was recently found to have a missense mutation in the JARID1C gene that codes for the H3K4 demethylase, which functions as a transcriptional repressor by removing methyl groups from trimethylated H3K4. Inactivation of this specific H3K4 demethylase gene by mutation would be consistent with sustained H3K4 trimethylation and overexpression of affected genes, including EN-2. These recent discoveries have launched the concept that histone H3 methylation/demethylation is a dynamic process that can reversibly mediate gene expression and repression in a cell-specific manner during programmed stages of cell differentiation and neuronal lineage commitment. Thus, the observed overexpression of EN-2 in the autism cerebellum may reflect a failure to downregulate EN-2 expression appropriately during perinatal cerebellar cell development.2
Based on the findings reported here, EN-2 may now be included in the several candidate genes that have both genetic and epigenetic associations with autism including brain-derived neurotrophic factor,50, 51 RELN,52, 53 oxytocin,54, 55 HOXA1,56, 57 MeCP2,58 and FXS.59 In addition, five independent family-based studies have identified polymorphic variation in EN-2 as an autism susceptibility gene.1, 60, 61, 62, 63 Whereas functional alterations due to DNA mutations would be expected to be irreversible, epigenetic factors have potential for modulation and reversibility with targeted interventions. In a major advance, a subject-specific approach was recently published on genome-wide mapping of H3K4 trimethylation in prefrontal cortex of 16 subjects with an autism spectrum disorder.62 A complex pattern emerged with hundreds of loci showing unique subject-specific alterations in H3K4 methylation in genes regulating neuronal connections and social behaviors, often in conjunction with altered gene expression. Several recent reports of altered histone methylation at gene promoters during brain development and the essential regulatory role of histone-specific methylases and demethylases have provided new insights into potential mechanisms underlying altered gene expression in complex neurodevelopmental disease.49, 64, 65
In summary, this is the first case–control study to evaluate the expression and epigenetics of the developmentally regulated homeobox gene EN-2 in the autism brain. The results suggest that the unexpected gene and protein overexpression in the presence of promoter DNA methylation may be partially explained by over-riding histone methylation patterns in H3K27 and H3K4. The observation of genome-wide and promoter-specific DNA hypermethylation in the autism cerebellum is contrary to that previously reported in peripheral lymphocytes from children with autism, and suggests that peripheral cells may not always reflect epigenetic alterations in the brain and brain-specific gene expression in autism. Research into the contribution of epigenetic abnormalities to autism pathogenesis and pathophysiology is an emerging and challenging new field that compliments and extends the search for autism susceptibility genes.
Acknowledgments
We would like to thank the families of individuals with autism for the thoughtful donation of post-mortem tissues to the Autism Tissue Program at the Harvard Brain Tissue Resource Center and the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland. This study was sponsored by National Institute of Child Health and Development; grant number: 1RO1HD051873, Arkansas Biosciences Institute and Jane Botsford Johnson Foundation.
The authors declare no conflict of interest.
References
- Benayed R, Choi J, Matteson PG, Gharani N, Kamdar S, Brzustowicz LM, et al. Autism-associated haplotype affects the regulation of the homeobox gene, ENGRAILED 2. Biol Psychiatry. 2009;66:911–917. doi: 10.1016/j.biopsych.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski J, Holst MI, Liebig C, Oberdick J, Baader SL. Engrailed-2 negatively regulates the onset of perinatal Purkinje cell differentiation. J Comp Neurol. 2004;472:87–99. doi: 10.1002/cne.20059. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Purpura DP. Autism, fever, epigenetics and the locus coeruleus. Brain Res Rev. 2009;59:388–392. doi: 10.1016/j.brainresrev.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst MI, Maercker C, Pintea B, Masseroli M, Liebig C, Jankowski J, et al. Engrailed-2 regulates genes related to vesicle formation and transport in cerebellar Purkinje cells. Mol Cell Neurosci. 2008;38:495–504. doi: 10.1016/j.mcn.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Zec N, Rowitch DH, Bitgood MJ, Kinney HC. Expression of the homeobox-containing genes EN1 and EN2 in human fetal midgestational medulla and cerebellum. J Neuropathol Exp Neurol. 1997;56:236–242. doi: 10.1097/00005072-199703000-00002. [DOI] [PubMed] [Google Scholar]
- Logan C, Hanks MC, Noble-Topham S, Nallainathan D, Provart NJ, Joyner AL. Cloning and sequence comparison of the mouse, human, and chicken engrailed genes reveal potential functional domains and regulatory regions. Dev Genet. 1992;13:345–358. doi: 10.1002/dvg.1020130505. [DOI] [PubMed] [Google Scholar]
- Liu J, Nyholt DR, Magnussen P, Parano E, Pavone P, Geschwind D, et al. A genomewide screen for autism susceptibility loci. Am J Hum Genet. 2001;69:327–340. doi: 10.1086/321980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon M, Cantor RM, Liu J, Gilliam TC, Geschwind DH. Evidence for a language quantitative trait locus on chromosome 7q in multiplex autism families. Am. J Hum Genet. 2002;70:60–71. doi: 10.1086/338241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney ER, Kemper TL, Bauman ML, Rosene DL, Blatt GJ. Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum. 2008;7:406–416. doi: 10.1007/s12311-008-0043-y. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr Opin Neurobiol. 1997;7:269–278. doi: 10.1016/s0959-4388(97)80016-5. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trend Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Kamiya J, Aoki Y. Associations between hyperglycaemia and somatic transversion mutations in mitochondrial DNA of people with diabetes mellitus. Diabetologia. 2003;46:1559–1566. doi: 10.1007/s00125-003-1215-4. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med. 1988;318:1349–1354. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- Freitag CM, Kleser C, Schneider M, von Gontard A. Quantitative assessment of neuromotor function in adolescents with high functioning autism and Asperger Syndrome. J Autism Develop Disord. 2007;37:948–959. doi: 10.1007/s10803-006-0235-6. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, et al. Consensus paper: pathological role of the cerebellum in autism. Cerebellum. 2012;11:777–807. doi: 10.1007/s12311-012-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Halloran CJ, Kinsella GJ, Storey E. The cerebellum and neuropsychological functioning: a critical review. J Clin Exp Neuropsychol. 2012;34:35–56. doi: 10.1080/13803395.2011.614599. [DOI] [PubMed] [Google Scholar]
- Baader SL, Sanlioglu S, Berrebi AS, Parker-Thornburg J, Oberdick J. Ectopic overexpression of engrailed-2 in cerebellar Purkinje cells causes restricted cell loss and retarded external germinal layer development at lobule junctions. J Neurosci. 1998;18:1763–1773. doi: 10.1523/JNEUROSCI.18-05-01763.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Sudarov A, Szulc KU, Sgaier SK, Stephen D, Turnbull DH, et al. The engrailed homeobox genes determine the different foliation patterns in the vermis and hemispheres of the mammalian cerebellum. Development. 2010;137:519–529. doi: 10.1242/dev.027045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillitoe RV, Vogel MW, Joyner AL. Engrailed homeobox genes regulate establishment of the cerebellar afferent circuit map. J Neurosci. 2010;30:10015–10024. doi: 10.1523/JNEUROSCI.0653-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuemerle B, Zanjani H, Joyner A, Herrup K. Pattern deformities and cell loss in Engrailed-2 mutant mice suggest two separate patterning events during cerebellar development. J Neurosci. 1997;17:7881–7889. doi: 10.1523/JNEUROSCI.17-20-07881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheh MA, Millonig JH, Roselli LM, Ming X, Jacobsen E, Kamdar S, et al. En2 knockout mice display neurobehavioral and neurochemical alterations relevant to autism spectrum disorder. Brain Res. 2006;1116:166–176. doi: 10.1016/j.brainres.2006.07.086. [DOI] [PubMed] [Google Scholar]
- Friso S, Choi SW, Dolnikowski GG, Selhub J. A method to assess genomic DNA methylation using high-performance liquid chromatography/electrospray ionization mass spectrometry. Anal Chem. 2002;74:4526–4531. doi: 10.1021/ac020050h. [DOI] [PubMed] [Google Scholar]
- Sutherland E, Coe L, Raleigh EA. McrBC: a multisubunit GTP-dependent restriction endonuclease. J Mol Biol. 1992;225:327–348. doi: 10.1016/0022-2836(92)90925-a. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kokubun S, Itoi E, Roach HI. Improved quantification of DNA methylation using methylation-sensitive restriction enzymes and real-time PCR. Epigenetics. 2007;2:86–91. doi: 10.4161/epi.2.2.4203. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Melnyk S, Fuchs GJ, Schulz E, Lopez M, Kahler SG, Fussell JJ, et al. Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism. J Autism Dev Disord. 2012;42:367–377. doi: 10.1007/s10803-011-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelkamm A, Vennemann B, Fracasso T, Lutz-Bonengel S, Schmidt U, Heinrich M. Validation of adequate endogenous reference genes for the normalisation of qPCR gene expression data in human post mortem tissue. Int J Legal Med. 2010;124:371–380. doi: 10.1007/s00414-010-0433-9. [DOI] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Hsieh J, Gage FH. Chromatin remodeling in neural development and plasticity. Curr Opin Cell Biol. 2005;17:664–671. doi: 10.1016/j.ceb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci. 2005;6:108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129 (Pt 2:290–292. doi: 10.1093/brain/awh729. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121 (Pt 4:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Sutera S, Pandey J, Esser EL, Rosenthal MA, Wilson LB, Barton M, et al. Predictors of optimal outcome in toddlers diagnosed with autism spectrum disorders. J Autism Dev Disord. 2007;37:98–107. doi: 10.1007/s10803-006-0340-6. [DOI] [PubMed] [Google Scholar]
- Nagarajan RP, Hogart AR, Gwye Y, Martin MR, LaSalle JM. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics. 2006;1:172–182. doi: 10.4161/epi.1.4.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M, Corn PG, Baylin SB, Herman JG. A gene hypermethylation profile of human cancer. Cancer Res. 2001;61:3225–3229. [PubMed] [Google Scholar]
- Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A, et al. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci USA. 2005;102:9341–9346. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynal NJ, Si J, Taby RF, Gharibyan V, Ahmed S, Jelinek J, et al. DNA methylation does not stably lock gene expression but instead serves as a molecular mark for gene silencing memory. Cancer Res. 2012;72:1170–1181. doi: 10.1158/0008-5472.CAN-11-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn RL, Pruitt K, Eguchi S, Baylin SB, Herman JG. hTERT is expressed in cancer cell lines despite promoter DNA methylation by preservation of unmethylated DNA and active chromatin around the transcription start site. Cancer Res. 2007;67:194–201. doi: 10.1158/0008-5472.CAN-06-3396. [DOI] [PubMed] [Google Scholar]
- Pruitt K, Zinn RL, Ohm JE, McGarvey KM, Kang SH, Watkins DN, et al. Inhibition of SIRT1 reactivates silenced cancer genes without loss of promoter DNA hypermethylation. PLoS Genet. 2006;2:e40. doi: 10.1371/journal.pgen.0020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryndyak VP, Beland FA, Pogribny IP. E-cadherin transcriptional down-regulation by epigenetic and microRNA-200 family alterations is related to mesenchymal and drug-resistant phenotypes in human breast cancer cells. Int J Cancer. 2010;126:2575–2583. doi: 10.1002/ijc.24972. [DOI] [PubMed] [Google Scholar]
- Burgold T, Spreafico F, De Santa F, Totaro MG, Prosperini E, Natoli G, et al. The histone H3 lysine 27-specific demethylase Jmjd3 is required for neural commitment. PloS one. 2008;3:e3034. doi: 10.1371/journal.pone.0003034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, et al. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–734. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- Rose S, Melnyk S, Trusty TA, Pavliv O, Seidel L, Li J, et al. Intracellular and extracellular redox status and free radical generation in primary immune cells from children with autism. Autism Res Treat. 2012;2012:986519. doi: 10.1155/2012/986519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynder C, Stalker L, Doughty ML. Role of H3K4 demethylases in complex neurodevelopmental diseases. Epigenomics. 2010;2:407–418. doi: 10.2217/epi.10.12. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Toro R, Proitsi P, Powell J, Paus T, FB P, et al. A functional polymorphism of the brain derived neurotrophic factor gene and cortical anatomy in autism spectrum disorder. J Neurodev Disord. 2009;1:215–223. doi: 10.1007/s11689-009-9012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchikami M, Yamamoto S, Morinobu S, Takei S, Yamawaki S. Epigenetic regulation of BDNF gene in response to stress. Psychiatry Investig. 2010;7:251–256. doi: 10.4306/pi.2010.7.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaar DA, Shao Y, Haines JL, Stenger JE, Jaworski J, Martin ER, et al. Analysis of the RELN gene as a genetic risk factor for autism. Mol Psychiatry. 2005;10:563–571. doi: 10.1038/sj.mp.4001614. [DOI] [PubMed] [Google Scholar]
- Lintas C, Persico AM. Neocortical RELN promoter methylation increases significantly after puberty. Neuroreport. 2010;21:114–118. doi: 10.1097/WNR.0b013e328334b343. [DOI] [PubMed] [Google Scholar]
- Munesue T, Yokoyama S, Nakamura K, Anitha A, Yamada K, Hayashi K, et al. Two genetic variants of CD38 in subjects with autism spectrum disorder and controls. Neurosci Res. 2010;67:181–191. doi: 10.1016/j.neures.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Vaituzis C, Tran L, Mackie S, Tiemeier H, et al. Allelic variation within the putative autism spectrum disorder risk gene homeobox A1 and cerebellar maturation in typically developing children and adolescents. Autism Res. 2012;5:93–100. doi: 10.1002/aur.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stodgell CJ, Ingram JL, O'Bara M, Tisdale BK, Nau H, Rodier PM. Induction of the homeotic gene Hoxa1 through valproic acid's teratogenic mechanism of action. Neurotoxicol Teratol. 2006;28:617–624. doi: 10.1016/j.ntt.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Lopez-Rangel E, Lewis ME. Loud and clear evidence for gene silencing by epigenetic mechanisms in autism spectrum and related neurodevelopmental disorders. Clin Genet. 2006;69:21–22. doi: 10.1111/j.1399-0004.2006.00543a.x. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Ono MY, Hagerman PJ. Recent advances in fragile X: a model for autism and neurodegeneration. Curr Opin Psychiatry. 2005;18:490–496. doi: 10.1097/01.yco.0000179485.39520.b0. [DOI] [PubMed] [Google Scholar]
- Gharani N, Benayed R, Mancuso V, Brzustowicz LM, Millonig JH. Association of the homeobox transcription factor, ENGRAILED 2, 3, with autism spectrum disorder. Mol Psychiatry. 2004;9:474–484. doi: 10.1038/sj.mp.4001498. [DOI] [PubMed] [Google Scholar]
- Sen B, Singh AS, Sinha S, Chatterjee A, Ahmed S, Ghosh S, et al. Family-based studies indicate association of Engrailed 2 gene with autism in an Indian population. Genes Brain Behav. 2010;9:248–255. doi: 10.1111/j.1601-183X.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- Carayol J, Schellenberg GD, Tores F, Hager J, Ziegler A, Dawson G. Assessing the impact of a combined analysis of four common low-risk genetic variants on autism risk. Mol Autism. 2010;1:4. doi: 10.1186/2040-2392-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Shu BC, Hallmayer JF, Lung FW. Intronic single nucleotide polymorphisms of engrailed homeobox 2 modulate the disease vulnerability of autism in a han chinese population. Neuropsychobiology. 2010;62:104–115. doi: 10.1159/000315441. [DOI] [PubMed] [Google Scholar]
- Stadler F, Kolb G, Rubusch L, Baker SP, Jones EG, Akbarian S. Histone methylation at gene promoters is associated with developmental regulation and region-specific expression of ionotropic and metabotropic glutamate receptors in human brain. J Neurochem. 2005;94:324–336. doi: 10.1111/j.1471-4159.2005.03190.x. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Huang HS. Epigenetic regulation in human brain-focus on histone lysine methylation. Biological Psychiatry. 2009;65:198–203. doi: 10.1016/j.biopsych.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]