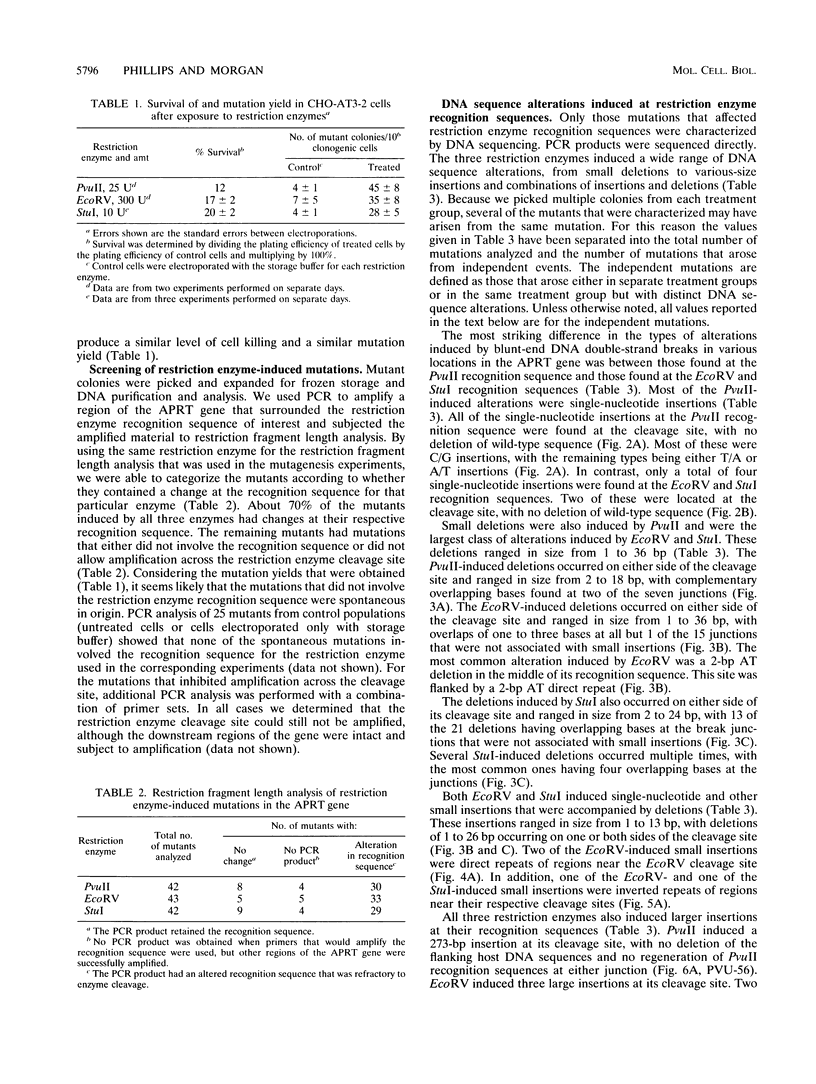
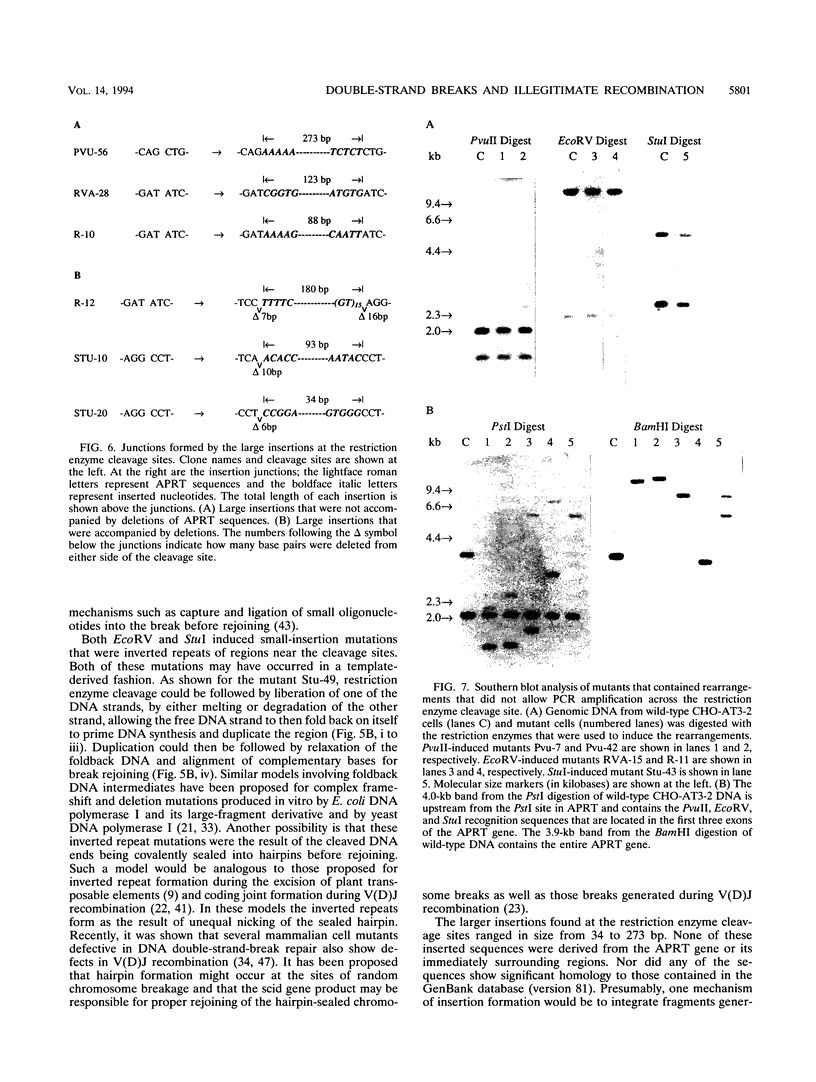
Abstract
We examined DNA double-strand-break-induced mutations in the endogenous adenine phosphoribosyl-transferase (APRT) gene in cultured Chinese hamster ovary cells after exposure to restriction endonucleases. PvuII, EcoRV, and StuI, all of which produce blunt-end DNA double-strand breaks, were electroporated into CHO-AT3-2 cells hemizygous at the APRT locus. Colonies of viable cells containing mutations at APRT were expanded, and the mutations that occurred during break repair were analyzed at the DNA sequence level. Restriction enzyme-induced mutations consisted of small deletions of 1 to 36 bp, insertions, and combinations of insertions and deletions at the cleavage sites. Most of the small deletions involved overlaps of one to four complementary bases at the recombination junctions. Southern blot analysis revealed more complex mutations, suggesting translocation, inversion, or insertion of larger chromosomal fragments. These results indicate that blunt-end DNA double-strand breaks can induce illegitimate (nonhomologous) recombination in mammalian chromosomes and that they play an important role in mutagenesis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abella Columna E., Giaccia A. J., Evans J. W., Yates B. L., Morgan W. F. Analysis of restriction enzyme-induced chromosomal aberrations by fluorescence in situ hybridization. Environ Mol Mutagen. 1993;22(1):26–33. doi: 10.1002/em.2850220106. [DOI] [PubMed] [Google Scholar]
- Adair G. M., Carver J. H., Wandres D. L. Mutagenicity testing in mammalian cells. I. Derivation of a Chinese hamster ovary cell line heterozygous for the adenine phosphoribosyltransferase and thymidine kinase loci. Mutat Res. 1980 Sep;72(2):187–205. doi: 10.1016/0027-5107(80)90035-4. [DOI] [PubMed] [Google Scholar]
- Ager D. D., Phillips J. W., Columna E. A., Winegar R. A., Morgan W. F. Analysis of restriction enzyme-induced DNA double-strand breaks in Chinese hamster ovary cells by pulsed-field gel electrophoresis: implications for chromosome damage. Radiat Res. 1991 Nov;128(2):150–156. [PubMed] [Google Scholar]
- Aplan P. D., Lombardi D. P., Ginsberg A. M., Cossman J., Bertness V. L., Kirsch I. R. Disruption of the human SCL locus by "illegitimate" V-(D)-J recombinase activity. Science. 1990 Dec 7;250(4986):1426–1429. doi: 10.1126/science.2255914. [DOI] [PubMed] [Google Scholar]
- Bryant P. E., Riches A. C. Oncogenic transformation of murine C3H 10T1/2 cells resulting from DNA double-strand breaks induced by a restriction endonuclease. Br J Cancer. 1989 Dec;60(6):852–854. doi: 10.1038/bjc.1989.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavolina P., Agnese C., Maddalena A., Sciandrello G., Di Leonardo A. Induction of CAD gene amplification by restriction endonucleases in V79,B7 Chinese hamster cells. Mutat Res. 1989 Jan-Feb;225(1-2):61–64. doi: 10.1016/0165-7992(89)90034-1. [DOI] [PubMed] [Google Scholar]
- Clark J. M. DNA synthesis on discontinuous templates by DNA polymerase I of Escherichia coli. Gene. 1991 Jul 31;104(1):75–80. doi: 10.1016/0378-1119(91)90467-p. [DOI] [PubMed] [Google Scholar]
- Clark J. M. Novel non-templated nucleotide addition reactions catalyzed by procaryotic and eucaryotic DNA polymerases. Nucleic Acids Res. 1988 Oct 25;16(20):9677–9686. doi: 10.1093/nar/16.20.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa N. D., Bryant P. E. The induction of DNA double-strand breaks in CHO cells by Pvu II: kinetics using neutral filter elution (pH 9.6). Int J Radiat Biol. 1990 May;57(5):933–938. doi: 10.1080/09553009014551051. [DOI] [PubMed] [Google Scholar]
- Costa N. D., Masson W. K., Thacker J. The effectiveness of restriction endonucleases in cell killing and mutation. Somat Cell Mol Genet. 1993 Sep;19(5):479–490. doi: 10.1007/BF01233253. [DOI] [PubMed] [Google Scholar]
- Dewey W. C., Miller H. H., Leeper D. B. Chromosomal aberrations and mortality of x-irradiated mammalian cells: emphasis on repair. Proc Natl Acad Sci U S A. 1971 Mar;68(3):667–671. doi: 10.1073/pnas.68.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Franklin N. C. Extraordinary recombinational events in Escherichia coli. Their independence of the rec+ function. Genetics. 1967 Apr;55(4):699–707. doi: 10.1093/genetics/55.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman T., Ståhl S., Hornes E., Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989 Jul 11;17(13):4937–4946. doi: 10.1093/nar/17.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. S., Fairley C. F., Morgan W. F. Bridging the gap. Joining of nonhomologous ends by DNA polymerases. J Biol Chem. 1994 May 6;269(18):13061–13064. [PubMed] [Google Scholar]
- King J. S., Valcarcel E. R., Rufer J. T., Phillips J. W., Morgan W. F. Noncomplementary DNA double-strand-break rejoining in bacterial and human cells. Nucleic Acids Res. 1993 Mar 11;21(5):1055–1059. doi: 10.1093/nar/21.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer K. M., Brock J. A., Bloom K., Moore J. K., Haber J. E. Two different types of double-strand breaks in Saccharomyces cerevisiae are repaired by similar RAD52-independent, nonhomologous recombination events. Mol Cell Biol. 1994 Feb;14(2):1293–1301. doi: 10.1128/mcb.14.2.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Hamatake R. K., Motto-Fox J., Fitzgerald M. P., Sugino A. Fidelity of DNA polymerase I and the DNA polymerase I-DNA primase complex from Saccharomyces cerevisiae. Mol Cell Biol. 1989 Oct;9(10):4447–4458. doi: 10.1128/mcb.9.10.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M. R. Site-specific recombination in the immune system. FASEB J. 1991 Nov;5(14):2934–2944. doi: 10.1096/fasebj.5.14.1752360. [DOI] [PubMed] [Google Scholar]
- Lieber M. R. The mechanism of V(D)J recombination: a balance of diversity, specificity, and stability. Cell. 1992 Sep 18;70(6):873–876. doi: 10.1016/0092-8674(92)90237-7. [DOI] [PubMed] [Google Scholar]
- Meuth M. The structure of mutation in mammalian cells. Biochim Biophys Acta. 1990 Jun 1;1032(1):1–17. doi: 10.1016/0304-419x(90)90009-p. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P. Studies on sequence recognition by type II restriction and modification enzymes. CRC Crit Rev Biochem. 1982;13(3):287–323. doi: 10.3109/10409238209114231. [DOI] [PubMed] [Google Scholar]
- Morgan W. F., Fero M. L., Land M. C., Winegar R. A. Inducible expression and cytogenetic effects of the EcoRI restriction endonuclease in Chinese hamster ovary cells. Mol Cell Biol. 1988 Oct;8(10):4204–4211. doi: 10.1128/mcb.8.10.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane J. P., Fuller L. F., Painter R. B. Establishment and characterization of a permanent pSV ori--transformed ataxia-telangiectasia cell line. Exp Cell Res. 1985 May;158(1):119–126. doi: 10.1016/0014-4827(85)90437-9. [DOI] [PubMed] [Google Scholar]
- Murnane J. P., Yezzi M. J., Young B. R. Recombination events during integration of transfected DNA into normal human cells. Nucleic Acids Res. 1990 May 11;18(9):2733–2738. doi: 10.1093/nar/18.9.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obe G., Von der Hude W., Scheutwinkel-Reich M., Basler A. The restriction endonuclease Alu I induces chromosomal aberrations and mutations in the hypoxanthine phosphoribosyltransferase locus, but not in the Na+/K+ ATPase locus in V79 hamster cells. Mutat Res. 1986 May;174(1):71–74. doi: 10.1016/0165-7992(86)90079-5. [DOI] [PubMed] [Google Scholar]
- Papanicolaou C., Ripley L. S. Polymerase-specific differences in the DNA intermediates of frameshift mutagenesis. In vitro synthesis errors of Escherichia coli DNA polymerase I and its large fragment derivative. J Mol Biol. 1989 May 20;207(2):335–353. doi: 10.1016/0022-2836(89)90258-1. [DOI] [PubMed] [Google Scholar]
- Pergola F., Zdzienicka M. Z., Lieber M. R. V(D)J recombination in mammalian cell mutants defective in DNA double-strand break repair. Mol Cell Biol. 1993 Jun;13(6):3464–3471. doi: 10.1128/mcb.13.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer P., Thode S., Hancke J., Vielmetter W. Mechanisms of overlap formation in nonhomologous DNA end joining. Mol Cell Biol. 1994 Feb;14(2):888–895. doi: 10.1128/mcb.14.2.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer P., Vielmetter W. Joining of nonhomologous DNA double strand breaks in vitro. Nucleic Acids Res. 1988 Feb 11;16(3):907–924. doi: 10.1093/nar/16.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J., Macelis D. Restriction enzymes and their isoschizomers. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2077–2109. doi: 10.1093/nar/19.suppl.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Chang X. B., Wilson J. H. Comparison of filler DNA at immune, nonimmune, and oncogenic rearrangements suggests multiple mechanisms of formation. Mol Cell Biol. 1989 Jul;9(7):3049–3057. doi: 10.1128/mcb.9.7.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Menetski J. P., Nakajima P. B., Bosma M. J., Gellert M. V(D)J recombination: broken DNA molecules with covalently sealed (hairpin) coding ends in scid mouse thymocytes. Cell. 1992 Sep 18;70(6):983–991. doi: 10.1016/0092-8674(92)90248-b. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Porter T. N., Wilson J. H. Mechanisms of nonhomologous recombination in mammalian cells. Mol Cell Biol. 1985 Oct;5(10):2599–2607. doi: 10.1128/mcb.5.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Proctor G. N., Stewart L. K., Wilson J. H. Oligonucleotide capture during end joining in mammalian cells. Nucleic Acids Res. 1991 Dec;19(25):7201–7205. doi: 10.1093/nar/19.25.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Wilson J. H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986 Dec;6(12):4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Bryant P. E. Induction of mutations at the thymidine kinase locus in CHO cells by restriction endonucleases. Mutagenesis. 1991 May;6(3):219–223. doi: 10.1093/mutage/6.3.219. [DOI] [PubMed] [Google Scholar]
- Sperry A. O., Blasquez V. C., Garrard W. T. Dysfunction of chromosomal loop attachment sites: illegitimate recombination linked to matrix association regions and topoisomerase II. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5497–5501. doi: 10.1073/pnas.86.14.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccioli G. E., Rathbun G., Oltz E., Stamato T., Jeggo P. A., Alt F. W. Impairment of V(D)J recombination in double-strand break repair mutants. Science. 1993 Apr 9;260(5105):207–210. doi: 10.1126/science.8469973. [DOI] [PubMed] [Google Scholar]
- Thacker J., Chalk J., Ganesh A., North P. A mechanism for deletion formation in DNA by human cell extracts: the involvement of short sequence repeats. Nucleic Acids Res. 1992 Dec 11;20(23):6183–6188. doi: 10.1093/nar/20.23.6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thode S., Schäfer A., Pfeiffer P., Vielmetter W. A novel pathway of DNA end-to-end joining. Cell. 1990 Mar 23;60(6):921–928. doi: 10.1016/0092-8674(90)90340-k. [DOI] [PubMed] [Google Scholar]
- Wake C. T., Gudewicz T., Porter T., White A., Wilson J. H. How damaged is the biologically active subpopulation of transfected DNA? Mol Cell Biol. 1984 Mar;4(3):387–398. doi: 10.1128/mcb.4.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. F. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- Winegar R. A., Lutze L. H., Rufer J. T., Morgan W. F. Spectrum of mutations produced by specific types of restriction enzyme-induced double-strand breaks. Mutagenesis. 1992 Nov;7(6):439–445. doi: 10.1093/mutage/7.6.439. [DOI] [PubMed] [Google Scholar]
- Winegar R. A., Phillips J. W., Youngblom J. H., Morgan W. F. Cell electroporation is a highly efficient method for introducing restriction endonucleases into cells. Mutat Res. 1989 Jan-Feb;225(1-2):49–53. doi: 10.1016/0165-7992(89)90032-8. [DOI] [PubMed] [Google Scholar]
- de Boer J. G., Drobetsky E. A., Grosovsky A. J., Mazur M., Glickman B. W. The Chinese hamster aprt gene as a mutational target. Its sequence and an analysis of direct and inverted repeats. Mutat Res. 1989 Aug;226(4):239–244. doi: 10.1016/0165-7992(89)90076-6. [DOI] [PubMed] [Google Scholar]



