Abstract
Harmful excessive use of alcohol has a severe impact on society and it remains one of the major causes of morbidity and mortality in the population. However, mechanisms that underlie excessive alcohol consumption are still poorly understood, and thus available medications for alcohol use disorders are limited. Here, we report that changing the level of chromatin condensation by affecting DNA methylation or histone acetylation limits excessive alcohol drinking and seeking behaviors in rodents. Specifically, we show that decreasing DNA methylation by inhibiting the activity of DNA methyltransferase (DNMT) with systemic administration of the FDA-approved drug, 5-azacitidine (5-AzaC) prevents excessive alcohol use in mice. Similarly, we find that increasing histone acetylation via systemic treatment with several histone deacetylase (HDAC) inhibitors reduces mice binge-like alcohol drinking. We further report that systemic administration of the FDA-approved HDAC inhibitor, SAHA, inhibits the motivation of rats to seek alcohol. Importantly, the actions of both DNMT and HDAC inhibitors are specific for alcohol, as no changes in saccharin or sucrose intake were observed. In line with these behavioral findings, we demonstrate that excessive alcohol drinking increases DNMT1 levels and reduces histone H4 acetylation in the nucleus accumbens (NAc) of rodents. Together, our findings illustrate that DNA methylation and histone acetylation control the level of excessive alcohol drinking and seeking behaviors in preclinical rodent models. Our study therefore highlights the possibility that DNMT and HDAC inhibitors can be used to treat harmful alcohol abuse.
Keywords: addiction, alcohol, chromatin, DNMT, epigenetic, ethanol, HDAC
Introduction
Alcohol use disorders are the second most detrimental neuropsychiatric disorder.1 Four percent of deaths worldwide are associated with alcohol abuse,2 and chronic harmful alcohol use is one of the four most common risk factors for multiple diseases such as cardiovascular diseases and diabetes, and is the third largest risk factor for reduced productivity.2 Finally, alcohol has been rated as the most harmful drug to oneself and others.3 However, the central molecular mechanisms underlying harmful excessive alcohol intake are not well understood and thus treatment options remain limited with modest efficacy.
The alteration in gene expression is one of the central features underlying neuroadaptations that result from chronic drug and alcohol use.4, 5 One means of controlling gene expression is the remodeling of chromatin structure.6 Specifically, chromatin condensation and decondensation block or allow, respectively, the accessibility of gene promoters to the transcriptional machinery.6 Chromatin is formed by a DNA molecule wrapped around an octomer of histones (H2A, H2B, H3 and H4).6 Histone acetylation promotes chromatin relaxation that allows gene expression, whereas histone deacetylation, catalyzed by histone deacetylases (HDACs), leads to chromatin condensation and to repression of transcription.6 Among the HDAC superfamily, classes I and IIa are the most abundant in the brain.7 DNA methylation occurs on specific cytosine-guanine dinucleotide domains (CpG islands) within gene promoters and is catalyzed by DNA methyltransferases (DNMT).8 Methylated-CpG islands are binding sites for Methyl CpG binding protein 2 (MeCP2), which forms a protein complex with HDAC that represses gene transcription by promoting chromatin condensation at the promoter.8, 9
Animal and human studies suggest that alterations in DNA methylation and histone acetylation contribute to mechanisms that underlie psychiatric disorders including drug addiction.10, 11 Specifically for alcohol, decreases in Neuropeptide Y expression in the central and medial amygdala induced by alcohol withdrawal in rats are due to a decrease in histone acetylation, which is linked to HDAC hyperactivation,12 and human studies have revealed that alcoholics display alterations in methylated DNA profile and DNMT levels.13, 14, 15, 16, 17 These data suggest that modifications in chromatin remodeling by alcohol may be a focal point in neuroadaptations resulting in continuous excessive alcohol seeking and intake. If so, modifiers of chromatin structure can be used for the treatment of alcohol use disorders.
Materials and methods
Information on reagents, animals, preparation of solutions, systemic administration of inhibitors, blood alcohol concentration measurements, mouse experiments (intermittent access to 20% alcohol or 0.03% saccharin 2-bottle choice, intermittent limited access to 20% alcohol or 0.03% saccharin in drinking in the dark (DID)), rat experiments (self-administration of 20% alcohol and 1.5% sucrose), sample preparations, western blot analysis and statistical analyses are available in Supplementary information.
Results
Systemic administration of 5-azacitidine reduces 20% alcohol drinking, but not saccharin intake, in mice
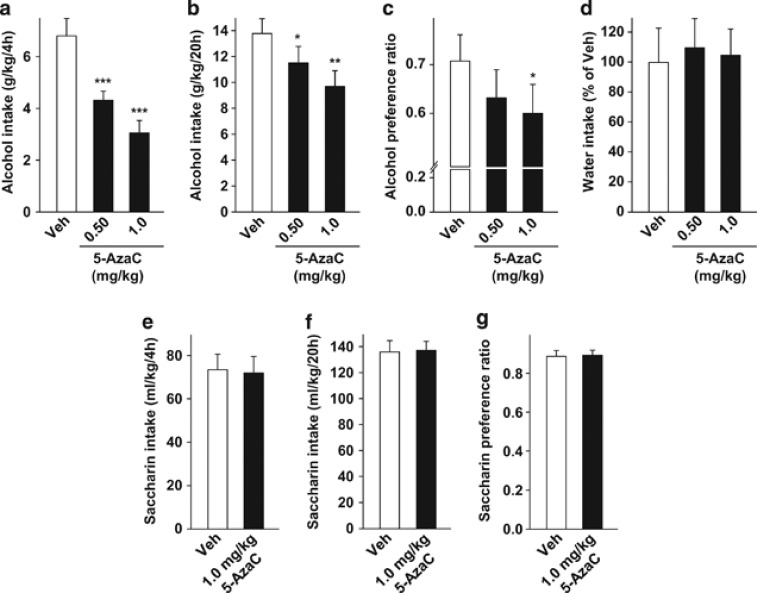
Inhibition of DNMT activity reduces the level of DNA methylation, which, in turn, increases gene expression by promoting chromatin decondensation at the promoter.8, 9, 18 We therefore determined whether reducing DNA methylation via inhibiting DNMT activity18 reduces alcohol intake. To do so, we tested the effect of the DNMT inhibitor, 5-azacitidine (5-AzaC), an FDA-approved drug for the treatment of certain forms of cancer, on excessive alcohol drinking in mice by using the intermittent access to 20% alcohol 2-bottle choice procedure. After 8 weeks of alcohol exposure, mice reached a high level of alcohol intake (∼7 g/kg during the first 4 h and ∼21 g/kg during the entire session; Supplementary Figure S1a) and preference ratio (∼0.75; Supplementary Figure S1b). Furthermore, with this procedure, mice experience repeated cycles of alcohol binge drinking and withdrawal similar to those of human abusers of alcohol.19 Previous studies showed that 5-AzaC is most effective when administered repeatedly in animal models for depression,20 therefore we used here a similar regime. Specifically, mice were treated systemically (intraperitoneal, i.p.) with 5-AzaC, 24, 18 and 2 h before the beginning of the test session (Supplementary Figure S2a). A within-subject design was used to test the effect of the drug, with mice receiving either 5-AzaC or vehicle once a week according to a Latin square experimental design. We found that 5-AzaC significantly decreased binge consumption of alcohol as measured during the first 4 h of alcohol access (one-way repeated measures analysis of variance (RM-ANOVA), F(2,18)=20.4, P<0.001; post hoc, Ps<0.001; Figure 1a). Our results also show that 5-AzaC reduced alcohol consumption (one-way RM-ANOVA, F(2,18)=7.4, P<0.01; Figure 1b) and preference (one-way RM-ANOVA, F(2,18)=3.9, P<0.05; Figure 1c) during a 24-h alcohol-drinking session but not during the subsequent drinking session (Supplementary Figure S3). Post hoc analysis revealed that 5-AzaC at 0.5 and 1.0 mg/kg significantly reduced alcohol drinking (Ps<0.05; Figure 1b), and the higher dose significantly decreased the preference for the alcohol solution (P<0.05; Figure 1c). 5-AzaC treatment did not modify water intake (Figure 1d), nor did it change the kinetics of blood alcohol clearance (Supplementary Figure S4).
Figure 1.
Systemic administration of 5-azacitidine (5-AzaC) reduces excessive alcohol intake, but not saccharin intake, in mice. (a–d) Mice undergoing intermittent access to 20% alcohol 2-bottle choice for 24 h were systemically administered (intraperitoneal, i.p.) with 5-AzaC (0.50–1.0 mg/kg) or its vehicle (Veh) 24, 18, and 2 h before the initiation of the test alcohol-drinking session. (a) Amount of alcohol (g/kg) consumed during the first 4 h of 20% alcohol access. (b) Amount of alcohol (g/kg) consumed during the last 20 h of 20% alcohol access. (c) Preference for alcohol is calculated as the ratio of the volume of alcohol solution intake/volume of total fluid intake during the last 20 h of 20% alcohol access. (d) Water intake during the last 20 h of 20% alcohol access. Results are expressed as mean±s.e.m., *P<0.05, **P<0.01 and ***P<0.001 compared with vehicle. (a–d) n=10. (e–g) Mice with intermittent access to 0.03% saccharin for 24 h in a 2-bottle choice procedure were systemically administered (i.p.) with 1 mg/kg 5-AzaC as described above. Amount of saccharin solution (ml kg−1) consumed during the first 4 h (e) and the last 20 h of access (f). (g) Preference ratio for saccharin solution during the last 20 h of access. Results are expressed as mean±s.e.m. (e–g) n=10.
Next, to evaluate whether the effects of 5-AzaC were specific to alcohol or were due to a generalized inhibition of reward, we tested the effect of the DNMT inhibitor on saccharin intake. Mice undergoing intermittent access to 0.03% saccharin (2-bottle choice; Supplementary Figures S5a and b) were systemically treated with 1 mg/kg 5-AzaC 24, 18 and 2 h before the beginning of the test session. A within-subject design, as detailed above for alcohol, was used to test the effect of the drug on saccharin intake (Supplementary Figure S2a). In contrast, we found that 5-AzaC does not modify the level of saccharin consumption (paired t-test: first 4 h t(9)=0.15, P=0.88; Figure 1e, and the last 20 h t(9)=−0.10, P=0.92; Figure 1f) or preference (paired t-test, t(9)=−0.09, P=0.93; Figure 1g). This result indicates that DNMT inhibition reduces both alcohol intake and preference, but that effect is not generalized to other rewarding substances. Together, these results suggest that promoting chromatin decondensation via inhibiting DNMT activity reduces alcohol drinking.
Systemic administration of HDAC inhibitors reduces binge-like alcohol drinking, but not saccharin, intake in mice
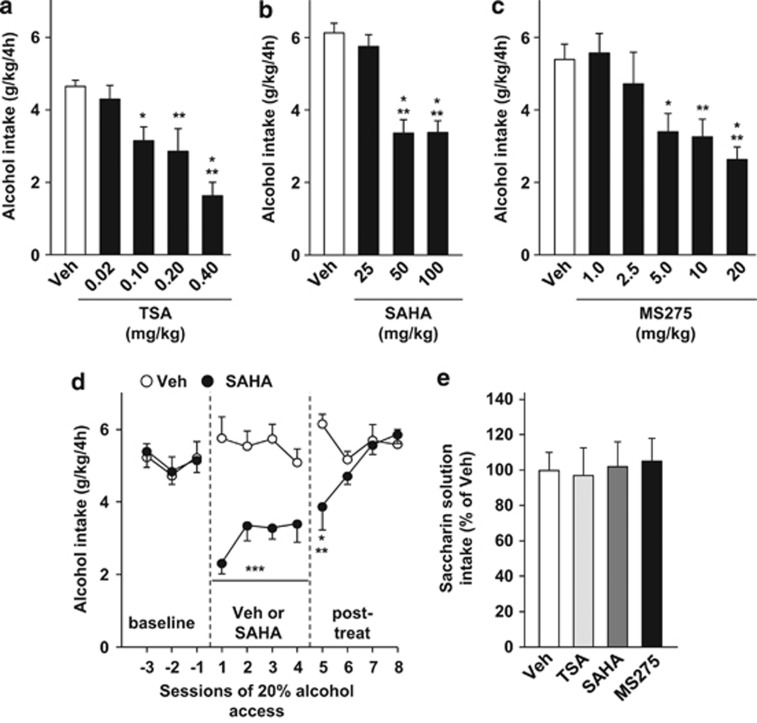
Another way to increase chromatin relaxation and induce gene expression is by enhancing histone acetylation via the inhibition of HDAC activity.21, 22, 23 We therefore evaluated whether the administration of HDAC inhibitors modifies the level of binge-like 20% alcohol drinking. We used a second well-established preclinical drinking model in mice, in which mice have intermittent access to a single bottle of 20% alcohol for 4 h beginning 2 h into the dark cycle.24, 25 This procedure also promotes high levels of consumption (∼7 g/kg per 4 h), and generates pharmacologically relevant blood alcohol concentrations of ∼100 mg%,24 which corresponds to the definition of binge drinking in humans.26 In this paradigm, mice experience periods of alcohol binge drinking and withdrawal similar to what human alcohol abusers encounter.19 Three different HDAC inhibitors were tested in three independent groups of mice (for baseline levels of alcohol intake, see Supplementary Table S1). A within-subject design was used, with mice receiving either vehicle or an HDAC inhibitor once a week according to a Latin square experimental design. HDAC inhibitors were administered 2 h before the beginning of alcohol access session (Supplementary Figure S2b). As shown in Figures 2a and b, systemic administration of pan HDAC class I and II inhibitors, TSA and SAHA, produced a significant dose-dependent decrease in 20% alcohol drinking in mice (one-way RM-ANOVA, TSA (F(4,31)=8.2, P<0.001), SAHA (F(3,42)=23.5, P<0.001)). Post hoc analysis revealed that all doses of TSA tested, except 0.02 mg/kg, reduced binge-like alcohol drinking (Ps<0.05; Figure 2a) and SAHA significantly inhibited alcohol binging at 50 and 100 mg/kg (Ps<0.001), but not at 25 mg/kg (Figure 2b).
Figure 2.
Systemic administration of HDAC inhibitors reduces binge-like alcohol drinking, but not saccharin consumption, in mice. Mice undergoing an intermittent access to 20% alcohol in drinking in the dark (DID) limited access procedure were systemically administered (intraperitoneal, i.p.) with vehicle (Veh), TSA (a), SAHA (b) or MS275 (c) 2 h before the beginning of the alcohol-drinking session. (a–c) Amount of alcohol (g/kg) consumed during the 4 h of 20% alcohol access after TSA (0.02–0.4 mg/kg), SAHA (25–100 mg/kg) or MS275 (1–20 mg/kg) treatment. Results are expressed as mean±s.e.m. alcohol consumed during a 4-h session in g/kg, *P<0.05, **P<0.01, ***P<0.001 compared with vehicle. (a) n=9, (b) n=15, (c) n=10. (d) Mice undergoing the 20% alcohol DID procedure were systemically administered with vehicle (Veh) or SAHA (50 mg/kg) daily for 7 days. Results are expressed as mean±s.e.m., **P<0.01, ***P<0.001 compared with vehicle. n=10. (e) Mice with access to a saccharin solution (0.03%, w/v) in the DID procedure were systemically administered (i.p.) with vehicle (Veh), TSA (0.4 mg/kg), SAHA (100 mg/kg) or MS275 (20 mg/kg) 2 h before the beginning of the saccharin-drinking session. Results are presented as mean±s.e.m. and expressed as percentage of the saccharin solution consumed by the vehicle group during a 4-h session. Veh n=17, TSA n=8, SAHA n=9, MS275 n=8.
Alcohol has been reported to activate HDAC2, a class I HDAC.27 We therefore sought to determine whether the selective inhibition of class I HDAC using MS275 prevents binge-like alcohol drinking, and found that systemic administration of the drug dose dependently inhibited the excessive levels of alcohol consumed by mice (one-way RM-ANOVA, F(5,43)=6.84, P<0.001) at doses higher than 2.5 mg/kg (Post hoc, Ps<0.05; Figure 2c).
As a single acute administration of all three HDAC inhibitors did not have a long-lasting effect on alcohol intake (Supplementary Figures S6a–c), we next tested the effect of a subchronic systemic administration of one of the inhibitors, SAHA, on binge-like alcohol drinking. To do so, mice received a daily i.p. administration of SAHA (50 mg/kg) or vehicle at 1000 h (that is, 2 h before the beginning of the alcohol-drinking session) every day for 7 consecutive days using a between-subject design (Supplementary Figure S2c). Daily SAHA administration began on a day of scheduled alcohol access, and alcohol consumption was recorded over the next four sessions of alcohol access (Supplementary Figure S2c). We found that daily systemic administration of SAHA significantly reduced binge-like alcohol drinking over the four sessions of access to alcohol (two-way RM-ANOVA, a main effect of treatment (F(1,18)=35.46, P<0.001), with no effect of session (F(3,54)=0.69, P=0.56), and no interaction (F(3,54)=0.69, P=0.56); Figure 2d). Interestingly, the effect of SAHA persisted through the first session of alcohol access following the end of treatment (two-way RM-ANOVA, treatment (F(1,18)=3.54, P=0.076), session (F(3,52)=2.66, P=0.058), interaction (F(3,52)=5.73, P<0.01); Figure 2d).
Next, to evaluate whether the effects of HDAC inhibitors were specific to alcohol or were due to a generalized inhibition of reward, TSA, SAHA and MS275 (0.4, 100 and 20 mg/kg, respectively) were administered systemically (i.p.) and the intake of 0.03% saccharin was measured. Mice experienced 2 weeks of abstinence from alcohol followed by 2 weeks of saccharin intake before the test (Supplementary Figure S2b, for baseline level of saccharin intake see Supplementary Table S1). A within-subject design was used to test the drugs, with mice receiving either the vehicle or an HDAC inhibitor once a week according to a Latin square experimental design. We found that the HDAC inhibitors did not alter the level of saccharin intake (one-way ANOVA, F(3,38)=0.05, P=0.98; Figure 2e). This result demonstrates that the reduction in alcohol intake induced by HDAC inhibition is selective for alcohol and not a consequence of a decrease in the total volume of fluid intake or a generalized attenuation of reward. Together, these results suggest that promoting chromatin relaxation by inhibiting the activity of HDACs, and specifically HDAC2, inhibits binge-like alcohol drinking.
Systemic administration of SAHA selectively reduces alcohol operant self-administration and seeking in rats
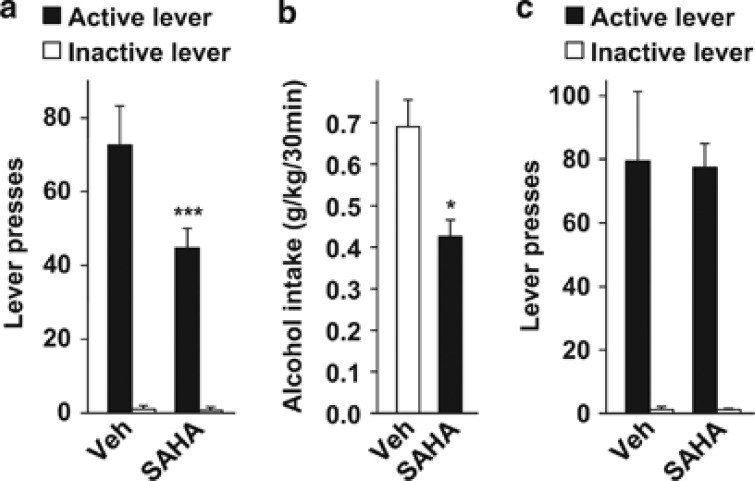
To test whether HDAC inhibition reduces the motivation to consume and/or seek alcohol, we evaluated the effect of systemic SAHA administration on alcohol operant self-administration in rats. Rats with history of intermittent access to 20% alcohol were trained to press a lever for a 20% alcohol aliquot (0.1 ml) under an FR3 reinforcement schedule (that is, three presses on the active lever resulted in one reward delivery) during 30-min sessions. Once rats reached a stable baseline responding (Supplementary Table S2), rats received a systemic administration (i.p.) of 50 mg/kg SAHA or its vehicle (Supplementary Figure S2d). A within-subject design was used to test the effect of SAHA, with rats receiving either vehicle or the HDAC inhibitor once a week according to a Latin square experimental design. We found that SAHA treatment reduced the number of presses for the active-lever without affecting inactive-lever presses (two-way RM-ANOVA, main effects of treatment (F(1,8)=10.1, P<0.05) and lever (F(1,8)=71.9, P<0.001), interaction (F(1,8)=7.8, P<0.05); Figure 3a). A significant reduction in the level of alcohol intake was also observed (paired t-test, t(8)=3.4, P<0.05; Figure 3b). Rats returned to baseline lever presses the next day (Supplementary Figure S7), further confirming that a single administration of SAHA does not have a long-term effect. These results indicate that HDAC inhibition suppresses alcohol self-administration in rats. Furthermore, analysis of the cumulative alcohol deliveries during the 30-min test session shows that rats treated with SAHA had a lower overall rate of alcohol drinking (two-way RM-ANOVA, main effects of the treatment (F(1,8)=10.2, P<0.05) and time (F(14,112)=64.8, P<0.001), interaction (F(14,112)=4.6, P<0.001); Supplementary Figure S8a). Post hoc analysis confirmed that rats treated with SAHA displayed a lower number of alcohol deliveries for all intervals later than 6–8 min compared with vehicle-treated rats (Ps<0.05). Moreover, the latency for the last 20% alcohol delivery occurred earlier in SAHA-treated rats as compared with controls (paired t-test, t(8)=3.0, P<0.05; Supplementary Figure S8b). In contrast, SAHA treatment did not affect the latency to the first alcohol delivery (Supplementary Figure S8c). The distribution of inter-response intervals was similar between both SAHA and vehicle groups, indicating that the interval of time between two consecutive active-lever presses is not affected by the treatment (two-way RM-ANOVA, F(1,8)=1.9, P=0.2; Supplementary Figure S8d). Together, these results show that SAHA induces an early termination of the drinking episode without altering the mobility of the animal.
Figure 3.
Systemic administration of SAHA selectively reduces operant self-administration of alcohol in rats. (a, b) Rats with a history of intermittent access to 20% alcohol were trained to self-administer 20% alcohol under an FR3 schedule during 30-min sessions. Two hours before the beginning of the test session, rats received an intraperitoneal (i.p.) administration of vehicle (Veh) or 50 mg/kg SAHA. (a) Number of lever presses during the 30-min test session. (b) Amount of alcohol (g/kg) consumed during the 30-min test session. Results are expressed as mean±s.e.m., *P<0.05 and ***P<0.001 compared with vehicle. n=9. (c) Rats were trained to self-administer 1.5% sucrose under an FR3 schedule during 30-min sessions. Two hours before the beginning of session, rats received an i.p. administration of vehicle (Veh) or 50 mg/kg SAHA. Data are represented as number of lever presses during the 30-min test session. Results are expressed as mean±s.e.m. n=6 per group.
We then asked whether the reduction in alcohol consumption seen with SAHA treatment was a result of a general reduction in drive for appetitive stimuli or change in the ability of the animal to move and perform various tasks. These possibilities have important potential implications for possible clinical trials. To answer this question, a between-subject design was used to test the effect of SAHA (50 mg/kg) on operant responding for 1.5% sucrose (Supplementary Table S3; Supplementary Figure S2d). Before the test session, the level of active-lever presses was 87.7±23.1 and 82.5±16.9 in the vehicle and SAHA groups (unpaired t-test, t(10)=0.18, P=0.86) respectively. We found that SAHA treatment did not alter the level of sucrose deliveries (two-way RM-ANOVA, no main effect of treatment (F(1,10)=0.8 × 10−4, P=0.98), main effect of lever (F(1,10)=40.9, P<0.001), no interaction (F(1,10)=0.02, P=0.89); Figure 3c). This result demonstrates that the effect of SAHA on operant self-administration is selective for alcohol and is not the consequence of impairments in locomotion.
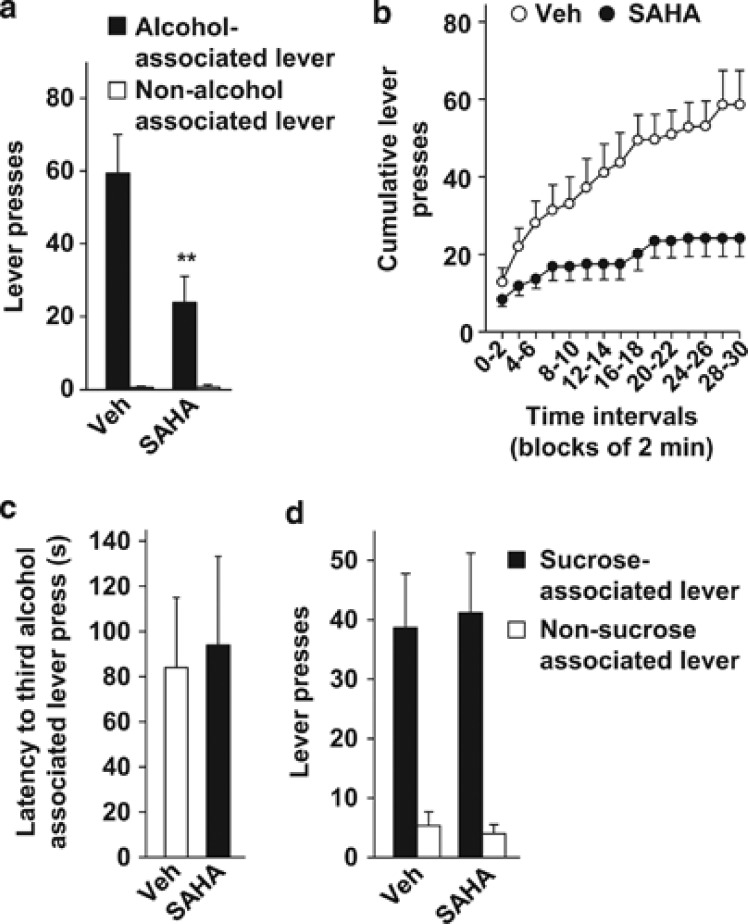
Next, to evaluate whether SAHA affects the motivation to seek alcohol, we tested its action on instrumental performance during an extinction session (that is, when active-lever presses did not result in alcohol delivery). In this condition, persistent pressing on the lever previously associated with reward delivery (for example, alcohol) during the test session is a measurement of reward seeking.24, 28 The more rats press on the reward-associated lever during the entire session of extinction, the more they display a high level of reward seeking. Alcohol-seeking behavior was tested after 2 weeks of self-administration without any manipulation (Supplementary Figure S2d). Before the first session of extinction, the levels of active-lever presses were 64.7±10 and 66.5±11.9 in the vehicle and SAHA groups, respectively (unpaired t-test, t(10)=−0.11, P=0.91). A between-subject design was used to test the effect of SAHA on alcohol seeking. We found that i.p. administration of SAHA (50 mg/kg) decreased alcohol seeking in rats as indicated by a reduction in the number of alcohol-associated lever (that is, the previously active lever) presses, without affecting the presses on the non-alcohol associated lever (that is, the previously inactive lever) (two-way RM-ANOVA, main effects of treatment (F(1,10)=6.8, P<0.05) and lever (F(1,10)=40.9, P<0.001), interaction (F(1,10)=8.1, P<0.05); Figure 4a). Furthermore, the analysis of cumulative lever presses revealed that SAHA reduced the overall rate of alcohol-associated lever presses during the 30-min test session (two-way RM-ANOVA, main effects of treatment (F(1,10)=5.0, P<0.05) and time (F(14,140)=24.3, P<0.001), interaction (F(14,140)=5.6, P<0.001); Figure 4b). Post hoc analysis confirmed that SAHA induced a decrease in the number of alcohol-associated lever presses for all intervals later than 10–12 min (Ps<0.05; Figure 4b). SAHA-treated rats stopped pressing the alcohol-associated lever earlier (743.2±231 s) compared with vehicle-treated rats (1306.6±105 s, unpaired t-test, t(10)=2.22, P=0.05). In contrast, SAHA treatment did not affect the latency to the third alcohol-associated lever press (that is, when the first alcohol delivery should occur) (Figure 4c). Together, these results suggest that SAHA-mediated HDAC inhibition represses alcohol seeking in rats both in the presence and in the absence of alcohol.
Figure 4.
Systemic administration of SAHA reduces alcohol seeking, but not sucrose seeking, in rats. (a–c) Rats underwent the same alcohol administration paradigm as described in the legend of Figure 3. Two hours before the beginning of the single extinction session, rats received an intraperitoneal (i.p.) administration of vehicle (Veh) or 50 mg/kg SAHA. (a) Number of lever presses during a 30-min extinction session. (b) Cumulative mean presses in bins of 2 min, indicative of the rate of alcohol-associated lever presses during a 30-min extinction session. (c) Latency to the third alcohol-associated lever presses. Results are expressed as mean±s.e.m., **P<0.01 compared with vehicle. (a–c) n=6 per group. (d) Rats trained to self-administer 1.5% sucrose under an FR3 schedule (30-min) received an i.p. administration of vehicle (Veh) or 50 mg/kg SAHA 2 h before the beginning of the first extinction session. Data represent the number of lever presses during the 30-min extinction session. Results are expressed as mean±s.e.m. Veh n=6 and SAHA n=7.
Finally, we determined whether SAHA affects the motivation to seek sucrose during a single extinction session. Sucrose-seeking behavior was tested in the same groups of rats after 2 weeks of regular self-administration without any drug manipulation (Supplementary Figure S2d). The basal levels of active-lever presses before the first session of extinction were 83.16±16.5 and 85.7±21.0 in the vehicle and SAHA groups, respectively (unpaired t-test, t(10)=−0.09, P=0.93). SAHA-treated rats showed the same level of sucrose seeking compared with vehicle-treated rats (two-way RM-ANOVA, no main effect of treatment (F(1,11)=1.2 × 10−4, P=0.97), main effect of lever (F(1,11)=31.4, P<0.001), no interaction (F(1,11)=0.02, P=0.88); Figure 4d). This result confirms that SAHA is selective for alcohol and demonstrates that its effect is not the consequence of a general reduction in motivation to obtain a reward or because of impairments in memory.
Excessive alcohol intake increases DNMT levels and reduces Histone H4 acetylation in the nucleus accumbens
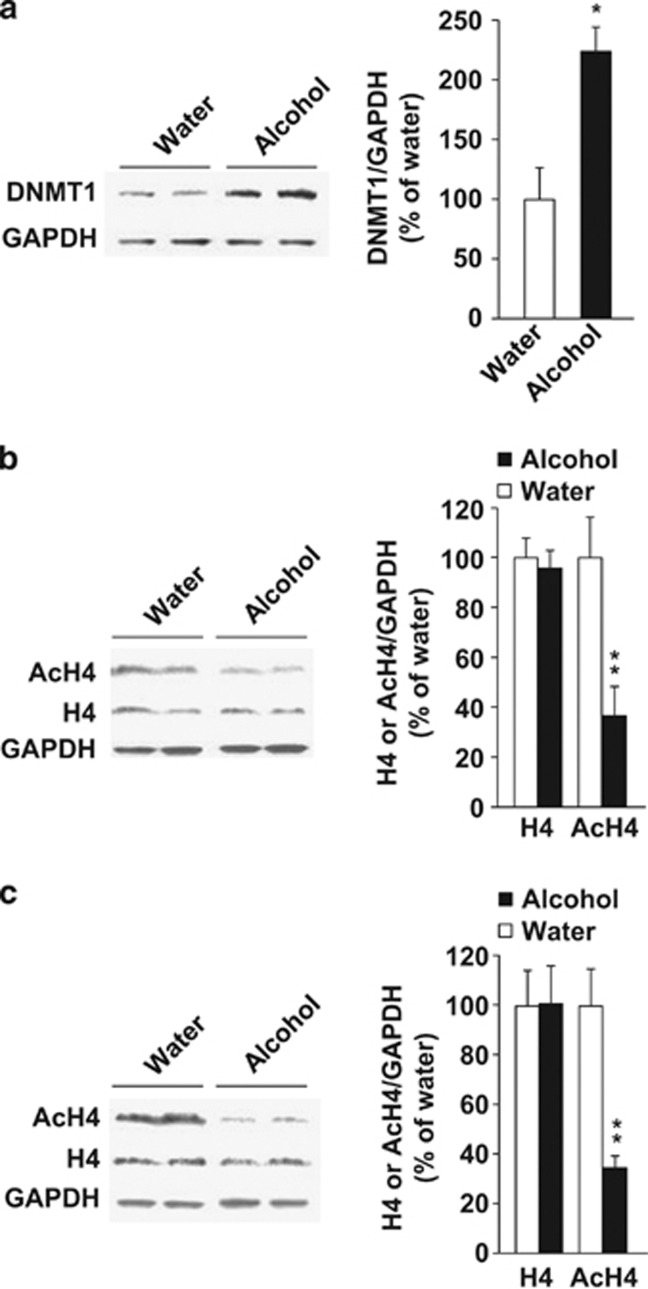
As described above, we found that inhibition of DNMT1 or HDAC activity reduced alcohol intake in rodents. We hypothesized that exposure of mice/rats to alcohol results in an increase in the level or activity of DNMT1 or HDACs, which is attenuated upon administration of the inhibitors. We therefore first tested whether excessive alcohol drinking alters DNMT1 levels in the nucleus accumbens (NAc), a key component of the brain's reward circuitry that has an important role in the expression of behavioral phenotypes associated with alcohol exposure.29 As shown in Figure 5a, we found that the levels of DNMT1 were significantly higher in the NAc of mice consuming alcohol compared with mice that consumed water only (water; unpaired t-test, t(4)=−3.74, P<0.05).
Figure 5.
Excessive alcohol intake increases DNA methyltransferase 1 (DNMT1) levels and reduces the level of Histone H4 acetylation. (a) Mice nucleus accumbens (NAc) slices were collected 4 h after the beginning of 20% alcohol access. DNMT1 level was determined by western blot analysis. Age-matched alcohol-naïve mice consuming only water were used as control. GAPDH was used as a loading control. Results are expressed as mean of image density of DNMT1/GAPDH±s.e.m., *P<0.05 compared with water. n=3. (b) Mice NAc slices were collected after a 4-h session of 20% alcohol access in the drinking in the dark (DID) procedure. Age-matched alcohol-naïve mice consuming only water were used as control. Total histone H4 (H4) and acetylated H4 (AcH4, pan-acetylated-lysine antibody) levels were determined by western blot analysis. GAPDH was used as a loading control. Results are expressed as mean of image density of H4 or AcH4/GAPDH±s.e.m., **P<0.01 compared with water. n=6–7. (c) Rat NAc slices were collected at the end of a 20% alcohol operant self-administration session of 30 min. The controls, age-matched alcohol-naïve rats, were concomitantly confined to the behavioral chamber for 30 min with access to the levers, but lever presses had no associated consequences. Results are expressed as mean H4 or AcH4/GAPDH±s.e.m., **P<0.01 compared with water. n=4–5.
The substrates of HDACs are histones, the major component of the chromatin structure.6 As the level of histone H4 acetylation is particularly associated with the effects of drugs of abuse,30, 31 we sought to determine whether binge-like drinking alters histone H4 acetylation in the NAc. As shown in Figure 5b, binge-like alcohol drinking reduced the level of acetylated histone H4 in the NAc of mice (unpaired t-test, t(11)=4.17, P<0.01) without affecting the total level of histone H4 (unpaired t-test, t(10)=0.41, P=0.69). Finally, we examined whether the reduction in the level of histone acetylation by alcohol is observed across species. To do so, we measured the level of acetylated histone in the NAc of rats that self-administered 20% alcohol and in age-matched alcohol-naïve rats. As shown in Figure 5c, rats undergoing operant self-administration procedure exhibit a reduced level of acetylated histone H4 in the NAc (unpaired t-test, t(6)=4.16, P<0.01) without affecting the total level of histone H4 (unpaired t-test, t(7)=−0.04, P=0.97). Together, these results show that excessive intake of alcohol increases the level of DNMT1 and produces hypoacetylation of histone H4 in the NAc, suggesting that HDAC activity is increased.
Discussion
Harmful excessive alcohol use has devastating consequences that can lead to physical dependence and recurring relapse episodes.1, 2, 3, 29 Unfortunately, pharmacotherapies available to treat or prevent alcohol abuse disorders are very limited.32 In the present study, we present data to suggest that agents that induce chromatin decondensation have the potential to be developed as medications to treat harmful excessive alcohol consumption, as well as alcohol seeking. Specifically, using preclinical murine models, we demonstrate that reducing DNA methylation via the systemic administration of a DNMT inhibitor reduces acute episodes of binge drinking, as well as excessive alcohol intake during a 24-h session in mice without affecting saccharine intake or alcohol metabolism. Similarly, we found that HDAC inhibition, which also produces relaxation of the chromatin structure, leads to an inhibition of binge-like alcohol, but not saccharin drinking. Both types of inhibitors reduced excessive consumption in mice experiencing successive cycles of alcohol drinking and withdrawal in a similar manner to human alcohol abusers,19 making the results particularly relevant in the context of studying alcohol abuse and harmful alcohol use. Importantly, we demonstrate that systemic administration of the FDA-approved drug, SAHA, produces a specific suppression in the motivation of rats to self-administer and seek excessive amounts of alcohol but not sucrose. We also show that SAHA produces a specific inhibition of alcohol-seeking behavior, a finding that is of particular interest because subjects with alcohol abuse disorders report high levels of craving for alcohol (seeking), which induces persistence in alcohol-drinking behavior that makes it difficult to initiate or maintain abstinence.29
Our findings are in agreement with previous reports indicating that HDAC inhibition suppresses behaviors related to drugs of abuse. For instance, systemic administration of HDAC inhibitors decreases cocaine taking- and cocaine-seeking behavior,33, 34, 35 as well as prevent nicotine-induced conditioned place preference.36 In contrast with our findings, Wolstenholme et al.37 reported that subchronic treatment with TSA increased alcohol intake 3 weeks after the end of the treatment. This discrepancy may stem from differences in the regimen of TSA administration (2 mg/kg, once a day for 5 days compared with an acute administration used herein), as well as differences in drinking procedure (2-bottle choice continuous access to 10% alcohol leading to an intake of moderate (∼4 g/kg) level of alcohol, compared with intermittent access to 20% alcohol leading to an intake of ∼7 g/kg herein). Importantly, we were able to replicate our results with three different HDAC inhibitors, in two different behavioral procedures and in two different species, providing strong preclinical evidence that chromatin relaxation reduces excessive binge-like alcohol-drinking behaviors.
Importantly, from a therapeutic perspective, we show that alteration of chromatin remodeling does not affect saccharin and sucrose intake indicating that the inhibitors do not produce a general effect on motivation to consume a rewarding solution.38 The selectivity of SAHA to reduce alcohol but not sucrose intake in rats is in contrast with the effects of naltrexone and acamprosate, both FDA-approved drugs currently used to treat alcohol craving. Naltrexone and acamprosate repress water and sucrose intake,39, 40, 41, 42 and compliance issues associated with the two drugs are likely to be due to a general reduction in motivation.43, 44
Finally, our study is of particular interest from a clinical perspective, as we tested the actions of two FDA-approved drugs, SAHA (Zolinza®, Merck & Co., Whitehouse Station, NJ, USA) and 5-AzaC (Vidaza®, Celgene Corporation, Summit, NJ, USA), which have been used as therapeutic agents for the treatment of several types of cancers45, 46, 47, 48 (see also http://www.ClinicalTrials.gov). In addition, phase II clinical trials using MS275 (Entinostat) are in progress for Hodgkin's lymphoma and, breast and lung cancer (http://www.ClinicalTrials.gov), and interestingly, several reports indicate that 5-AzaC, SAHA, and MS275 show a potential antidepressant use.20, 23, 49
Methylated DNA at gene promoters represses transcription by condensing the chromatin,8, 9 and reducing DNA methylation by inhibiting DNMT activity has been shown to increase gene expression.18, 49, 50 Conversely, histone acetylation leads to chromatin decondensation, which, in turn, enhances gene expression making promoters accessible for transcriptional machinery.6 Inhibition of HDAC activity spares the acetylated form of the histones, thus contributing to enhanced gene expression.21, 22, 23 Our results suggest that the level of gene expression controlled by chromatin structure accounts for the amount of alcohol intake and that a reduction in excessive alcohol intake requires an increase in specific gene products. It is also possible that DNMT and HDAC inhibitors produce an increase in genes whose expression is reduced by alcohol intake. In line with this possibility, we found that excessive alcohol drinking leads to increased levels of DNMT1 and hypoacetylation of histone H4 in the NAc of rodents. Theses alterations may lead to the condensation of chromatin and a repression of gene expression. Thus, it is plausible that DNMT and HDAC inhibitors reduce alcohol drinking and seeking by adjusting the activity of DNMT1 and the level of histone H4 acetylation in the NAc, and possibly other brain regions. This possibility is in line with recent publications analyzing the effect of alcohol intake on the transcriptome in different brain regions that suggest that while alcohol intake increases the expression of some genes, it also reduces the expression of others.51, 52, 53 Furthermore, rodents that were bred based on their high alcohol consumption exhibit not only upregulation but also downregulation of gene expression in the brain compare with their respective low drinking lines of mice and rats.54, 55, 56, 57 Another intriguing possibility is that inhibition of DNMT1 and HDAC activity leads to increase in endogenous factors that act to repress the level of alcohol drinking behaviors. For example, the expression of the growth factors, brain-derived neurotrophic factor (BDNF) and the glial cell line-derived neurotrophic factor (GDNF), are regulated by epigenetic modifications including DNA methylation and histone acetylation,49, 58, 59 and both BDNF and GDNF are endogenous inhibitors of alcohol intake that prevent the escalation from moderate to excessive consumption.60, 61, 62, 63, 64, 65, 66, 67, 68, 69 It is therefore plausible that HDAC and DNMT inhibition induces increases in BDNF and GDNF expression, which may, in turn, strengthen their action to inhibit excessive alcohol drinking. Finally, it is also possible that HDAC and DNMT inhibitors produce an increase in the expression of genes, which, in turn, acts to reduce the expression of alcohol-responsive genes. Further studies will be needed to identify specific genes and pathways controlled by chromatin remodeling that allow the robust reduction in excessive alcohol intake and alcohol seeking.
In contrast to our finding that the level of DNMT1 is increased in the NAc of mice undergoing cycles of excessive alcohol intake and withdrawal, Ponomarev et al.13 conducted a post-mortem study and found that alcoholic subjects show a DNA hypomethylation associated with a reduced expression of DNMT1 in the amygdala and the superior frontal cortex, as compared with control subjects. The difference between our studies and Ponomarev et al. could be due to differences in brain regions. In addition, our study does not differentiate whether the molecular changes are due to the repeated cycles of excessive alcohol intake and withdrawal which could also be a potential reason for the differences between our results and the human studies. Further studies are needed to determine whether alcohol exposure leads to long-term epigenetic changes after the cessation of alcohol exposure.
Another possibility for the differences between the present study and Ponomarev et al.'s results is the fact that mice and rats used in our study were not physically dependent on alcohol. Our paradigms model ‘problem drinkers', people who engage in harmful excessive alcohol use but do not yet meet criteria for alcohol use disorders.70 However, problem drinkers are at great risk for developing severe alcohol use disorders. Intervening in this vast population may be a way of preventing the development and progression to full-fledged alcohol use disorders. It is also very plausible that the molecular changes in these two disease states are very different. It is also possible that treatment with chromatin remodeling inhibitors can prevent the escalation to physical dependence.
In summary, it is plausible that chromatin structure modifiers currently being used in the clinic or in clinical trials represent a new therapeutic strategy to treat problem drinkers who engage in harmful excessive alcohol use as well as people with full-fledged alcohol use disorders. The lack of a specific receptor target for alcohol in the brain positions a potential treatment strategy that targets chromatin structure as particularly promising.
Acknowledgments
We thank Wenheng Zhu and Mimi Zou for technical assistance and Somayeh Ahmadiantehrani for reviewing the manuscript. This work was supported by NIH-NIAAA R01 AA016848 (DR), NIH-NIAAA P50 AA017072 (DR) and by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco (DR).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, et al. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) Global Status Report on Alcohol and Health. World Health Organization: Geneva, Switzerland; 2011. [Google Scholar]
- Nutt DJ, King LA, Phillips LD. Drug harms in the UK: a multicriteria decision analysis. Lancet. 2010;376:1558–1565. doi: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Yuan W, Li MD. Genes and pathways co-associated with the exposure to multiple drugs of abuse, including alcohol, amphetamine/methamphetamine, cocaine, marijuana, morphine, and/or nicotine: a review of proteomics analyses. Mol Neurobiol. 2011;44:269–286. doi: 10.1007/s12035-011-8202-4. [DOI] [PubMed] [Google Scholar]
- Ron D, Messing RO. Signaling pathways mediating alcohol effects. Curr Top Behav Neurosci. 2011;13:87–126. doi: 10.1007/7854_2011_161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A. Dynamic epigenetic regulation in neurons: enzymes, stimuli and signaling pathways. Nat Neurosci. 2010;13:1330–1337. doi: 10.1038/nn.2671. [DOI] [PubMed] [Google Scholar]
- Broide RS, Redwine JM, Aftahi N, Young W, Bloom FE, Winrow CJ. Distribution of histone deacetylases 1–11 in the rat brain. J Mol Neurosci. 2007;31:47–58. doi: 10.1007/BF02686117. [DOI] [PubMed] [Google Scholar]
- Chen ZX, Riggs AD. DNA methylation and demethylation in mammals. J Biol Chem. 2011;286:18347–18353. doi: 10.1074/jbc.R110.205286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield RD. Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J Neurosci. 2012;32:1884–1897. doi: 10.1523/JNEUROSCI.3136-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleich S, Lenz B, Ziegenbein M, Beutler S, Frieling H, Kornhuber J, et al. Epigenetic DNA hypermethylation of the HERP gene promoter induces down-regulation of its mRNA expression in patients with alcohol dependence. Alcohol Clin Exp Res. 2006;30:587–591. doi: 10.1111/j.1530-0277.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- Bonsch D, Lenz B, Fiszer R, Frieling H, Kornhuber J, Bleich S. Lowered DNA methyltransferase (DNMT-3b) mRNA expression is associated with genomic DNA hypermethylation in patients with chronic alcoholism. J Neural Transm. 2006;113:1299–1304. doi: 10.1007/s00702-005-0413-2. [DOI] [PubMed] [Google Scholar]
- Bonsch D, Lenz B, Kornhuber J, Bleich S. DNA hypermethylation of the alpha synuclein promoter in patients with alcoholism. Neuroreport. 2005;16:167–170. doi: 10.1097/00001756-200502080-00020. [DOI] [PubMed] [Google Scholar]
- Bonsch D, Lenz B, Reulbach U, Kornhuber J, Bleich S. Homocysteine associated genomic DNA hypermethylation in patients with chronic alcoholism. J Neural Transm. 2004;111:1611–1616. doi: 10.1007/s00702-004-0232-x. [DOI] [PubMed] [Google Scholar]
- Szyf M. Epigenetics, DNA methylation, and chromatin modifying drugs. Annu Rev Pharmacol Toxicol. 2009;49:243–263. doi: 10.1146/annurev-pharmtox-061008-103102. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Sales AJ, Biojone C, Terceti MS, Guimaraes FS, Gomes MV, Joca SR. Antidepressant-like effect induced by systemic and intra-hippocampal administration of DNA methylation inhibitors. Br J Pharmacol. 2011;164:1711–1721. doi: 10.1111/j.1476-5381.2011.01489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess-Stumpp H, Bracker TU, Henderson D, Politz O. MS-275, a potent orally available inhibitor of histone deacetylases--the development of an anticancer agent. Int J Biochem Cell Biol. 2007;39:1388–1405. doi: 10.1016/j.biocel.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Lopez-Atalaya JP, Barco A. Selective boosting of transcriptional and behavioral responses to drugs of abuse by histone deacetylase inhibition. Neuropsychopharmacology. 2009;34:2642–2654. doi: 10.1038/npp.2009.125. [DOI] [PubMed] [Google Scholar]
- Covington HE, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci USA. 2010;107:20093–20098. doi: 10.1073/pnas.1005554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprow GM, Thiele TE. The neurobiology of binge-like ethanol drinking: Evidence from rodent models. Physiol Behav. 2012;3:325–331. doi: 10.1016/j.physbeh.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA . Council Appoves Definition of Binge Drinking. NIAAA News Letter. Vol. 3. NIAAA: Rockville, USA; 2004. [Google Scholar]
- Agudelo M, Gandhi N, Saiyed Z, Pichili V, Thangavel S, Khatavkar P, et al. Effects of alcohol on histone deacetylase 2 (HDAC2) and the neuroprotective role of trichostatin A (TSA) Alcohol Clin Exp Res. 2011;35:1550–1556. doi: 10.1111/j.1530-0277.2011.01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Bie B, Wang Y, Cai YQ, Zhang Z, Hou YY, Pan ZZ. Upregulation of nerve growth factor in central amygdala increases sensitivity to opioid reward. Neuropsychopharmacology. 2012;37:2780–2788. doi: 10.1038/npp.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Romieu P, Deschatrettes E, Host L, Gobaille S, Sandner G, Zwiller J. The inhibition of histone deacetylases reduces the reinstatement of cocaine-seeking behavior in rats. Curr Neuropharmacol. 2011;9:21–25. doi: 10.2174/157015911795017317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu P, Host L, Gobaille S, Sandner G, Aunis D, Zwiller J. Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats. J Neurosci. 2008;28:9342–9348. doi: 10.1523/JNEUROSCI.0379-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Host L, Dietrich JB, Carouge D, Aunis D, Zwiller J. Cocaine self-administration alters the expression of chromatin-remodelling proteins; modulation by histone deacetylase inhibition. J Psychopharmacol. 2011;25:222–229. doi: 10.1177/0269881109348173. [DOI] [PubMed] [Google Scholar]
- Pastor V, Host L, Zwiller J, Bernabeu R. Histone deacetylase inhibition decreases preference without affecting aversion for nicotine. J Neurochem. 2011;116:636–645. doi: 10.1111/j.1471-4159.2010.07149.x. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Warner JA, Capparuccini MI, Archer KJ, Shelton KL, Miles MF. Genomic analysis of individual differences in ethanol drinking: evidence for non-genetic factors in C57BL/6 mice. PLoS ONE. 2011;6:e21100. doi: 10.1371/journal.pone.0021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci USA. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher T, Mittleman G. Schedule-induced alcohol drinking: non-selective effects of acamprosate and naltrexone. Addict Biol. 2006;11:55–63. doi: 10.1111/j.1369-1600.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Delory MJ. Acamprosate and naltrexone treatment effects on ethanol and sucrose seeking and intake in ethanol-dependent and nondependent rats. Psychopharmacology (Berl) 2009;204:335–348. doi: 10.1007/s00213-009-1465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beczkowska IW, Bowen WD, Bodnar RJ. Central opioid receptor subtype antagonists differentially alter sucrose and deprivation-induced water intake in rats. Brain Res. 1992;589:291–301. doi: 10.1016/0006-8993(92)91289-q. [DOI] [PubMed] [Google Scholar]
- Bouza C, Angeles M, Munoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S, Grant S. Endogenous modulators and pharmacological inhibitors of histone deacetylases in cancer therapy. Oncogene. 2012;31:537–551. doi: 10.1038/onc.2011.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurn KT, Thomas S, Moore A, Munster PN. Rational therapeutic combinations with histone deacetylase inhibitors for the treatment of cancer. Future Oncol. 2011;7:263–283. doi: 10.2217/fon.11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless MW, Norris S, O'Byrne KJ, Gray SG. Targeting histone deacetylases for the treatment of disease. J Cell Mol Med. 2009;13:826–852. doi: 10.1111/j.1582-4934.2008.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintas-Cardama A, Santos FP, Garcia-Manero G. Therapy with azanucleosides for myelodysplastic syndromes. Nat Rev Clin Oncol. 2010;7:433–444. doi: 10.1038/nrclinonc.2010.87. [DOI] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobayashi A, Otsuki K, Yamagata H, Hobara T, et al. Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 2011;69:359–372. doi: 10.1016/j.neuron.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan MK, Rhodes JS, Crabbe JC, Mayfield RD, Adron Harris R, Ponomarev I. Molecular profiles of drinking alcohol to intoxication in C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:659–670. doi: 10.1111/j.1530-0277.2010.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Kimpel MW, Schultz JA, McClintick JN, Edenberg HJ, Bell RL. Changes in gene expression in regions of the extended amygdala of alcohol-preferring rats after binge-like alcohol drinking. Alcohol. 2010;44:171–183. doi: 10.1016/j.alcohol.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Kimpel MW, Edenberg HJ, Bell RL, Strother WN, McClintick JN, et al. Differential gene expression in the nucleus accumbens with ethanol self-administration in inbred alcohol-preferring rats. Pharmacol Biochem Behav. 2008;89:481–498. doi: 10.1016/j.pbb.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, et al. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci USA. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Strother WN, McClintick JN, Tian H, Stephens M, Jerome RE, et al. Gene expression in the hippocampus of inbred alcohol-preferring and -nonpreferring rats. Genes Brain Behav. 2005;4:20–30. doi: 10.1111/j.1601-183X.2004.00091.x. [DOI] [PubMed] [Google Scholar]
- Kimpel MW, Strother WN, McClintick JN, Carr LG, Liang T, Edenberg HJ, et al. Functional gene expression differences between inbred alcohol-preferring and -non-preferring rats in five brain regions. Alcohol. 2007;41:95–132. doi: 10.1016/j.alcohol.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Kimpel MW, McClintick JN, Ding ZM, Hyytia P, Colombo G, et al. Gene expression in the ventral tegmental area of 5 pairs of rat lines selectively bred for high or low ethanol consumption. Pharmacol Biochem Behav. 2012;102:275–285. doi: 10.1016/j.pbb.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD. Epigenetic gene regulation in the adult mammalian brain: multiple roles in memory formation. Neurobiol Learn Mem. 2011;96:68–78. doi: 10.1016/j.nlm.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DY, Neasta J, Ron D. Epigenetic regulation of BDNF expression via the scaffolding protein RACK1. J Biol Chem. 2010;285:19043–19050. doi: 10.1074/jbc.M110.100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, Carnicella S, Kharazia V, Janak PH, Ron D. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J Neurosci. 2009;29:13494–13502. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, McGough NN, Logrip ML, Phamluong K, Janak PH, et al. The dopamine D3 receptor is part of a homeostatic pathway regulating ethanol consumption. J Neurosci. 2006;26:1457–1464. doi: 10.1523/JNEUROSCI.3786-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J. 2008;22:2393–2404. doi: 10.1096/fj.07-099135. [DOI] [PubMed] [Google Scholar]
- McGough NN, He DY, Logrip ML, Jeanblanc J, Phamluong K, Luong K, et al. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci. 2004;24:10542–10552. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Carnicella S, Yowell QV, Ron D. Glial cell line-derived neurotrophic factor reverses alcohol-induced allostasis of the mesolimbic dopaminergic system: implications for alcohol reward and seeking. J Neurosci. 2011;31:9885–9894. doi: 10.1523/JNEUROSCI.1750-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ahmadiantehrani S, Janak PH, Ron D. GDNF is an endogenous negative regulator of ethanol-mediated reward and of ethanol consumption after a period of abstinence. Alcohol Clin Exp Res. 2009;33:1012–1024. doi: 10.1111/j.1530-0277.2009.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Escalating ethanol intake is associated with altered corticostriatal BDNF expression. J Neurochem. 2009;109:1459–1468. doi: 10.1111/j.1471-4159.2009.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci USA. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash A, Zhang H, Pandey SC. Innate differences in the expression of brain-derived neurotrophic factor in the regions within the extended amygdala between alcohol preferring and nonpreferring rats. Alcohol Clin Exp Res. 2008;32:909–920. doi: 10.1111/j.1530-0277.2008.00650.x. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Misra K. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci. 2006;26:8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Goldman D. Problem drinking and alcoholism: diagnosis and treatment. Am Fam Physician. 2002;65:441–448. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.