Abstract
Introduction:
Osteogenesis distraction (OD) is a mainstream technique in maxillofacial surgical reconstruction with varied applications. OD technique employs a distractor with the aim to get new bone in the site of interest. Osseous maturation time is necessary before the device can be removed and few patients’ complaint of related discomfort, especially when these devices are external, and induces superficial infections, paresthesia, hypertrophic scars and social relationship difficulties. The use of Low Level Laser Therapy (LLLT) has been proved beneficial to soft tissue and osseous repairs.
Materials and Method:
12 rabbits were randomly divided in to two groups. In all animals, distractor was placed and one group was exposed to LLLT while the other group served as control. After consolidation, animals were sacrificed, the new bone formed were subjected to investigations including histomorphometric, physical analysis and tomographical analysis. Statistical analyses were performed using SPSS software.
Result:
Newly formed bone was significantly different between the groups. The physical properties of the neobone were comparatively better when the animals were exposed to LLLT with varying statistical significance.
Conclusion:
The results obtained with smaller sample size in this study need to be interpreted with care. The results of this preliminary pilot study encourage the use of LLLT during healing period. However the histological, tomographical and physical findings need to be ascertained using a larger sample size to study the bio-stimulatory effects with laser therapy from basics to clinical relevance on wound and bone healing.
Keywords: Bone regeneration, low level laser therapy, osteogenesis distraction
INTRODUCTION
Osteogenesis distraction (OD) is presented as an advanced method of oral and maxillofacial surgery reconstruction and can be applied in congenital deformities, trauma and after oncology surgery. The OD technique uses a distractor device with the objective of getting new growing bone in a deformed site.[1,2] Correct bone maturation time is necessary to remove the device and some patients experience discomfort when these devices are external, and induce superficial infections, paresthesia, hypertrophic scars and social relationship difficulties.[2] The researches are focusing on solutions to accelerate the bone maturation process and improve the physical properties of the newly formed bone.[3,4] The use of low level laser therapy (LLLT) has demonstrated benefits in soft tissues and bone repair. The OD involves metabolic activities that are passive of biomodulation by LLLT use and can reduce the time on whole treatment.[5,6]
Bone tissue evaluation studies may use different tests for accuracy and complexity of execution to determine quantitative and qualitative differences in bone tissues among the studied groups. The computed tomography is an important tool to evaluate the bone geometry, density details, quality and quantity of neoformed bone with precision and with minimal error in direct relation with histological findings.[7,8]
Histological analysis with hematoxylin-eosin (HE) is a widely used test to evaluate bone alterations and tissue repair.[9]
Fluorescence spectroscopies and X-ray diffraction (XRF and XRD) are physical analyses that involve high technology equipments in different material characterizations in Bio-medicine.[10]
However, important applications can be made on bone tissues research on specifical minerals characteristics as, crystal type and perfection (crystalline structure) and mineral contents (Calcium, Phosphorous and other chemical elements).
Osteogenesis distraction in maxillofacial surgery is a well-known technique, but one should have a precision indication, characteristics, length properties and limitations of the new bone. Laboratory experiments like Instrumental hardness tests (IHT) permit the determination of the physical properties of the bone submitted under the simulation of mechanical tests. In this context, during IHT we can measure the hardness coefficient and elasticity of the materials that constitute the new formed bone when under force and elastic resistance deformation.[11,12]
The purpose of this study was to evaluate the biological and physical properties of the newly formed bone in OD and compare it with LLLT exposed OD cases.
MATERIALS AND METHODS
Samples
12 rabbits of Lagomorpha order, Oryctolagos cuniculus, species, New Zealand race, males, weighing 3.5–4.5 kg, free of congenital or acquired malformations were selected.
Appropriate permissions were secured from institutional review board and appropriate animal care was provided as per guidelines.
Surgical Step
All surgical procedures were done under sterilized conditions. Anesthesia was obtained by association of drugs: Solution of xylazine hydrochloride 2% (Anacedan®) and Zolazepam hydrochloride with tiletamine hydrochloride (Zoletil®).
Under general anesthesia, the right submandibular region was prepared for surgery. Local analgesia infiltration with 0.9 ml of Lidocaine (2%) and Epinephrine 1:100000 was given. A 3 cm long incision was placed on the inferior mandibular border to access the mandibular bone. Periosteal debridment, with muscular insertions and vestibular corticotomy between pre-molar and mental foramen, with carbide burs 701 were performed. Stabilization and fixation of distractor device (PROMM® – Surgical Materials Industry LTDA, Porto Alegre/RS) in medial and distal segments were done. The surgical wound was sutured as per plan with nylon monofilament no. 4-0. Isolated sutures were placed in the deep planes and simple sutures in the epidermis.
Distraction protocol
After the latency period of three days, the activations on the distractor device were started, and with rhythm of 0.7 mm of extention per day for one week (approximately 4.9 mm of totally extension). The distractor device was left in the animal for the next 10 days as a rigid fixation to achieve maturation of newly formed bones.
Samples division
The specimens were randomly divided into control groups that did not receive laser therapy and experimental group, with LLLT applied. The samples then were divided into four groups in accordance with histological analysis or physical analysis, and subjected or not to OD and LLLT irradiations [Tables 1 and 2].
Table 1.
Distribution of experimental group and control, submitted to hystological analysis

Table 2.
Distribution of experimental and control submitted to physical analysis and computer tomograph.
Irradiation with LLLT
Irradiation in selected groups were given with Thera Laser®, 830 nm laser, using a well calibrated apparatus, and with an accurate dosage of 10 J/cm2 of LLLT, with 40 mW potency. Continuous emission was given directly over the bone site under OD, during the bone consolidation period, and 48 hrs, with a total of 50 J/cm2 per animal.
Samples preparation
After the bone consolidation period, animals were sacrificed, the mandibles were dissected, isolated, and segmented in the body region. The specimens for histological evaluation were immersed in 10% formalin and the others in 2% glutaraldehyde until the preparation.
Histological and histomorphometrical analysis
4 μm thick sections of each segment were taken on the latero-medial position with a total of three microscopic slides of each specimen. They were stained using hematoxylin and eosin.
However, out of the measured areas on the neoformed bone, each slide was subdivided in experimental units (EU), viewed under 100× optical microscope and images were acquired using a digital camera connected with microscope.
Neoformed area was measured in square pixels, with free software ImageTool® for Windows 3.0 (The University of Texas Health Science Center in San Antonio, United States). The neoformed bone percentual (% NF) was obtained from the ratio with total neoformed area / EU total area.
Physical analysis and tomography
The right hemi-mandibles of four rabbits were dissected, removed, and stored in 2% glutaraldehyde. The samples embedded in resin, received manual polish with sandpaper of coarseness 180 to 4000 microns in ascending grades, under constant irrigation for posterior X-ray incidence in spectrometer. After polishing, the samples were ready to be tomographed and undergo the Instrumentation Hardness test.
Tomographic analysis
The acquired images from the samples were obtained by Siemens Somaton Plus 4 Tomograph, with 1 mm thick axial section. The images were stored as Digital Imaging and Communications in Medicine (DICOM) and exported to software OsiriX Imaging Software 3 for tridimensional reconstruction. The software produced images with determined numbers of CT as the numeric information contained in each pixel of a CT image. It is related to the composition and nature of the tissue imaged and is used to represent the density of tissue. Also called CT number, or Hounsfield Unit (HU).
With the purpose of obtaining density from the images, the software ImageJ, freeware, developed by National Institutes of Health, USA was used. We selected the anterior section and an outline was done to obtain the Hounsfield Units (HU). The numbers were obtained and were tabulated, then exported to Origin 6.0 (Microcal SoftwareTM) program to obtain the density variations with experimental units, in HU.
Instrumental Hardness Test
The region of interest was divided in 14 other regions, with distance of 500 micrometers on the anteroposterior in between them. To evaluate the nanohardness and elasticity model, 10 measures in each of these 14 regions with distance of 50 micrometers between each point were taken to avoid any influences of each measurement [Figure 1]. Each indentation was achieved with only one load circle and discharge from 0 to 1 Newton, with a penetrator Vickers and Fischercope HV 100 apparatus. The obtained data was then exported to software Origin 6.0 (Microcal Software™) to obtain the variations all over the sample.
Figure 1.
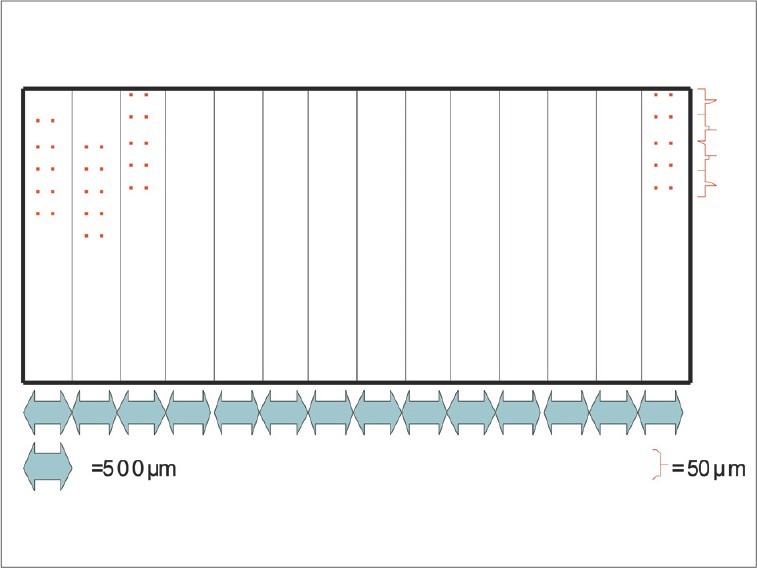
Interested region divided in 14 regions with distance of 500 micrometers between them. In each region, 10 measurements were taken, with minimum distance of 50 micrometers between each of them
Spectroscopy by X-Ray Fluorescence
The blocks were placed on spectrometer model XRF-1800 (Sequential X-ray Spectrometer, Shimadzu), for the analysis of the elements calcium and phosphorus. The samples were measured by Rh K (X-Ray) radiation, and with regimen of 40 kV and 95 mA. To quantify the Calcium and Phosphorous elements, we employed crystal diffractors of Lithium fluoride (LiF) and Germanium (Ge), to specifically filter the fluorescence. The measurements were taken in vacuum under 25 Pascal (Pa) pressure.
Measurements were taken in different points on length vector and analyzed by a millimeter mesh with respect to different variations of mature bone levels, as a result of lengthening by osteogenesis distraction technique. The values for quantification of Ca and P, in each point were calculated by Ca/P ratio, put through statistical analysis and organized in tables.
X-Ray diffraction spectroscopy
After the data obtained from XRF, the samples were transversally sectioned in a microtome on an anterior and medial portion of OD site, ground to a fine powder and inserted in glass slide for machine pressing. The X-ray analysis was performed by XRD Maxima 7000 (X-ray Diffractometer, Shimadzu). Radiation measurement Cu-Kα (λ= 1,5406 Å, 40 kV and 30 mA) was used. In each sample a diffractogram with peaks was obtained, and a ratio with signal amplitude and noise were identified and compared with well known structure of hydroxyapatite by using specific software on the same machine with appropriate adjustments to obtain the crystallinity grade.
Statistical analysis
For histological evaluation, we used the software Biostat 4.0 to evaluate the interclass replicability of the examiners. The normal existence of the histomorphometric measurements of the new formed bone, was evaluated by Kolmogorov-Smirnov test. The Student-T test was used to compare the neoformation percentile among the groups. SPSS (Statistic Package for Social Science, Chicago, USA) version 16 was employed for performing other statistic tests.
RESULTS AND DISCUSSION
Experimental studies in different animal groups permitted a new comprehension of biological and biomechanical principles of OD. The improvement of technique with the establishment of an ideal rhythm on device activations is crucial in the process of the new formed bone.[13,14] In the present study the protocol activation of the device was 0.7 mm/day that is suggested by literature. Activations of 0.5 to 1 mm/day result in new bone formation, but when within large distraction sites and with a shorter duration, OD has been shown to form fibrous connections,[15,16] and this situation was not observed in this present investigation. The results of these investigations have been tabulated in Table 3.
Table 3.
Analysis and physical properties of studied groups
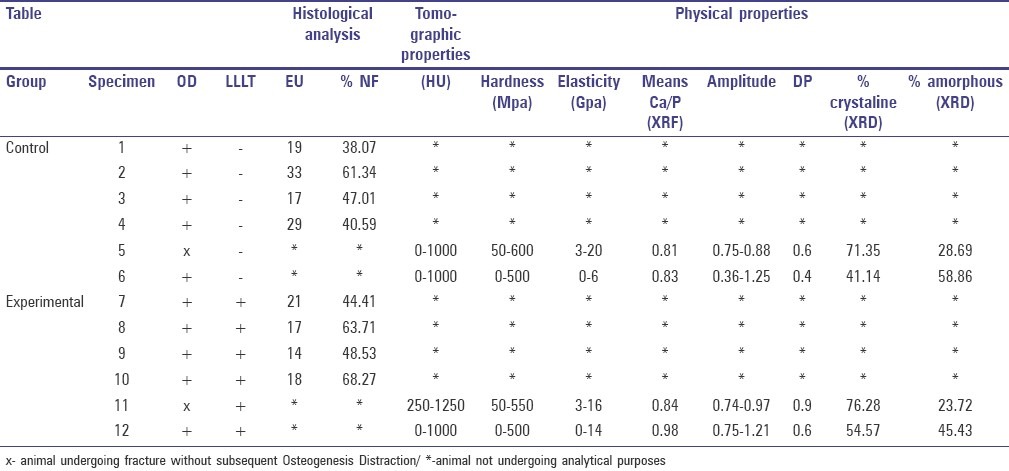
Effect of laser irradiation on lengthening during OD has been well established.[5,6,17,18] However, the physical and biological characteristics of such newly formed bone warranted investigation with and without LLLT.
Histological analysis and histomorphometric (Group 1)
On length, the area was verified for bone formation with presence of blood vessels, newformed osteocytes, as well as intense osteoblastic activity, with intense osteogenic activity. This tissue was characterized by a presence of trabecular tissue with connective tissue on interim. The bone trabeculae were presented as parallel structures, but perpendicular on the fracture line, presenting a physiologic repair with the objective to fill in the bone defect. These results corroborate with literature findings.[5–7,17] In agreement with the study by Djasim et al.,[16] we found new bone trabeculae formation in all groups, filled with fibrovascular tissues, and where the bone trabeculae were aligned in the direction vector of OD we found on the central area a minor quantity of bone formation, that indicates the limits/border of preexisting bone, and diminishing on the central area direction.
For the new bone formed, the measurements of EU were submitted to Kolmogorov-Smirnov statistical test to verify the normality of histomorphometric measurements and distribution (P < 0.05). For the subsequent analysis, parametric tests were used.
Neoformed bone mean values were obtained from the measurements on experimental units of each slide. These measurements were submitted to parametrical Student t-test for independent samples to observe the major percentile of newbone formed in experimental group (57.89%) as compared to control group (46.75%) with P = 0.006. These results showed a positive action of LLLT on the experimental group, as presented in the literature and same results were demonstrated by researchers’ findings of major newbone formation in irradiate group.[5,6]
Tomographic and Physical analysis (Group 2)
Tomographic exam (HU)
The rabbits number 5 and 11 from group 2 suffered fracture, although not lengthened by OD. The former did not receive laser irradiation and the latter was irradiated by laser. The tomographic analysis revealed variations of density from 0 to 1,000 HU for the rabbit number 5 and from 250 to 1,250 HU for the rabbit number 11.
The rabbit number 5 presented minor bone densities other than normal bone, and in accordance of the mentioned values of density in literature and corroborated by studies.[17–19] These results are similar to fractured bone callus produced on rabbits tibiae, but not irradiated with laser and researchers found density values of 297 HU from researchers.[19,20] In spite of the obtained values being inferior in comparison to normal bone, union had occurred in the segmented bone by neobone formation, and clinically verified and sustained by previous histological findings. The rabbit number 11, presented peaks values of bone density similar to the rabbit number 11 and as in the literature for normal bone.[18–21] These values follow the observed results in literature about bone callus produced by fractures in rabbits tibiae, with mean values of about 691 HU.[21] Similar to the rabbit number 5, union was found in the bone segmented area and clinically verified and confirmed by previous histological findings in neoformed bone area. This augmented on density in relation to the fractured group, but was not irradiated by laser was supported by a positive biomodulation induced by this technology. These effects have been confirmed by many studies, and demonstrated the advantages of LLLT use in healing wounds or bone fractures.[5,6,21,22]
The LLLT utilization on rabbit 11 produced an exuberant bone callus, clinically verified and observed augmentation of density in CT studies. The rabbit number 6 and 12 from the Group 2 suffered fractures and subsequent lengthening by OD. The first did not receive irradiation by LLLT, while the second was irradiated with LLLT. The CT analysis revealed density variations from 0 to 1,000 HU for both groups.
We believe that difficult healing process in these groups was the reason for specific bone length for each turn of distractor activation that result in disorganized tissue, and additional local trauma that could retard or make tissue repair difficult. This situation could be the principal referred cause in literature of instabilities and time consolidations.[23–27]
Nanohardness tests IHT
The nanohardness tests of the specimens under fractures presented superior values compared to those undergoing lengthening with OD. The variations among these groups, possibly by the favorable fracture, positioning and fixation without increase in bone length would create a possibility of a biological silence period in post-trauma and adequate wound healing.
The tomographic results and physical analysis are all in consonance with result findings by earlier researchers who observed with mechanical tests, and found no statistical difference with respect to tension forces among groups with and without LLLT that suffered fractures.[21] These results suggest the utilization of LLLT can be favorable on bone callus formation in early stages of cicatrization process, but with beneficial doubts in biomechanical properties.[21]
Relative to mechanical properties, both rabbit number 6 and rabbit number 12 presented nanohardness and elasticity model inferior compared to the other groups. For these groups, the nanohardness was between 0 until 550 MPa. The non-irradiated rabbit (6) presented elasticity module between 0 and 6 GPa, the irradiated rabbit (12) presented elasticity module from 0-14 GPa. These results indicate that the bone lengthened by OD and irradiated by LLLT presented advanced mechanical properties than of non-irradiated bone. Tomographic evaluation failed to identify difference between density among distracted bone, with or without LLLT, while the physical tests identified variation on elasticity model among the same animals. Therefore, the IHT can be more sensitive test, and can identify differences.
Spectroscopy by X-Ray fluorescence (XRF)
From this analysis, ratios were calculated between the percent mass of Calcium and Phosphorus to indicate the tendency of major mineralization in groups irradiated with LLLT. The observed ratio was lowest from expected pure sources of hydroxyapatite.[1,2] However the ratio was in the same levels as in bone formation with other nanoanalysis methods reported in the literature.[28,29]
The ratio among Calcium and Phosphorus from rabbit number 11 compared with rabbit number 12, both irradiated, indicates that the effect of LLLT occurs in early stages of osteogenesis, and corroborates with findings by Saito and Shimizu (1997).[28]
The imperfections in bone tissue could be responsible for higher solubility, promoting ionic exchanges necessary for homeostasis. The ratios found among these elements indicate that the mechanical properties of the neo bone tissue, expresses its organization and mature status.[29,30]
Spectroscopy by X-Ray diffraction (XRD)
The crystalline calculus are based on methodology standards from ASTM D5357 and D5758 (American Society for Testing and Materials), used for crystallinity calculus by XRD by many minerals with porous structure. From this result, we could conclude that the more advanced the structure maturity, more organized, and higher values of crystallinity. Then, with this premise, the crystallinity indicates the degree of maturation of bone tissue. The percentuals of crystallinity of the study indicates with precision the biomodulation effects of LLLT, as the rabbits bone with more per cents of crystallinity with and without OD express a superior size, order, and perfection quality crystal properties. The present result indicates that LLLT could induce faster bone regeneration.
The analysis of rabbits without OD was comparable with rabbits undergoing a surgical procedure, and used as negative control on the experiment. The methodological design was earlier employed by Pampu et al.. They had analyzed the influence of plasma rich platelet in OD, with latent period and with immediate activation of distractor device.[31] The crystallinity results corroborates with the histomorphometric studies, and indicates a possible coincidence on the mature grade with samples’ crystallinity. The histological findings are in agreement with our earlier findings.[32]
CONCLUSION
The results of this pilot study obtained with smaller sample size in the present study are to be interpreted carefully. However, this preliminary report has strong encouraging result of employing LLLT as a potential bio-stimulator. The histological, tomographic and physical findings warrants a deeper study with larger sample size as the understanding would reflect on the effects of LLLT from basics to clinical relevance on wound, and bone healing repair.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Samchukov ML, Cherkashin AM, Cope JB. Distraction osteogenesis: History and biologic basis of new bone formation. In: Lynch SE, Genco RJ, Marx RE, editors. Tissue engineering: Applications in maxillofacial surgery and periodontics. Illinois: Quintessence; 1999. pp. 131–46. [Google Scholar]
- 2.Mofid MM, Manson PN, Robertson BC, Tufaro AP, Elias JJ, Vander Kolk CA. Craniofacial distraction osteogenesis: A review of 3278 cases. Plast Reconstr Surg. 2001;108:1103–14. doi: 10.1097/00006534-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Kessler P, Neukam FW, Wiltfang J. Effects of distraction forces and frequency of distraction on bony regeneration. Br J Oral Maxillofac Surg. 2005;43:392–8. doi: 10.1016/j.bjoms.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Cakarer S, Olgac V, Aksakalli N, Tang A, Keskin C. Acceleration of consolidation period by thrombin peptide 508 in tibial distraction osteogenesis in rats. Br J Oral Maxillofac Surg. 2010;48:633–6. doi: 10.1016/j.bjoms.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Cerqueira A, Silveira RL, Oliveira MG, Sant’ana Filho M, Heitz C. Bone tissue microscopic findings related to the use of diode Laser (830 nm) in ovine mandible submitted to distraction osteogenesis. Acta Cir Bras. 2007;22:92–7. doi: 10.1590/s0102-86502007000200003. [DOI] [PubMed] [Google Scholar]
- 6.Miloro M, Miller JJ, Stoner JA. Low-level laser effect on mandibular distraction osteogeneis. J Oral Maxillofac Surg. 2007;65:168–76. doi: 10.1016/j.joms.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann CE, Harris G, Thurmüller P, Troulis MJ, Perrott DH, Rahn B, et al. Assessment of bone formation in a porcine mandibular distraction wound by computed tomography. Int J Oral Maxillofac Surg. 2004;33:569–74. doi: 10.1016/j.ijom.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Swennen GR, Eulzer C, Schutyser F, Hüttmann C, Schliephake H. Assessment of the distraction regenerate using three-dimensional quantitative computer tomography. Int J Oral Maxillofac Surg. 2005;34:64–73. doi: 10.1016/j.ijom.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Elshahat A, Inoue N, Marti G, Safe I, Manson P, Vanderkolk C. Role of guided bone regeneration principle in preventing fibrous healing in distraction osteogenesis at high speed: Experimental study in rabbit mandibles. J Craniofac Surg. 2004;15:916–21. doi: 10.1097/00001665-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Brundle CR, Evans CA, Wilson S. Encycolpedia of materials characterization. Greenwich: Butterworth-Heinemann; 1992. [Google Scholar]
- 11.Blando E. Technical study on indentation to evaluate materials on nano region and microhardness. [Thesis] Porto Alegre (RS): Pontifícia Universidade Católica do Rio Grande do Sul; 2001. [Google Scholar]
- 12.Newey D, Pollock HM, Wilkins MA. The ultra-microhardness of íon-implanted iron and steel at sub-micron depths and its correlation with wear-resistance. J Mater Sci. 1983;12:157–66. [Google Scholar]
- 13.Swennen G, Dempf R, Schliephake H. Craniofacial distraction osteogenesis: A review of the literature. Part II. Experimental studies. Int J Oral Maxillofac Surg. 2002;31:123–35. doi: 10.1054/ijom.2002.0225. [DOI] [PubMed] [Google Scholar]
- 14.Williams BE, King GJ, Liu ZJ, Rafferty KL. Sequential histomorphometric analysis of regenerate osteogenesis following mandibular distraction in the rat. Arch Oral Biol. 2005;50:497–506. doi: 10.1016/j.archoralbio.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Boccaccio A, Pappalettere C, Kelly DJ. The influence of expansion rates on mandibular distraction osteogenesis: A computational analysis. Ann Biomed Eng. 2007;35:1940–60. doi: 10.1007/s10439-007-9367-x. [DOI] [PubMed] [Google Scholar]
- 16.Djasim UM, Mathot BJ, Wolvius EB, van Neck JW, van der Wal KG. Histomorphometric comparison between continuous and discontinuous distraction osteogenesis. J Craniomaxillofac Surg. 2009;37:398–404. doi: 10.1016/j.jcms.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Hübler R, Blando E, Gaião L, Kreisner PE, Post LK, Xavier CB, et al. Effects of low-level laser therapy on bone formed after distraction osteogenesis. Lasers Med Sci. 2010;25:213–9. doi: 10.1007/s10103-009-0691-2. [DOI] [PubMed] [Google Scholar]
- 18.Bontrager KL. Tratado de técnica radiológica e base anatômica. 5th ed. Rio de Janeiro: Guanabara Koogan; 2003. p. 814. [Google Scholar]
- 19.Turkyilmaz I, Tözüm TF, Tumer C. Bone density assessments of oral implant sites using computerized tomography. J Oral Rehabil. 2007;34:267–72. doi: 10.1111/j.1365-2842.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- 20.Park HS, Lee YJ, Jeong SH, Kwon TG. Density of the alveolar and basal bones of the maxilla and the mandible. Am J Orthod Dentofacial Orthop. 2008;133:30–7. doi: 10.1016/j.ajodo.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 21.Kazem Shakouri S, Soleimanpour J, Salekzamani Y, Oskuie MR. Effect of low-level laser therapy on the fracture healing process. Lasers Med Sci. 2010;25:73–7. doi: 10.1007/s10103-009-0670-7. [DOI] [PubMed] [Google Scholar]
- 22.Chun YS, Lim WH. Bone density at interradicular sites: Implications for orthodontic mini-implant placement. Orthod Craniofac Res. 2009;12:25–32. doi: 10.1111/j.1601-6343.2008.01434.x. [DOI] [PubMed] [Google Scholar]
- 23.Pretel H, Lazarelli RF, Ramalho LT. Effect of low-level laser therapy on bone repair: Histological study in rats. Lasers Surg Med. 2007;39:788–96. doi: 10.1002/lsm.20585. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy JG, Schreiber J, Karp N, Thorne CH, Grayson BH. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg. 1992;89:1–8. [PubMed] [Google Scholar]
- 25.Douglas LR, Douglas JB, Smith PJ. Intraoral mandibular distraction osteogenesis in a patient with severe micrognathia secondary to TMJ ankylosis using a tooth and boneanchored device (PIT Device): A case report. J Oral Maxillofac Surg. 2000;58:1429–33. doi: 10.1053/joms.2000.18283. [DOI] [PubMed] [Google Scholar]
- 26.Marquez IM, Fish LC, Stella JP. Two-years follow-up of distraction osteogenesis: Its effect on mandibular ramus height in hemifacial microsomia. Am J Orthod Dentofacial Orthop. 2000;117:130–9. doi: 10.1016/s0889-5406(00)70223-x. [DOI] [PubMed] [Google Scholar]
- 27.Krawczyk A, Kuropka P, Kuryszko J, Wall A, Dragan S, Kulej M. Experimental studies on the effect of osteotomy technique on the bone regeneration in distraction osteogenesis. Bone. 2007;40:781–91. doi: 10.1016/j.bone.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Saito S, Shimizu N. Stimulatory effects of low-power Laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am J Orthod Dentofacial Orthop. 1997;111:525–32. doi: 10.1016/s0889-5406(97)70152-5. [DOI] [PubMed] [Google Scholar]
- 29.Bohic S, Heymann D, Pouëzat JA, Gauthier O, Daculsi G. Transmission FT-IR microspectroscopy of mineral phases in calcified tissues. C R Acad Sci III. 1998;321:865–76. doi: 10.1016/s0764-4469(99)80027-4. [DOI] [PubMed] [Google Scholar]
- 30.Shea JE, Miller SC. Skeletal function and structure: Implications for tissue targeted therapeutics. Adv Drug Deliv Rev. 2005;57:945–57. doi: 10.1016/j.addr.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Pampu AA, Dolanmaz D, Tüz HH, Avunduk MC, Kişnişci RS. Histomorphometric evaluation of the effects of zoledronic acid on mandibular distraction osteogenesis in rabbits. J Oral Maxillofac Surg. 2008;66:905–10. doi: 10.1016/j.joms.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Kreisner PE, Blaya DS, Gaião L, Maciel-Santos ME, Etges A, Santana-Filho M, de Oliveira MG. Histological evaluation of the effect of low-level laser on distraction osteogenesis in rabbit mandibles. Med Oral Patol Oral Cir Bucal. 2010;15:e616–8. doi: 10.4317/medoral.15.e616. [DOI] [PubMed] [Google Scholar]


