Abstract
Aim:
To present the outcome measures of the use of iliac bone graft, rhBMP-2 and rhBMP-2 with zygoma shavings in alveolar cleft defect closure.
Materials and Methods:
Retrospective analysis of details of treated alveolar defect cases (5-10 years during January 2008–December 2010) from records with 4 months follow-up in accordance with the inclusion criteria.
Predictor Variables:
Type of graft used (iliac crest graft/rhBMP-2/rhBMP-2 with zygoma shaving).
Outcome Variables:
Duration of the operation, blood loss, postoperative drugs used, donor site morbidity, efficiency of bone deposition (radiologically) at 4 months.
Statistics:
Descriptive statistics, one-way ANOVA. P < 0.05 was taken as significant.
Results:
Forty two cases met the inclusion criteria. Mean efficiency of bone deposition was 89.97 ± 4.79%. Mean efficiency of bone formation in rhBMP-2 (n=13), rhBMP-2 with zygoma shavings (n=9) and iliac crest graft (n=20) was 89.42%, 95.38% and 87.91%, respectively (P=0.000). Drugs usage and postoperative morbidity differed significantly between groups (P < 0.05).
Conclusion:
The use of rhBMP-2 evades the need for additional surgery and overcomes the postoperative morbidity that is associated with the conventional iliac grafting technique. The rhBMP-2 with zygoma shavings showed maximum benefits.
Keywords: Bone morphogenetic protein, zygoma shavings, iliac graft
INTRODUCTION
Autogenous iliac crest graft was first advocated for alveolar cleft repair by Boyne in 1974. The use of autogenous bone grafts was successful in restoring form and function.[1] The drawbacks are the significant donor site morbidity, pain, injury to the lateral cutaneous nerve, hernia and gait disturbances that are associated with harvesting of the iliac crest bone graft. These potential complications can be overcome with the use of a variety of grafts and graft substitutes. These bone substitutes are osteoconductive in nature, but they lack osteoinductive properties.
With the limitations associated with both autogenous and allogeneic bone, focus was turned on to fabricate completely synthetic bioimplants that have osteoinductive properties. By the late 1980s, bone morphogenetic protein (BMP), a potent osteoinductive protein, was identified.[2,3] The BMP has the ability to induce pluripotent mesenchymal stem cells to differentiate into osteoblasts that will lay down new bone. These proteins (BMP-2 and BMP-7) were synthetically fabricated in 2006, both of which are now available for clinical use.[4]
Various clinical and animal studies have also proved its osteoinductive properties to replicate embryonic bone formation.[3,4] This has led to the use of rhBMP-2 in certain oral and maxillofacial bone grafting procedures, sinus augmentation, alveolar cleft grafting procedures, and localized alveolar ridge augmentation besides spine and fracture treatment.[4] The aim of our study was to conduct a large-scale study comparing the standard iliac bone grafting, rhBMP-2, and rhBMP-2 with zygomatic shavings for closure of alveolar cleft defects.
MATERIALS AND METHODS
Case records of all patients with non-syndromic cleft within the age group of 5 to 10 years, operated from January 2008 to December 2010, were included in the study. The inclusion criteria included, all patients undergoing reconstruction of alveolar clefts with iliac crest grafts, rhBMP2 and those with rhBMP2 with fresh zygoma shavings (taken using Ebner's knife) [Figures 1–5 clinical photographs of procedure]. To compare the age and type of cleft and type of grafting materials, the following criteria were also included in the study.
Figure 1.
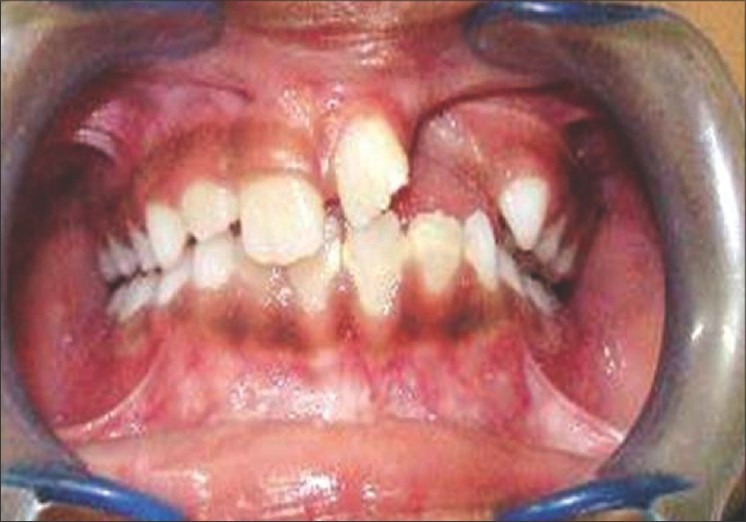
Oro-nasal fistula in a case of alveolar cleft
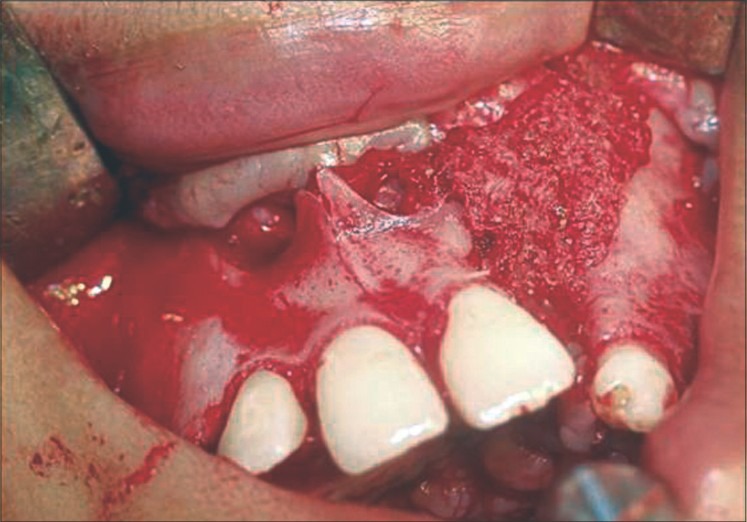
Figure 5.
Freeze-dried rhBMP-2 graft with zygoma shavings
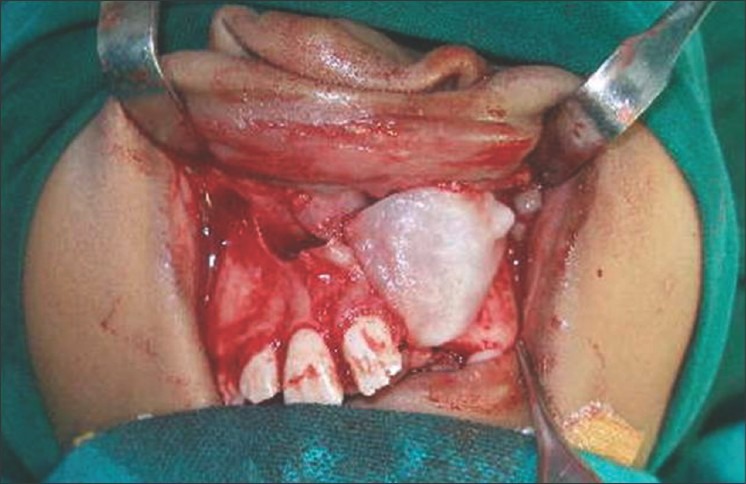
Figure 2.
Exposure of the oronasal cleft and closure of the nasal lining
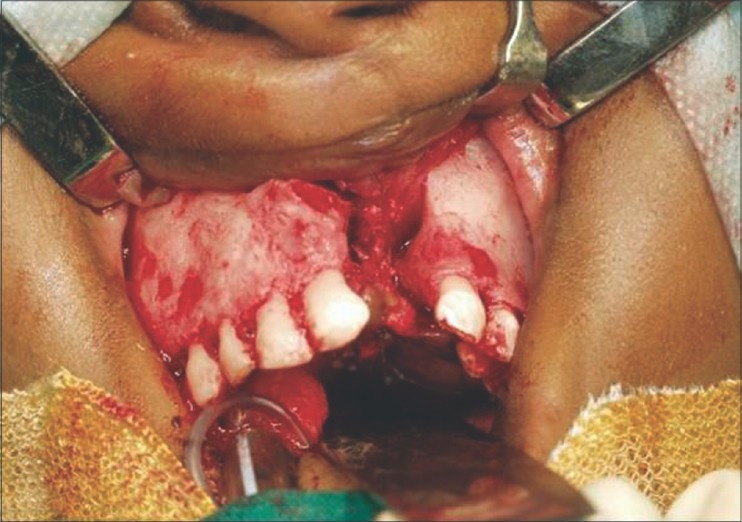
Figure 3.
Filling the cleft with cancellous bone graft harvested from the anterior iliac crest
Figure 4.
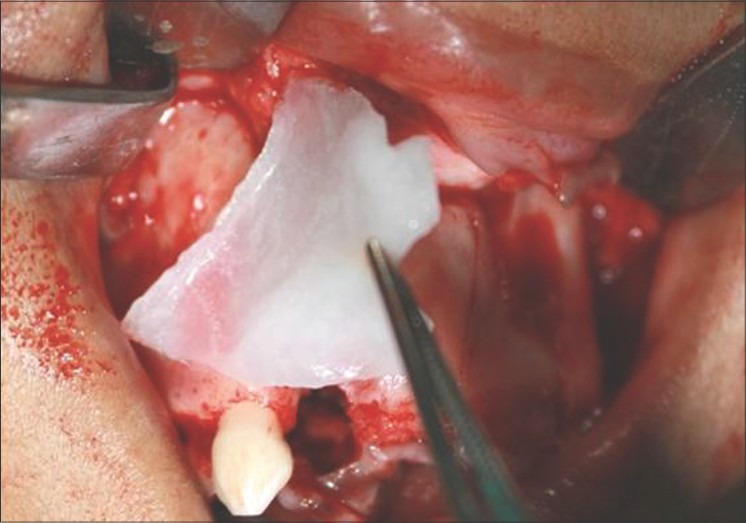
Freeze-dried rhBMP-2 graft reconstituted with sterile saline bound to absorbable collagen sheet graft
A minimum radiographic cleft area of 15 mm2 as seen in the orthopantomogram with a significant loss of clinical form, function, and/or esthetics [Figures 6a, 7a, 8a].
Patients with a minimum of 4 months radiographic (orthopantomogram) follow-up [Figures 6b, 7b, 8b].
Figure 6a.
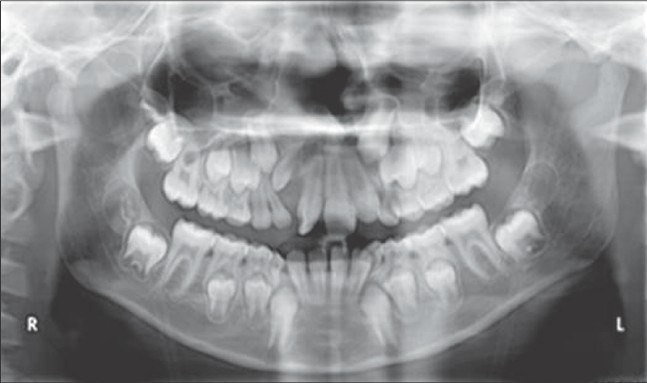
Preoperative radiograph before iliac bone grafting
Figure 7a.
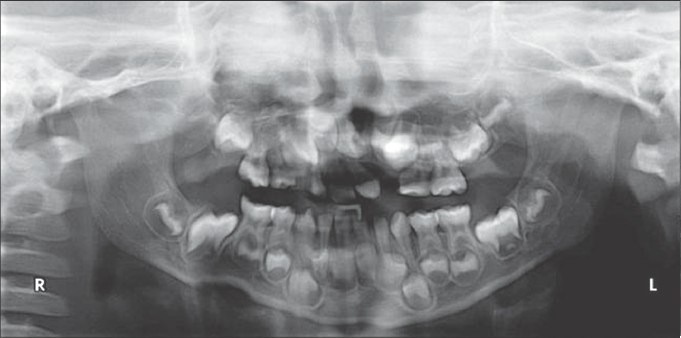
Preoperative radiograph before rhBMP-2 placement
Figure 8a.
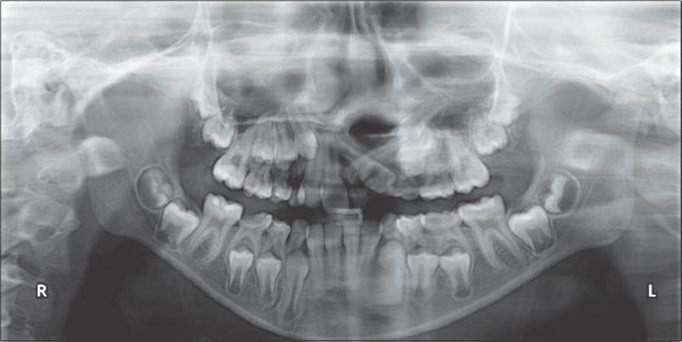
Preoperative radiograph before placement of rhBMP-2 and zygoma shavings
Figure 6b.
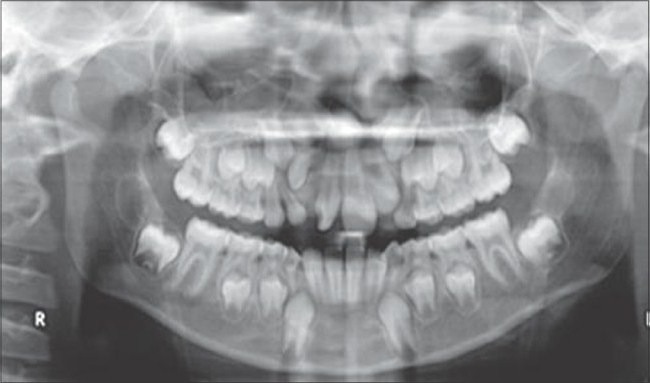
Postoperative radiograph after iliac bone grafting (4 months)
Figure 7b.
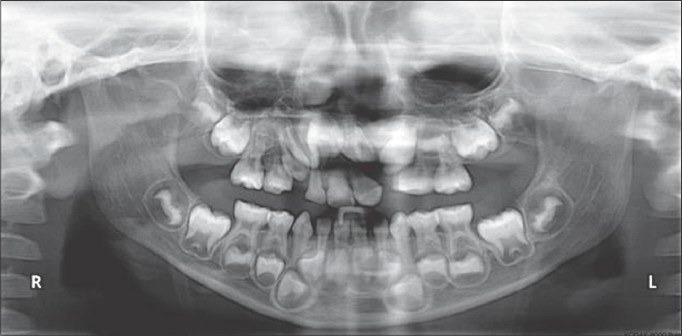
Postoperative radiograph after placement of rhBMP-2 (4 months)
Figure 8b.
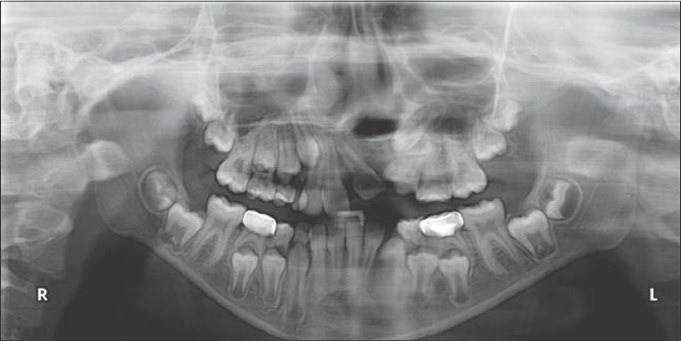
Postoperative radiograph after placement of rhBMP-2 and zygoma shavings (4 months)
Cases with previously repeated or corrective surgeries and patients with a follow-up period shorter than 4 months were excluded from the study.
The parameters used in the previously published literature were used.[5] Age, gender, type of the defect (unilateral/bilateral), type of graft (iliac crest graft, rhBMP-2/rhBMP-2 with zygoma shavings), total surgery time (in minutes), blood loss at surgical and harvest site at the end of surgery, length of scar (at the fourth post-operative month in millimeters), number of days of antibiotics, serratiopeptidase and nonsteroidal anti-inflammatory drugs (NSAIDs) use, number of days elapsed between surgery and removal of non-resorbable sutures placed at surgical sites, number of days to recover normal masticatory function, pain visual analog score (VAS) on the fifth postoperative day and pain at harvest site on a scale of 10, duration of edema, number of days the patient took to resume normal walk without any gait disturbances.
Radiographic (orthopantomogram) evaluation before and after 16 weeks postoperatively were used to measure the size of the defect as stated in the previously published literature.[5] The preoperative size of the defect was traced on a standard tracing sheet and the area involved was calculated after positioning it on to a regular graph sheet with a single small square denoted as 1 mm2. All the involved squares with at least 50% of involved area were taken as a single mm2 area. The procedure was repeated by two independent assistant surgeons apart from the author himself. The discrepancy in the measurements was overcome by taking an average of all three measured values. In postoperative orthopantomograms, areas with distinguishable radiolucency were only considered. Efficiency of healing after 16 weeks is calculated according to the following equation:
[(Preoperative radiolucency area – 4th month postoperative radiolucency area) / preoperative deficiency] × 100
All recorded data were entered and analyzed using the Statistical Package for Social Science (SPSS) 16.0 version. Descriptive statistics of all variables and comparison of means were tabulated for evaluating the difference in the various surgical techniques. One-way ANOVA was performed and a P value of <0.05 was considered to be statistically significant.
RESULTS
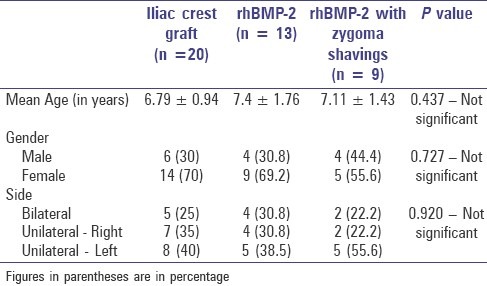
A total of 42 non-syndromic alveolar cleft defect cases were considered in the study. The study group consisted of 28 (66.7%) females and 14 (33.3%) males [Table 1]. The mean age was 7.05 ± 1.34 years. There were 11 bilateral alveolar cleft cases (26.2%) and the rest were unilateral alveolar clefts (73.8%). Among the unilateral cases, 18 clefts involved the right side and 13 involved the left side alveolus. The ratio of right to left side was about 1:1.38.
Table 1.
Descriptive statistics of the study population
Of the 42 cases, 13 cases (31%) were treated with rhBMP-2 alone, 9 cases with rhBMP-2 and zygomatic bone shavings, and 20 cases (47.6%) with iliac crest graft.
The mean age of patients who underwent alveolar cleft closure with rhBMP-2 was 7.4 ± 1.76 years, while the mean age of patients who underwent alveolar cleft closure with iliac crest graft was 6.79 ± 0.94 years, and those with rhBMP-2 and zygoma shavings were 7.11±1.43 years. There was no statistical difference between the mean of ages and the grafting procedures (P = 0.437). The distribution of gender and cleft side between the study populations were also not significant [Table 1].
Descriptive statistics of surgical and postoperative parameters of the three study groups is depicted in Table 2. The results were statistically significant between the iliac crest graft, rhBMP-2 and rhBMP-2 with zygoma shavings groups, in terms of antibiotic, serratiopeptidase and NSAIDs use, surgical site pain, duration of edema, number of working days lost for attendees, and the number of days the patient could walk unaided.
Table 2.
Comparison of surgical and post-surgical parameters between the surgical modalities
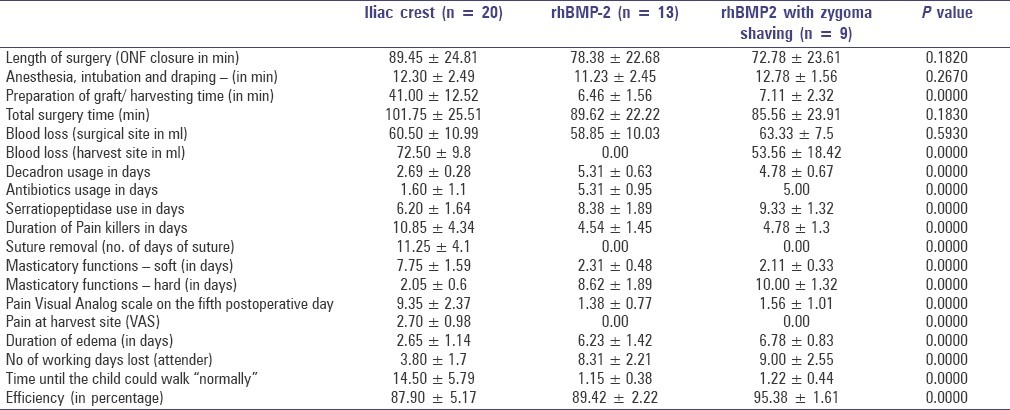
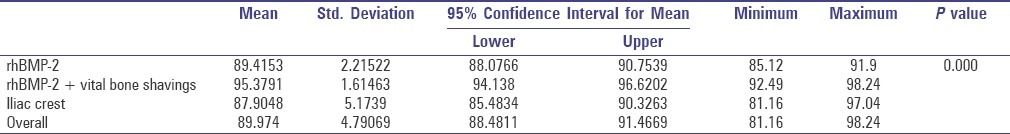
Table 3 shows the mean efficiency of bone deposition as revealed by the loss in radiolucency for all the three surgical techniques, measured by one-way ANOVA. The mean efficiency of bone formation at the end of 16 weeks in the rhBMP-2, rhBMP-2 with zygoma shavings, and iliac crest graft cases was 89.42%, 95.38% and 87.91% respectively [Graph 1]. This difference between groups was statistically significant (P = 0.000). The mean efficiency of bone deposition as revealed by the loss in radiolucency for gender and alveolar cleft side predilection was not statistically significant in both conditions (P = 0.663 and 0.376, respectively).
Table 3.
One-way ANOVA comparing efficiency of the three surgical modalities (in percentage)
Graph 1.
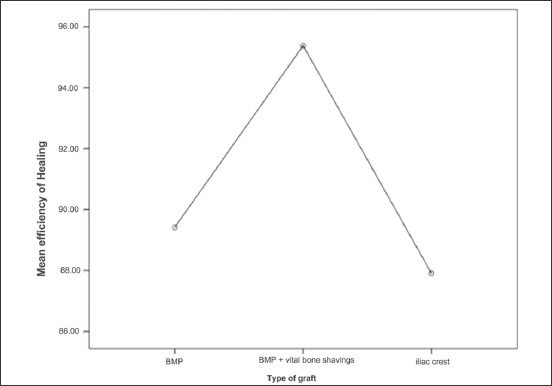
Showing the mean difference of efficiency percentage between three surgical modalities with 95% confidence interval
DISCUSSION
Reconstruction of alveolar cleft defects with autogenous bone grafts have been the standard choice of treatment. The additional surgical site for harvesting the graft carries an inherent risk of substantial complication, significant donor site morbidity, and resultant scar, longer duration of surgery, donor site pain, additional blood loss and further potential complications as a result of the procedure.[1]
Wozney et al., (1988) sequenced rhBMP-2 and cloned it paving the pathway for its mass production endorsing its large-scale clinical and laboratory use. This led to a series of clinical research studies involving spine fusions and long bone non-unions substantiating its efficacy and safety for human use. At the host's implantation site, it acts in situ by concentrating the host stem cells to differentiate into osteoblasts.[1] This physiological healing process summarizes all the sequence of events that occur during embryological bone formation that is demonstrated to be normal in the required quantity and quality.[6,7] The introduction of rhBMP-2 for alveolar cleft repair procedures is very recent, therefore there is a lack of long-term follow-up studies and the success rate of this material is still sparse in English literature.[1]
Two-dimensional radiographs are routinely used for evaluating the efficacy of secondary alveolar cleft bone grafting, but these may encompass some inherent distortion factors. Three-dimensional computed tomograms (CTs) might be a better alternative but the higher cost, increased radiation exposure particularly in young children, along with patient inconvenience and accessibility are the limiting factors.[8,9] Considering the cost and radiographic exposure, orthopantomograms prove to be a better imaging modality to study the efficiency of bone formation at an early postoperative period such as 4 months.[3] Planimetry has also been used in a number of studies to ascertain the efficiency of rhBMP-2 for healing in long bones.[5,6]
The difference between cases in the three groups of surgical modalities and type of clefts (P = 0.376), gender (P =0.633), mean age (P = 0.437) were not statistically significant, which clearly indicates that the distribution of cases between the three groups is not significant despite minor differences.
From Table 2 it is evident that the outcome of the surgical and postoperative parameters is greatly influenced by the type of grafting modality that is advocated with a high statistical significance. Graph 1 depicts the mean difference of efficiency percentage between the three grafting modalities with 95% confidence interval. As depicted in Table 3, rhBMP-2 group with zygoma shavings had higher mean efficiency percentage as compared to the rhBMP-2 alone and iliac crest graft group with a high statistical significance of P = 0.000. Conversely, the mean efficiency of bone deposition as revealed by the loss in radiolucency for genders and side was not statistically significant (P = 0.493 and 0.745, respectively).
Chin et al.,[1] radiographically depicted satisfactory bone formation in 3 months in severe cleft cases with good bone consolidation. Loss of radiolucency is a good evidence of consolidation of the defect. Successful studies of rhBMP-2/ACS have been conducted in animals followed by its use in humans for maxillary sinus floor augmentation and alveolar ridge augmentation. An open-label feasibility study for maxillary sinus floor augmentation was done using 1.77–3.4 mg rhBMP-2, which was implanted in an absorbable collagen sheet (ACS). In the present study, CT scans also showed significant new bone growth in all evaluated patients and this would further allow canine eruption or implant placement in the future.[5,9]
Addition of fresh vital bone shavings especially from zygoma does not require an additional surgery or donor site. These shavings can be taken using an Ebner's knife and is probably a potent source of progenitor cells. This would accelerate the formation of osteoid matrix at a very early stage. Such a procedure has been reported in the literature.[5]
The recovery of masticatory function for both soft and regular diet was comparatively late and the duration of edema was increased for rhBMP-2 and rhBMP-2 with zygoma shavings cases because it is reported that diffuse soft tissue swelling in the postoperative period at the surgical site is not unusual with rhBMP-2 use and the cause of this phenomenon is unknown. However, it is believed that an exaggerated inflammatory reaction is probably responsible for both the success in promoting bone formation as well as the adverse effect of soft-tissue swelling. This may very well be a dose-dependent phenomenon that still needs to be investigated for future use of rhBMP-2.[5,10,11] On contrast, pain in surgical site on a visual analog scale on the fifth postoperative day at the surgical site was significant as inflammatory mediators rush to the surgical site owing to the inflammatory response to rhBMP-2.
Statistically significant difference in the efficiency of the grafting modalities clearly indicates that grafting with rhBMP-2 and fresh zygoma shavings together has a better bone formation as indicated by obliteration of radiolucency in orthopantomograms. The results were similar to three-dimensional volumetric studies conducted in alveolar clefts by Herford et al.[12] and Dickinson et al.,[13] The zygoma shavings would have probably acted as a source of progenitor cells. Reports in the literature have highlighted such a phenomenon.[5]
It is reported that in spine surgeries, rhBMP-2 use as compared to autogenous iliac crest graft reduces the operating time and anesthesia time by 30 min, reduced recovery room time by 15 minutes besides less blood loss, reduced need for blood transfusion, and autograft extenders and harvesters. Use of rhBMP-2 with or without the zygomatic shavings reduced the length of stay by about 0.5 days. Our present findings were consistent with the study conducted by Polly et al.,[14] in spine surgeries. The reported literature of overall complication rates for autogenous iliac crest graft ranged from 15% to 49%, and major complications were 2 to 39%. These complications included donor site pain, infection, hematoma, wound dehiscence, vascular injuries, and iliac crest fracture. Literature documented an iliac crest fracture rate of 3.7–4%. Post-surgical hematoma accounted to about 4–10%. Donor site pain is a common complication after autogenous iliac crest bone graft harvesting. The suggested cause includes direct nerve injury, sacroiliac joint violation, sharp edges at harvest site and a generalized pain syndrome. They reported a visual analog scale of 6–7 after 1 month at the donor iliac graft site.[14] It should be remembered that the amount of graft that is required for spinal surgeries is at least two-folds more than that required for cleft surgeries. Bone graft harvest time from iliac crest takes a variable time of 30–45 min, with shortest time being reported as 20 min.[14] With the use of rhBMP-2, the time spent for harvesting the graft can completely be avoided.[5]
Furthermore, studies done on rhBMP-2 in spine surgeries have shown preliminary results suggesting that from a payer perspective, the high cost of rhBMP-2 is a concerning factor which can be entirely offset by reductions in the use of other medical resources making its cost neutral. The cost offsets can be overlooked when considering the low levels of pain, donor site morbidity, donor site scar, fusion failures and complications associated with autogenous iliac crest bone graft.[5,15]
CONCLUSION
This study clearly indicates that there is an obvious advantage of rhBMP-2 over the conventional gold standard iliac crest graft for restoration of maxillary alveolar clefts in terms of surgical, postoperative, and radiographic planimetry parameters, preferably with a minor addition of the vital zygoma bone shavings. Sparing young children from the invasive procedure of iliac crest harvesting is by itself a compelling reason to favor rhBMP-2 use as it avoids the need for an additional surgical site, blood loss, and postoperative morbidity in the iliac crest harvested site. The efficacy of the rhBMP-2 in establishing a bony union for the alveolar discontinuity defects is clear. As the surgical procedure of alveolar cleft repair using this innovative material involves only manipulation of the oral mucosa, it is possible in the distant future that patients would be able to tolerate this kind of surgery without the need for general anesthesia. Such an alveolar repair method may provide an easy access to perform these surgeries even at areas where facilities for general anesthesia are limited. Partial displacement of rhBMP-2 into the adjacent tissue may result in ectopic bone formation or may affect the nerves that come in contact with rhBMP-2. However no such effects have been observed in the literature in the craniofacial region or in our study. The long-term effects on this type of grafting procedure needs to be observed.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chin M, Ng T, Tom WK, Carstens M. Repair of alveolar clefts with recombinant Human Bone morphogenetic Protein (rhBMP2) in patients with Clefts. J Craniofac Surg. 2005;16:778–89. doi: 10.1097/01.scs.0000166802.49021.01. [DOI] [PubMed] [Google Scholar]
- 2.Wozney JM. Bone morphogenetic proteins. Prog Growth Factor Res. 1989;1:267–80. doi: 10.1016/0955-2235(89)90015-x. [DOI] [PubMed] [Google Scholar]
- 3.Wozney JM, Rosen V, Byrne M, Celeste AJ, Moutsatsos I, Wang EA. Growth factors influencing bone development. J Cell Sci Suppl. 1990;13:149–56. doi: 10.1242/jcs.1990.supplement_13.14. [DOI] [PubMed] [Google Scholar]
- 4.Clokie CM, Sandor GK. Reconstruction of 10 Major Mandibular defects using bioimplants containing BMP7. J Can Dent Assoc. 2008;74:67–72. [PubMed] [Google Scholar]
- 5.Balaji SM. Use of recombinant human bone Morphogenetic protein (rhBMP2) in reconstruction of Maxillary alveolar clefts. J Maxillofac Oral Surg. 2009;8:211–7. doi: 10.1007/s12663-009-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasko AW, Lane JM, Fellinger EJ, Rosen V, Wozney JM, Wang EA. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2).A radiographic, histological, and biomechanical study in rats [published erratum appears in J Bone Joint Surg Am 1992;74:1111] J Bone Joint Surg Am. 1992;74:659–70. [PubMed] [Google Scholar]
- 7.Sykaras N, Opperman LA. Bone morphogenetic proteins (BMPs): How do they function and what can they offer the clinician? J Oral Sci. 2003;45:57–73. doi: 10.2334/josnusd.45.57. [DOI] [PubMed] [Google Scholar]
- 8.Kawakami S, Hiura K, Yokozeki M, Takahashi T, Seike T, Nakanishi H, et al. Longitudinal evaluation of secondary bone grafting into the alveolar cleft. Cleft Palate Craniofac J. 2003;40:569–76. doi: 10.1597/02-052. [DOI] [PubMed] [Google Scholar]
- 9.Boyne PJ, Marx RE, Nevins M, Triplett G, Lazaro E, Lilly LC, et al. A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int J Periodontics Restorative Dent. 1997;17:11–25. [PubMed] [Google Scholar]
- 10.Balaji SM. Mandibular cystic defect: A composite approach with rhBMP-2 and rib graft. J Maxillofac Oral Surg. 2009;8:27–30. doi: 10.1007/s12663-009-0007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perri B, Cooper M, Lauryssen C, Anand N. Adverse swelling associated with use of rh-BMP-2 in anterior cervical discectomy and fusion: A case study. Spine J. 2007;7:235–9. doi: 10.1016/j.spinee.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Vig KW. Alveolar bone grafts: The surgical/orthodontic management of the cleft maxilla. Ann Acad Med Singapore. 1999;28:721–7. [PubMed] [Google Scholar]
- 13.Dickinson BP, Ashley RK, Wasson KL, O’Hara C, Gabbay J, Heller JB, et al. Reduced morbidity and improved healing with bone morphogenic protein-2 in older patients with alveolar cleft defects. Plast Reconstr Surg. 2008;121:209–17. doi: 10.1097/01.prs.0000293870.64781.12. [DOI] [PubMed] [Google Scholar]
- 14.Polly DW, Ackerman SJ, Shaffrey CI, Ogilvie JW, Wang JC, Stralka SW, et al. A cost analysis of bone morphogenic protein versus autogenous iliac crest bone graft in single-level anterior lumbar fusion. Orthopaedics. 2003;26:1027–37. doi: 10.3928/0147-7447-20031001-12. [DOI] [PubMed] [Google Scholar]
- 15.Ackerman SJ, Mafilios MS, Polly DW. Economic Evaluation of bone morphogenetic protein versus autogenous iliac crest bone graft in single-level anterior lumbar fusion an evidence-based modeling approach. Spine. 2002;27:S94–9. doi: 10.1097/00007632-200208151-00017. [DOI] [PubMed] [Google Scholar]


