Abstract
Osteopetrosis is a rare genetic disorder that causes generalized sclerosis of the bone due to defect in bone resorption and remodeling. Albergs-Schonberg disease or autosomal dominant osteopetrosis type II is a rare form of osteopetrosis. Osteomyelitis is a well-documented complication of osteopetrosis. Any associated dental abnormality may be attributed to the pathological changes in bone remodeling. This case report discusses a case of osteopetrosis with osteomyelitis as a complication in a 8-year-old boy.
Keywords: Osteomyelitis, osteopetrosis, osteosclerosis
INTRODUCTION
Osteopetrosis (Marble bone disease, Osteosclerosis, and Fragalis Generalisata) is a very rare form of inherited bone disorder due to defective osteoclastic function where bones are rendered fragile and prone to infections in spite of being radiodense. Globally the estimated prevalence of osteopetrosis is 1 in 100000 to 500000.[1] Autosomal dominant osteopetrosis type II or Albergs-Schonberg disease is the most common form of osteopetrosis. Classically, it has been termed as milder or benign osteopetrosis. Nontraumatic long bone fractures, hip osteoarthritis, and mandibular osteomyelitis are some of the distinguished complications of autosomal dominant osteopetrosis type II. Most studies have reported that osteopetrosis is associated with hepatosplenomegaly, anemia, increased susceptibility to infections, respiratory tract infections, cardiac disorders, multiple fractures, etc. Dental abnormalities may be attributed to the pathological changes in osteopetrosis. Patients with this disease seem to be especially susceptible to osteomyelitis of the mandible.[2] In a review of 141 cases of jaw osteomyelitis in Nigeria, Adekeye and Cornath found odontogenic infections to be the cause of 38% of mandibular and 25% of maxillary involvement.[3] In these patients, especially in areas of exposed mandibular bone, 0.2% chlorhexidine mouthwash is recommended to improve oral hygiene, and topical fluoride applications help to decrease the susceptibility to dental caries.[1]
CASE REPORT
A young 8-year-old boy had reported to the Department of Oral and Maxillofacial Surgery with the chief complaint of swelling on the right side of mandible since 4 months [Figure 1].
Figure 1.
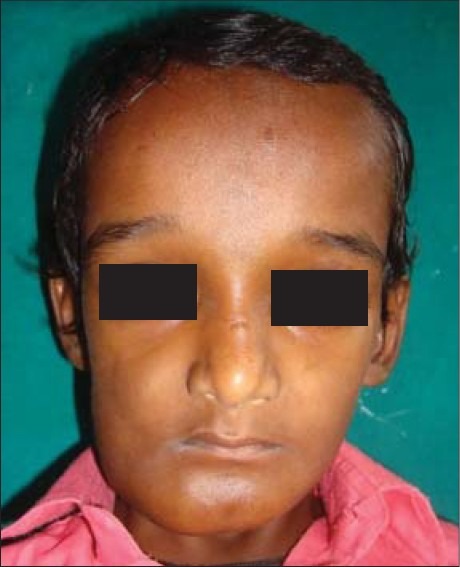
Clinical photograph showing swelling in the mandibular right posterior region
About 5 months ago, the patient had visited a local dental clinic due to pain in the lower right first molar. The tooth, 46, was diagnosed to be carious and subsequently extracted. The healing was not satisfactory with the presence of denuded bone in that area, which was subsequently curetted out later. An extraoral swelling extraoral swelling appeared in the same region, but no definitive diagnosis was made. Gradually, the patient had developed an extraoral draining sinus and was referred to our institute.
On general examination, there was frontal bossing and bowed legs [Figures 2–4]. Mild hepatoslenomegaly was detected with no relevant medical and family history. Necrotic bone was evident in 45, 46 region during intraoral examination.
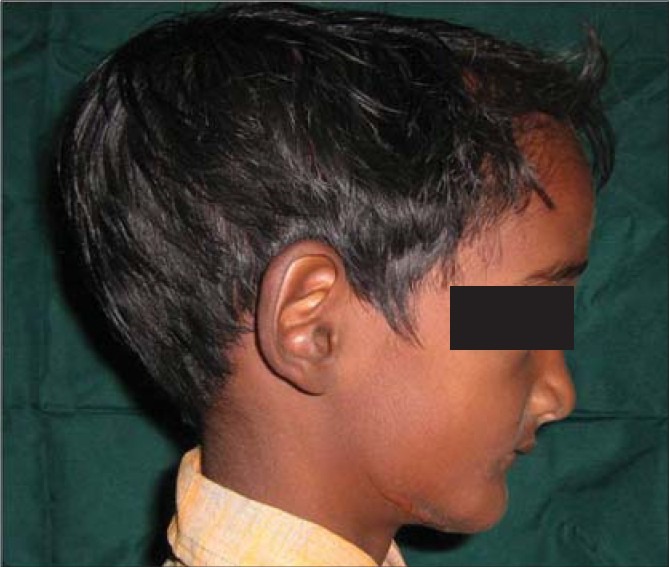
Figure 2.
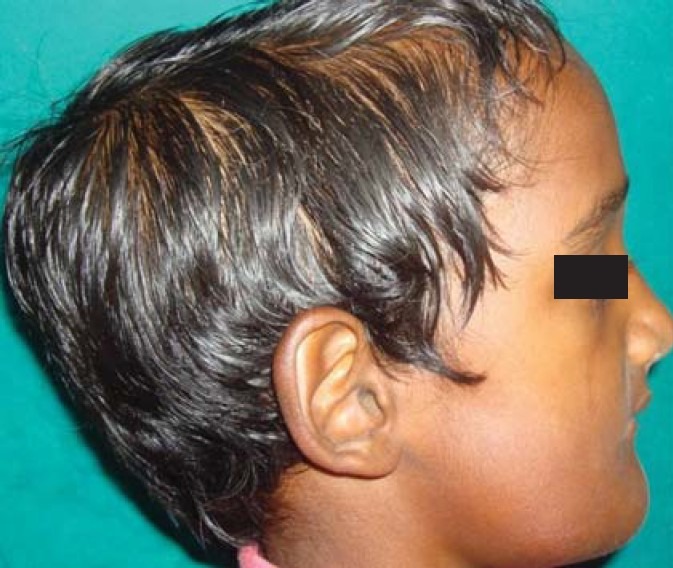
Lateral profile view showing frontal bossing
Figure 4.
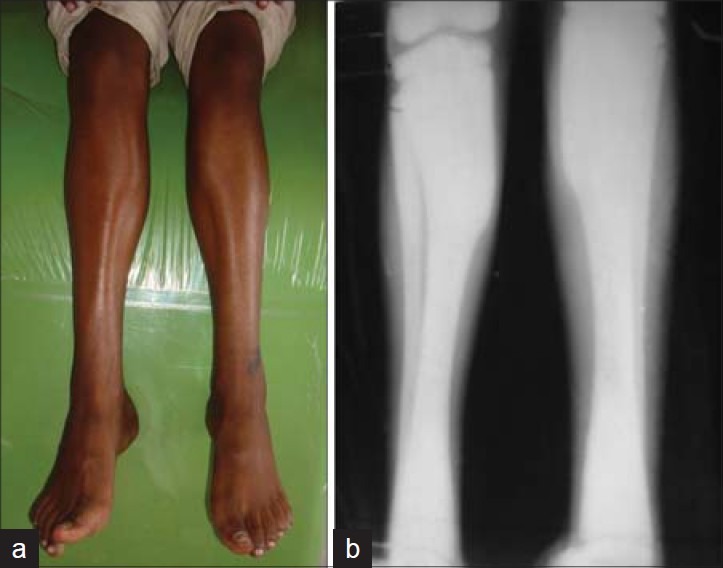
a) Clinical photograph showing bowing of legs. b) Radiographic view showing increased radiodensity with bowing of legs
OPG revealed the presence of a sequestrum on the right mandibular molar region with erosions of the mandibular cortical margins. Generalized alveolar bone loss and multiple carious teeth were also seen [Figure 5]. Blood picture showed Hb 9.2 gm%, TLC 8000 cu/mm (neutrophils 6.8%, lymphocytes 30%), and alkaline phosphatase 8 K.A.U/100 ml. Radiographic examination of the skull and tibia/fibula revealed a generalized increase in bone density [Figures 3 and 4].
Figure 5.
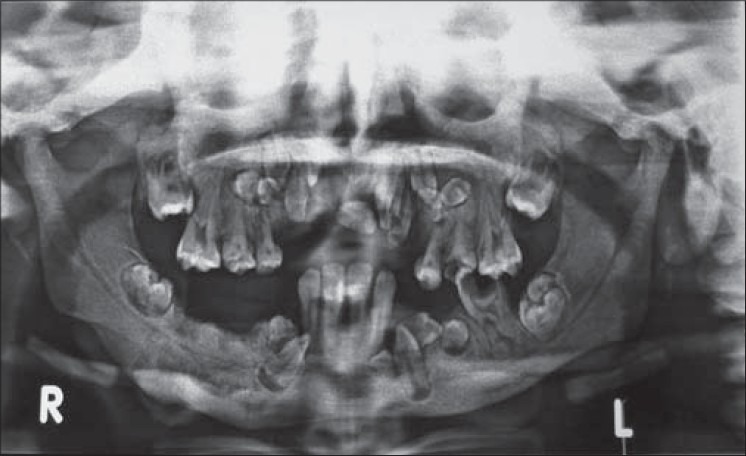
OPG showing sequestrum formation at lower border of mandible
Figure 3.
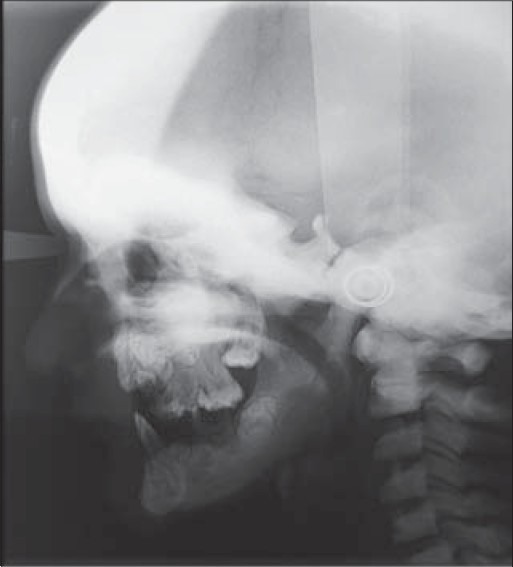
Lateral skull view showing increased radiodensity with frontal bossing
The patient was diagnosed as a case of osteopetrosis with mandibular osteomyelitis on the basis of clinical and radiographic findings.
As the facility of hyperbaric oxygen therapy was not available in our setup, segmental mandibulectomy and reconstruction with reconstruction plate were performed under general anesthesia [Figures 6–10]. Patient tolerated the procedure well and wound healing was uneventful [Figures 11 and 12].
Figure 6.
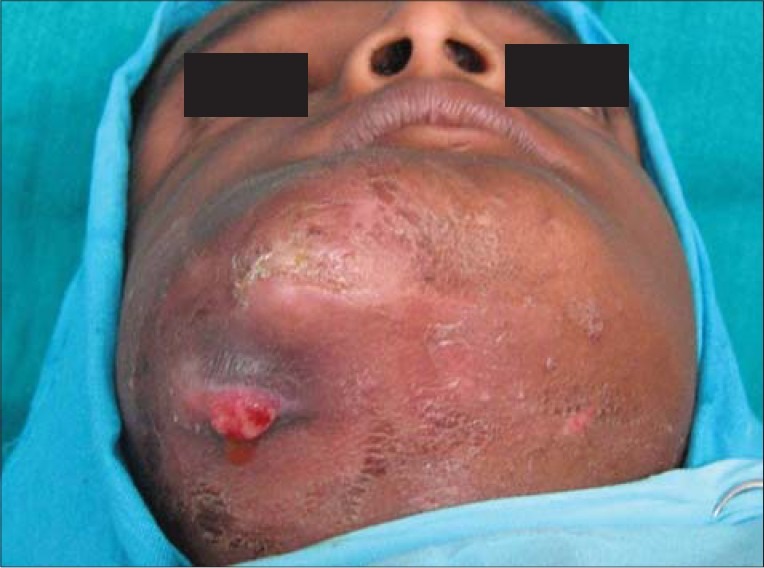
Clinical photograph showing draining sinus extraorally in mandibular right posterior region
Figure 10.
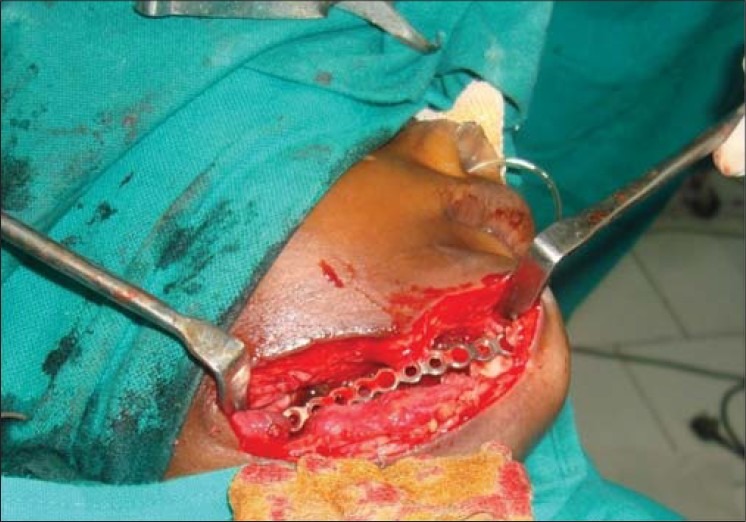
Clinical photograph showing reconstruction of mandible with reconstruction plate
Figure 11.
Clinical photograph showing healing extraorally after 2 weeks
Figure 12.
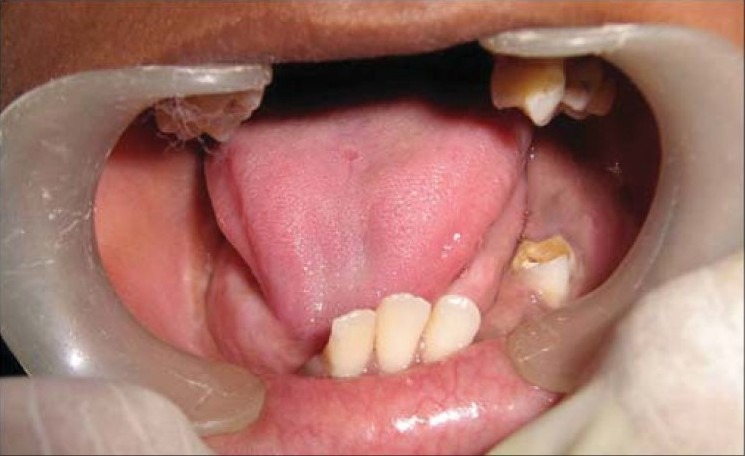
Clinical photograph showing healing intraorally after 2 weeks
Figure 7.
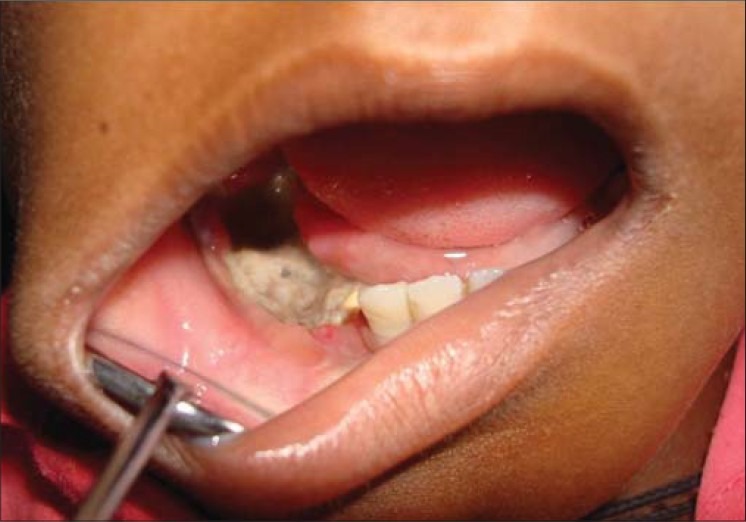
Clinical photograph showing large area of denuded bone in 45, 46 region
Figure 8.
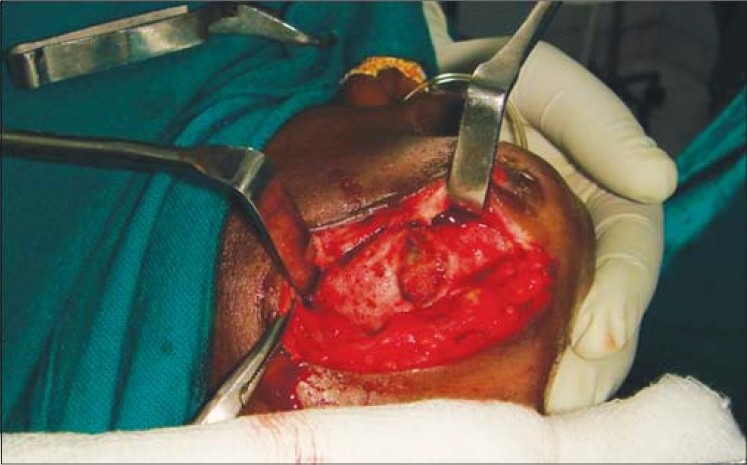
Clinical photograph showing exposure of lower border of mandible through submandibular approach
Figure 9.
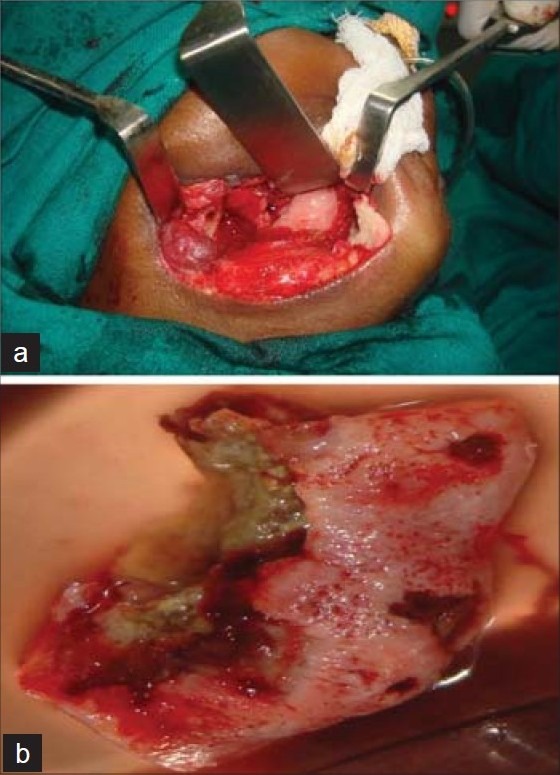
a) Clinical photograph showing segmental resection of mandible. b) Photograph showing resected mandible
DISCUSSION
Osteopetrosis is a rare metabolic bone disease characterized by a generalized increase in skeletal mass. This inherited disorder results from congenital defects in the development or function of osteoclasts.[4]
Osteopetrosis is classified as:
Juvenile malignant type: which is fatal within first few years of life (in the absence of effective therapy).
Intermediate type: appears during the first decade of life but does not follow the malignant course.
Autosomal dominant type I and type II with full life expectancy but many orthopedic problems.[5] The disease represents a spectrum of clinical variants because of the heterogenecity of genetic defects resulting in osteoclastic dysfunction.[6]
Pathological changes in osteopetrosis also include dental abnormalities. These patients are especially susceptible to caries. Constriction of canals housing neurovascular bundles that supply teeth and jaws, along with obliteration of the marrow cavities and the dental pulp chambers, is the most likely contributing factor to bone necrosis and dental caries. Other dental changes may include delayed eruption and early loss of teeth, enamel hypoplasia, malformed roots and crowns, and thickening of the lamina dura.[7] It has been reported that osteomyelitis, due to dental caries (10%); is a well-recognized complication in osteopetrosis. It occurs due to the obliteration and fibrosis of the marrow resulting in reduced blood circulation to bone. It is a potentially severe infection that runs a protracted course, due to the accompanying severe anemia and neutropenia.[8]
Radiograph shows a uniform increase in bone density without corticomedullary demarcation. The long bones have a dense chalky appearance. The most common complication of the osteopetrosis is pathologic fractures; those with congenital presentation are likely to have most fractures. Femoral shaft fractures either with transverse or short oblique pattern are most common. Other common locations are inferior neck of femur and posterior tibia. Upper extremity fractures are also reported. Bowing of the long bones and coxa vara may be present due to multiple fractures.[9] Fracture healing seems to occur at a normal rate, but the onset of callus formation after injury is variable.[10] There is also an increase in density of bone at the base of skull especially especially in relation to anterior cranial fossa. Under pneumatization of the mastoid, sphenoid, and frontal sinuses may occur. Neural foramina may be encroached upon. The vertebral column has a “sandwich” or “rugger jersey” appearance with dense sclerotic bone at each end plate of the vertebral body. Spondylosis of the lumbar spine has been reported.[9] A “bone within a bone” or endobone phenomenon may be seen in small bones of the hands but with increased density around the periphery.
Management of the patients with osteopetrosis requires a comprehensive approach to characteristic clinical problems including hematological and metabolic abnormalities, fractures, skeletal deformity, back pain, bone pain, osteomyelitis, and neurological squealae. Medical management of osteopetrosis is based on efforts to the host mesenchymal cells to diffrentiate into normal osteoclasts. Stimulation of host osteoclast has been attempted with calcium restriction, calcitriol, steroids, parathyroid hormone, and interferon. Hyperbaric oxygen has been shown to be beneficial in the treatment of mandibular osteomyelitis.[11] It has a bactericidal and bacteriostatic effect in vitro and in vivo, but the bone infection is not the only factor. Reduced tissue tissue resistance due to avascularity of the marrow spaces, and the partial obliteration of the mandibular canal appeared to be the basic problem. The beneficial effect is probably resulted from the improved vascular supply and increased oxygen perfusion to the ischemic areas of infection.[12] Bone marrow transplantation is the only permanent cure for osteopetrosis, but an appropriately matched donor is usually available for only about 50% of those affected, and engraftment is successful in about 45% of transplants.[13]
As osteopetrosis likely represents a spectrum of underlying etiologies resulting in osteoclast dysfunction, effective therapies need to be individualized. These patients should receive increased attention and prophylactic dental treatment to maintain their oral health status. Frequent oral hygiene procedures with emphasis on chlorhexidine mouthwash should be advocated. Preventive measures must be continuously and rigorously maintained in patients of osteopetrosis for the prevention against dental caries. Fluorides would be the mainstay for long-term maintenance of oral health.[1] Presence of bony sequestrum and cysts is a common finding of this entity. Soft tissue infections, periodic exacerbations and remission, and pus through numerous sinus tracts often make surgical intervention necessary.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Malik P, Punia H, Aggrawal A. Osteopetrosis: A case report. Indian J Dent Sci. 2010;2:24–6. [Google Scholar]
- 2.Ahmad I, Abbas SZ, Haque F, Rashid M, Ahmad SA. Osteomylitis of mandible: A rare presentation of osteopetrosis. Indian J Radiol Imaging. 2006;2:253–6. [Google Scholar]
- 3.Adekeye EO, Cornath J. Osteomyelitis of the jaws: A review of 141 cases. Br J Oral Maxillofac Surg. 1985;23:24–35. doi: 10.1016/0266-4356(85)90075-0. [DOI] [PubMed] [Google Scholar]
- 4.Felix R, Hofstetler N, Cecchini MG. Recent developments in the pathophysiology of osteopetrosis. Eur J Endocrinol. 1996;134:143–56. doi: 10.1530/eje.0.1340143. [DOI] [PubMed] [Google Scholar]
- 5.Filho AM, Domingos AC, Freitas DQ, Whaites EJ. Osteopetrosis: A review and report of 2 cases. Oral Dis. 2005;11:46–9. doi: 10.1111/j.1601-0825.2004.01046.x. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro F. Osteopetrosis: Current clinical considerations. Clin Orthop. 1993;294:87–97. [Google Scholar]
- 7.Dick HM, Simpson J. Dental changes in osteopetrosis. Oral Surg. 1972;34:408. doi: 10.1016/0030-4220(72)90316-7. [DOI] [PubMed] [Google Scholar]
- 8.Lawoyin DO, Daramola JO, Ajabge HA, Nyako EA, Lawoyin JO. Osyeomyelitis of the mandible associated with osteopetrosis: Report of a case. Br J Oral Maxillofac Surg. 1988;26:330–5. doi: 10.1016/0266-4356(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 9.Benichow OD, Laredo JD, Deverenejoul MC. Type II autosomal osteopetrosis. Bone. 2000;26:87–93. doi: 10.1016/s8756-3282(99)00244-6. [DOI] [PubMed] [Google Scholar]
- 10.Stoker DJ. Osteopetrosis. Semin Musculoskelet Radiol. 2002;6:299–305. doi: 10.1055/s-2002-36728. [DOI] [PubMed] [Google Scholar]
- 11.Mainous EG, Boyne PJ, Hart GB. Hyperbaric oxygen treatment of mandibular osteomyelitis: Report of three cases. J Am Dent Assoc. 1973;87:1426. doi: 10.14219/jada.archive.1973.0625. [DOI] [PubMed] [Google Scholar]
- 12.Mainous EG, Hart GB, Soffa DJ, Graham GA. Hyperbaric oxygen treatment of mandibular osteomyelitis in osteopetrosis. J Oral Surg. 1975;33:288–91. [PubMed] [Google Scholar]
- 13.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and Maxillofacial Pathology. 2nd ed. China: Saunders; 2002. Bone pathology; pp. 533–87. [Google Scholar]


